

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Rattus norvegicus) | BDBM50366382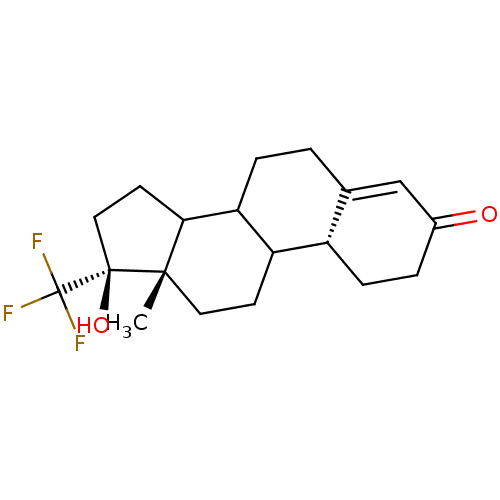 (CHEMBL57078) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]R5020 binding to cytosolic progesterone receptor (PRc) of rat uterus | Bioorg Med Chem Lett 5: 1899-1902 (1995) Article DOI: 10.1016/0960-894X(95)00320-S BindingDB Entry DOI: 10.7270/Q2KP82N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]R5020 binding to cytosolic progesterone receptor (PRc) of rat uterus | Bioorg Med Chem Lett 5: 1899-1902 (1995) Article DOI: 10.1016/0960-894X(95)00320-S BindingDB Entry DOI: 10.7270/Q2KP82N5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50366381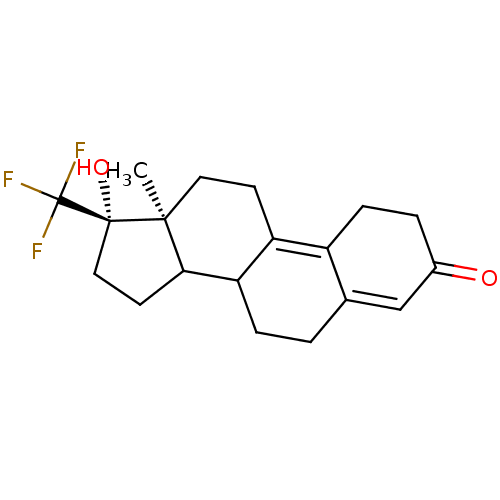 (CHEMBL301446) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]R5020 binding to cytosolic progesterone receptor (PRc) of rat uterus | Bioorg Med Chem Lett 5: 1899-1902 (1995) Article DOI: 10.1016/0960-894X(95)00320-S BindingDB Entry DOI: 10.7270/Q2KP82N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Rattus norvegicus) | BDBM50366380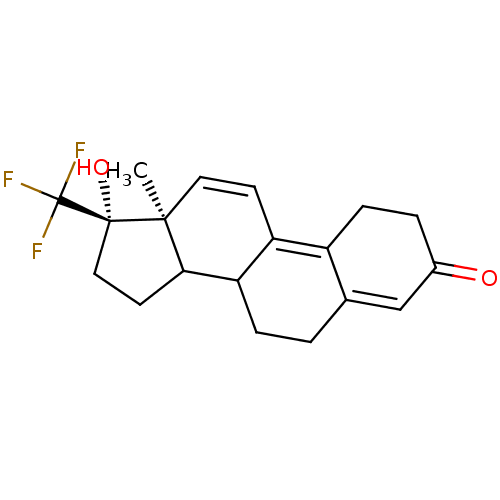 (CHEMBL56509) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]R5020 binding to cytosolic progesterone receptor (PRc) of rat uterus | Bioorg Med Chem Lett 5: 1899-1902 (1995) Article DOI: 10.1016/0960-894X(95)00320-S BindingDB Entry DOI: 