

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402859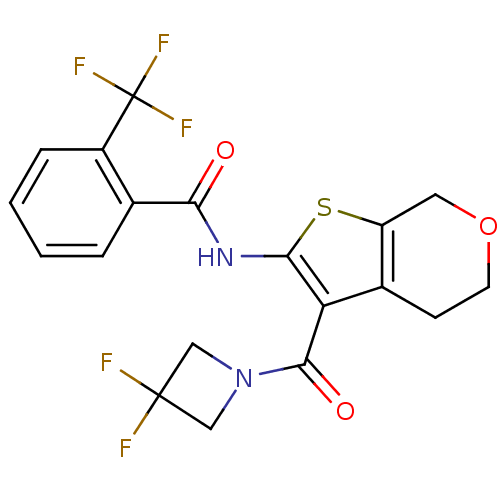 (CHEMBL2205591) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402858 (CHEMBL2205592) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402864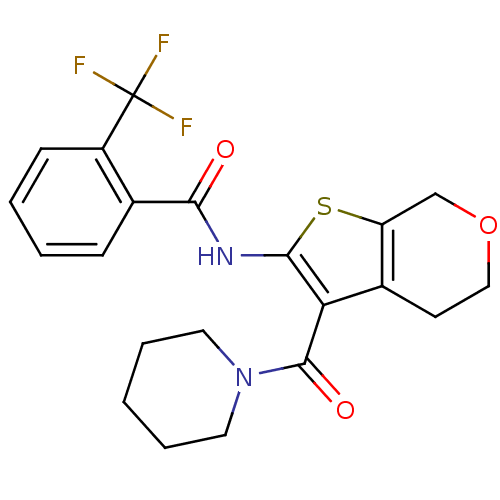 (CHEMBL2205615) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402863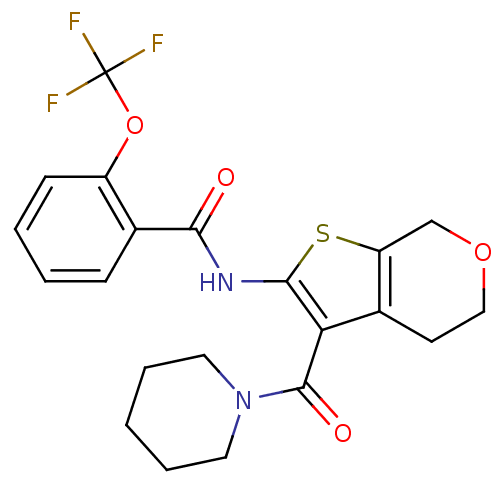 (CHEMBL2205616) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402857 (CHEMBL2205593) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402859 (CHEMBL2205591) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402857 (CHEMBL2205593) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402860 (CHEMBL2205588) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402861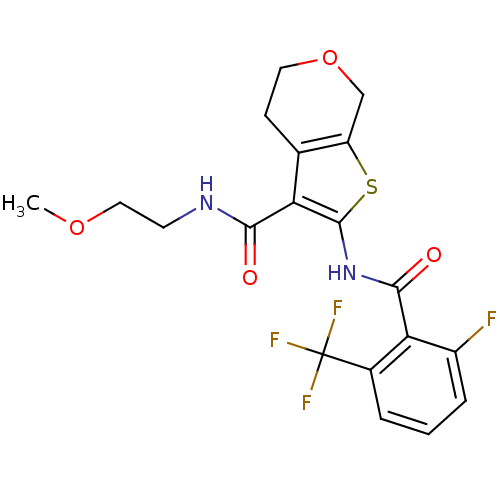 (CHEMBL2205584) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50402862 (CHEMBL2205620) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402864 (CHEMBL2205615) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402861 (CHEMBL2205584) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402863 (CHEMBL2205616) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402862 (CHEMBL2205620) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402860 (CHEMBL2205588) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50402858 (CHEMBL2205592) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402859 (CHEMBL2205591) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402857 (CHEMBL2205593) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402864 (CHEMBL2205615) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402857 (CHEMBL2205593) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402864 (CHEMBL2205615) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402863 (CHEMBL2205616) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402863 (CHEMBL2205616) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402861 (CHEMBL2205584) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402860 (CHEMBL2205588) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402859 (CHEMBL2205591) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402858 (CHEMBL2205592) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50402862 (CHEMBL2205620) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from rat CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402862 (CHEMBL2205620) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402861 (CHEMBL2205584) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402860 (CHEMBL2205588) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50402858 (CHEMBL2205592) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor after 60 mins by microscintillation counting analysis | Bioorg Med Chem Lett 22: 7314-21 (2012) Article DOI: 10.1016/j.bmcl.2012.10.087 BindingDB Entry DOI: 10.7270/Q2QR4Z9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180790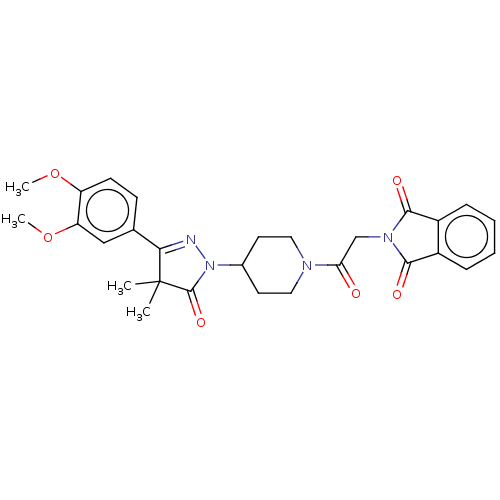 (US8865745, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM60633 (US8865745, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180793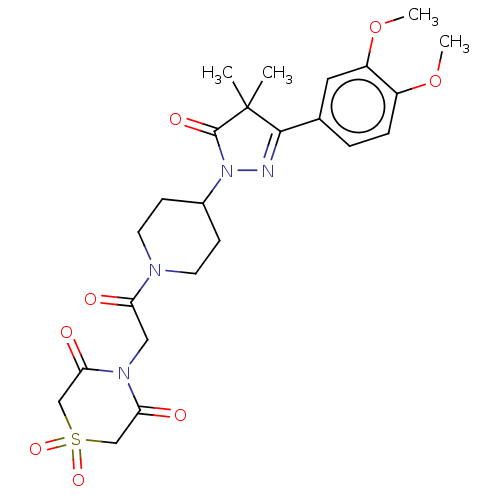 (US8865745, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180779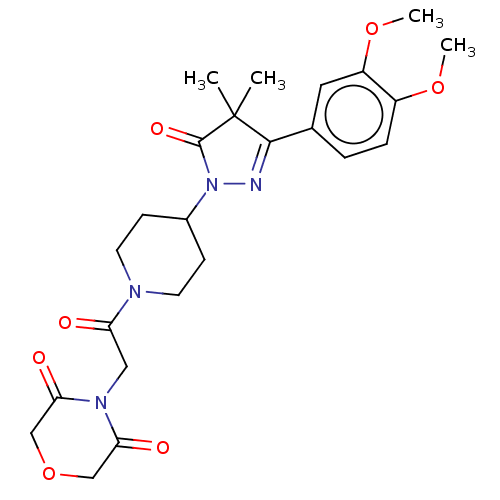 (US8865745, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM60635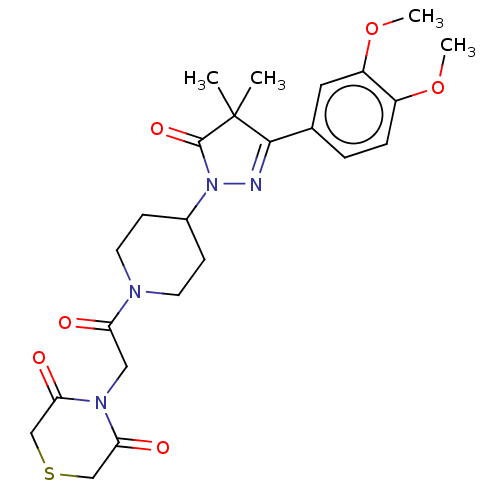 (US8865745, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.37 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180795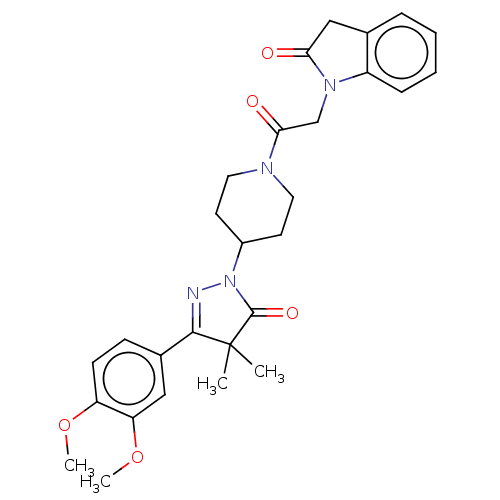 (US8865745, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.75 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM60634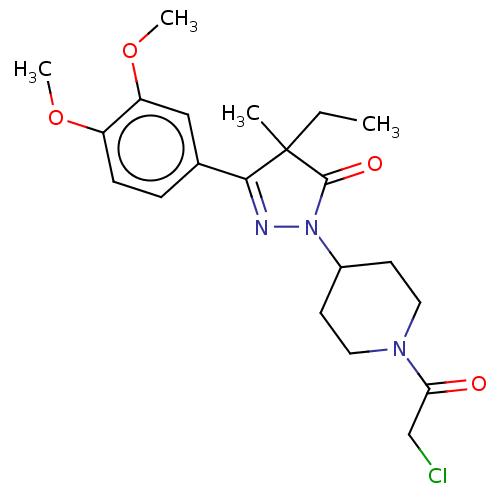 (US8865745, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.89 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180780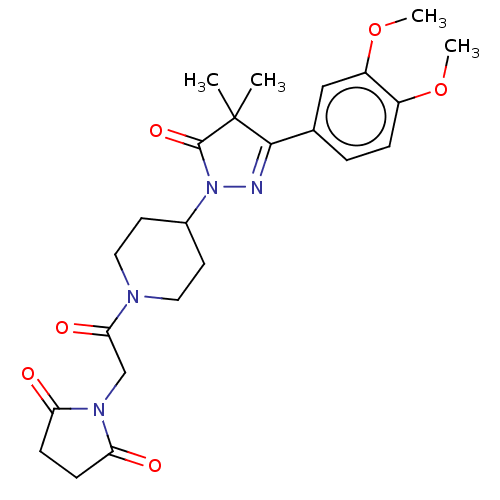 (US8865745, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.13 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180787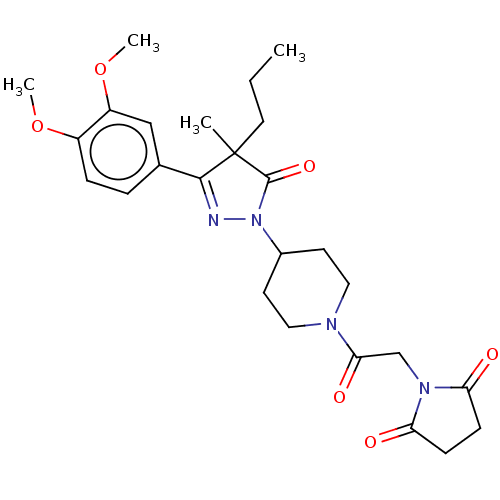 (US8865745, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180791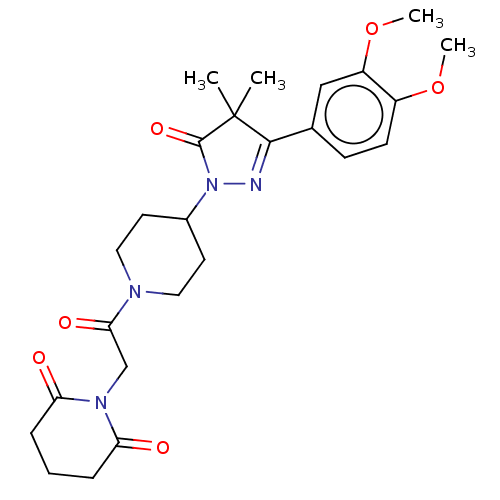 (US8865745, 15) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180789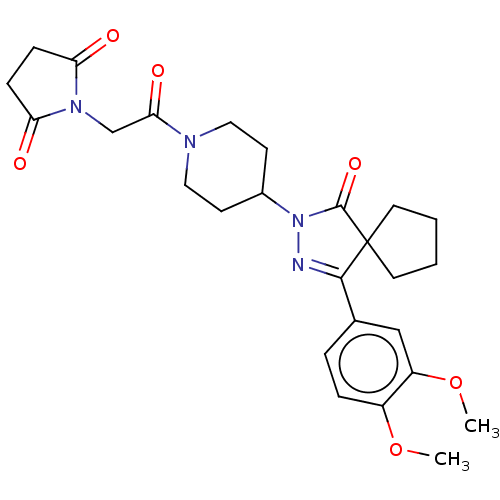 (US8865745, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 16.2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180781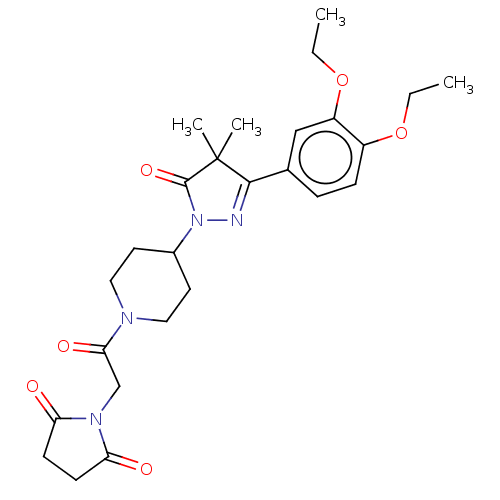 (US8865745, 3) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180782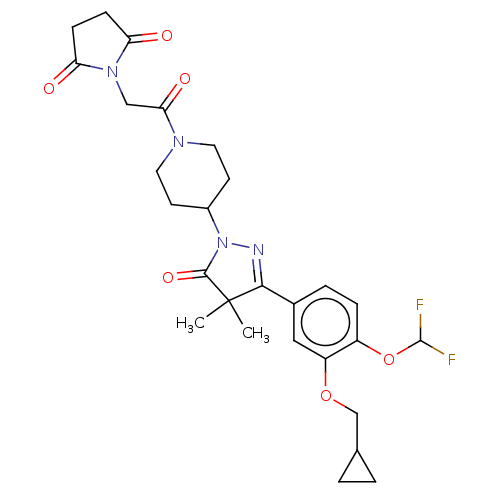 (US8865745, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180785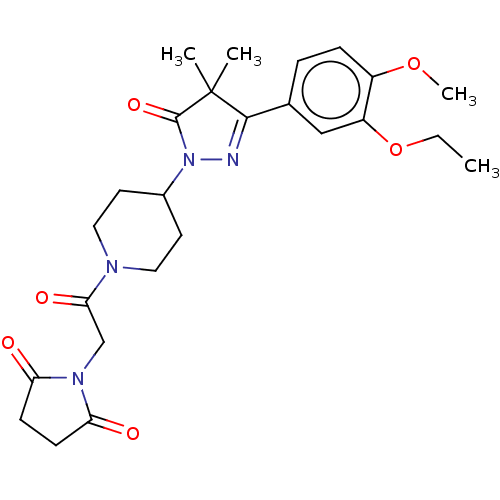 (US8865745, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22.4 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180794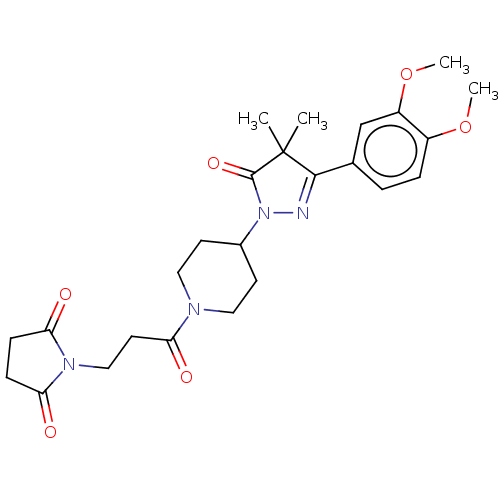 (US8865745, 19) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180792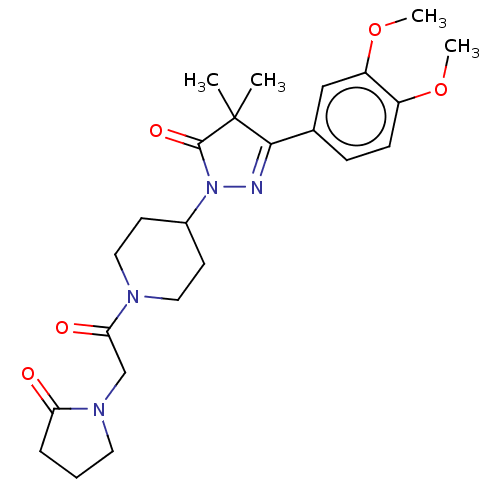 (US8865745, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 33.1 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180788 (US8865745, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 34.7 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM180783