10.7270/Q2KP82N5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50440614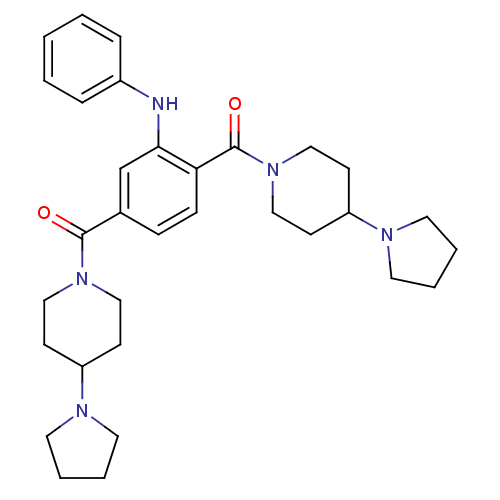 (CHEMBL2426364) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496581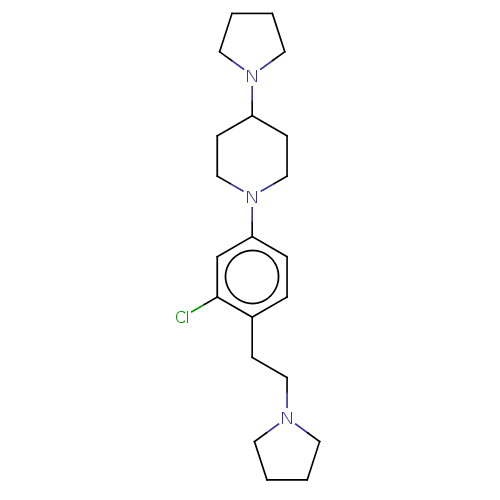 (CHEMBL3134130) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863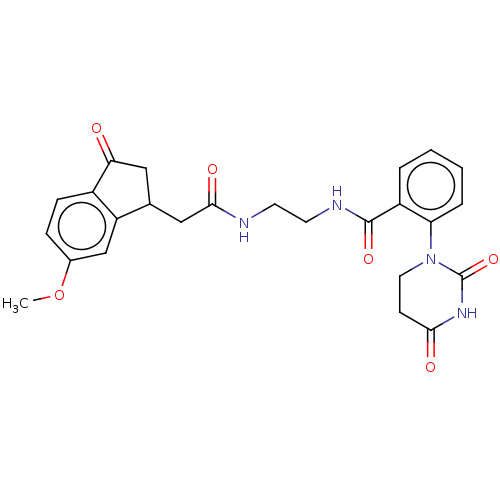 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496591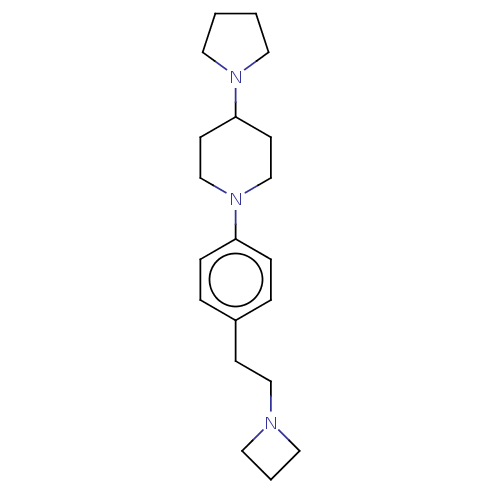 (CHEMBL3134144) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496584 (CHEMBL3134146) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496593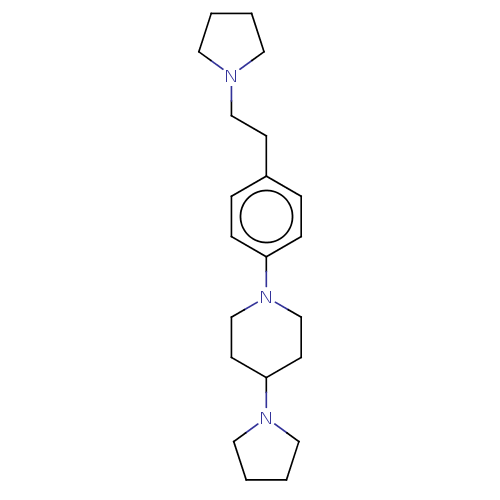 (CHEMBL3134131) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496596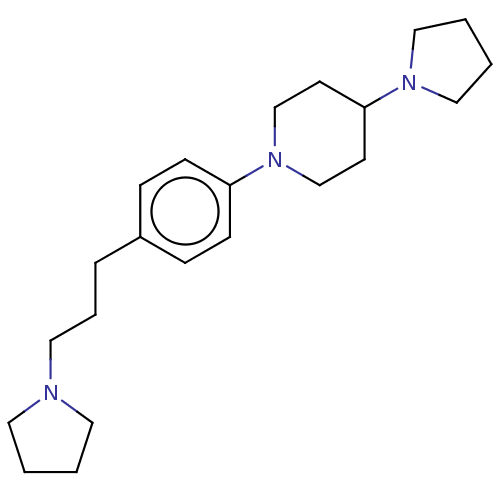 (CHEMBL3134134) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496589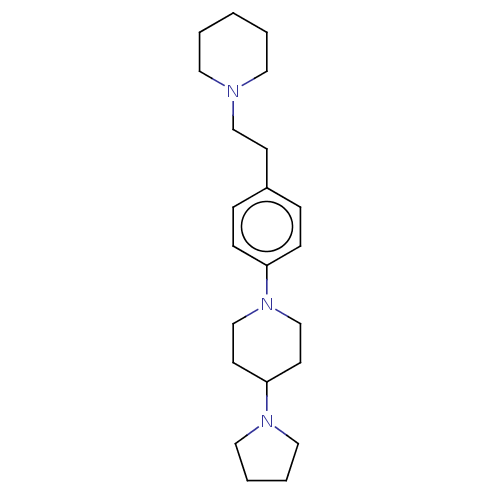 (CHEMBL3134126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496583 (CHEMBL3134125) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089867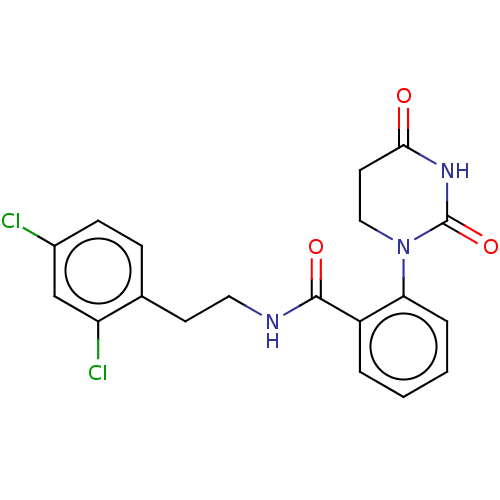 (CHEMBL3577626) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089867 (CHEMBL3577626) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496599 (CHEMBL3134136) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496592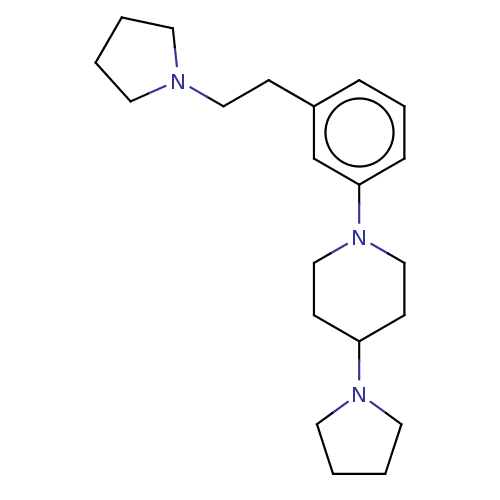 (CHEMBL3134135) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 224 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089865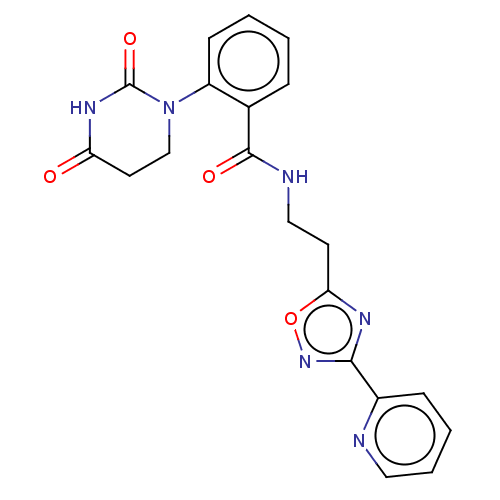 (CHEMBL3577624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50089865 (CHEMBL3577624) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of full length PARP1 (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase activity u... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496597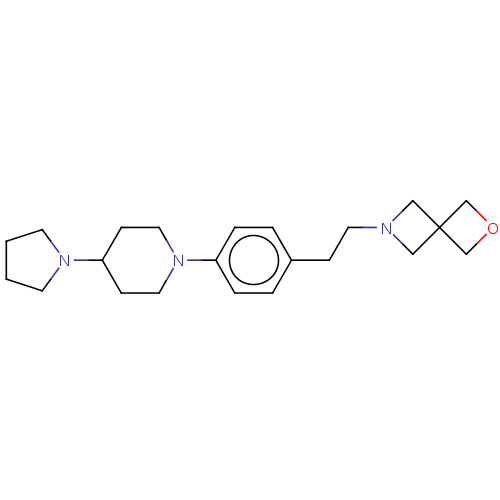 (CHEMBL3134129) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 288 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089863 (CHEMBL3577701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496598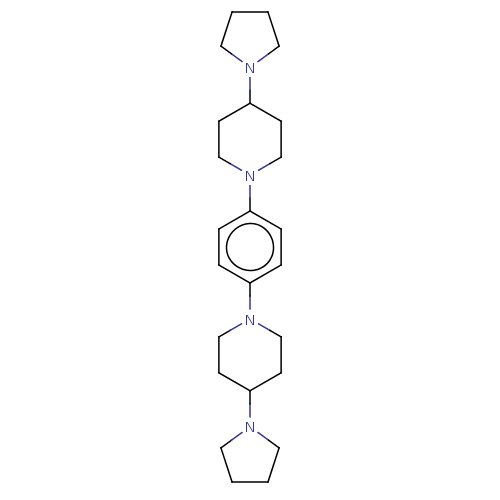 (CHEMBL3134140) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089866 (CHEMBL3577625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089864 (CHEMBL3577702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 331 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496579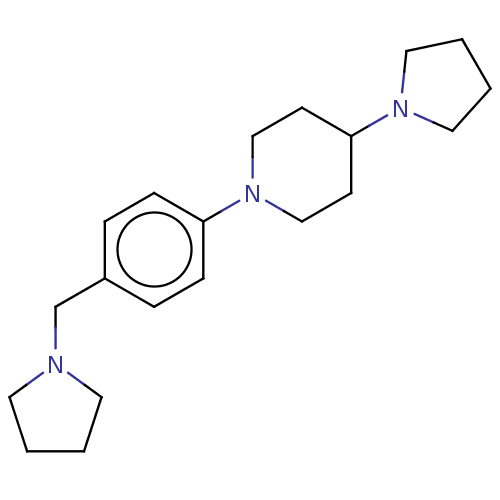 (CHEMBL3134133) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089858 (CHEMBL3577627) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496586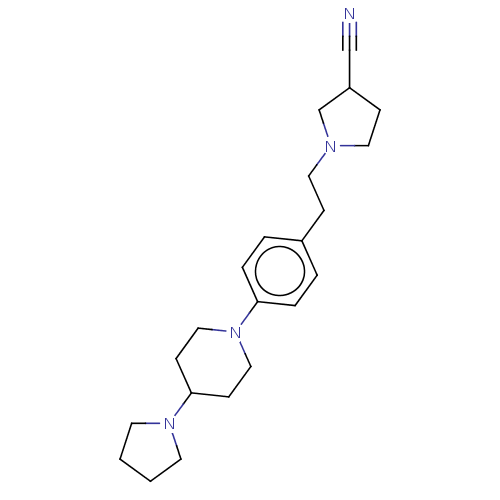 (CHEMBL3134142) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496585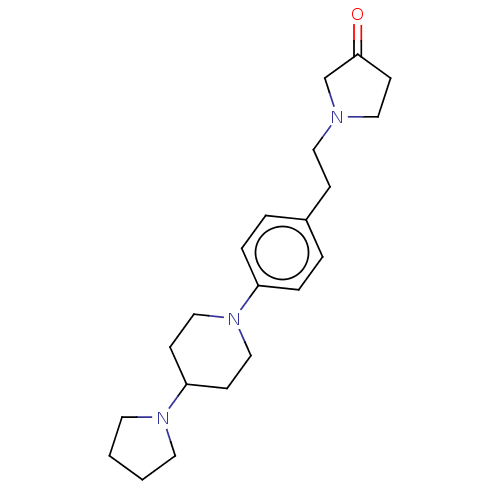 (CHEMBL3134143) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50089862 (CHEMBL3577700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of tankyrase 2 catalytic domain (unknown origin) expressed in Escherichia coli BL21 (DE3) assessed as reduction in ADP-ribosyl transferase... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089859 (CHEMBL3577628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50089859 (CHEMBL3577628) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 575 | n/a | n/a | n/a | n/a | n/a | n/a |
£Philochem AG Curated by ChEMBL | Assay Description Inhibition of hexa-histidine tagged recombinant tankyrase 1 biotinylated catalytic domain (amino acids 1106 to 1325) (unknown origin) expressed in Es... | J Med Chem 58: 5143-9 (2015) Article DOI: 10.1021/acs.jmedchem.5b00432 BindingDB Entry DOI: 10.7270/Q2R2134J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lethal(3)malignant brain tumor-like protein 3 (Homo sapiens (Human)) | BDBM50496580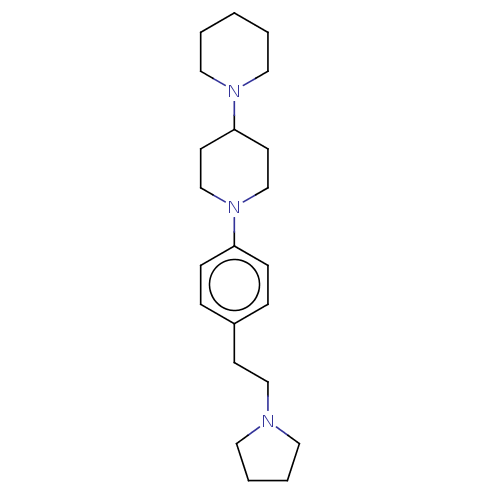 (CHEMBL3134132) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibition of His-tagged L3MBTL3 (unknown origin) using H4K20Me2 as substrate incubated for 30 mins at room temperature followed by incubation under ... | Medchemcomm 4: 1501-1507 (2013) Article DOI: 10.1039/c3md00197k BindingDB Entry DOI: 10.7270/Q2BV7KKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 272 total ) | Next | Last >> |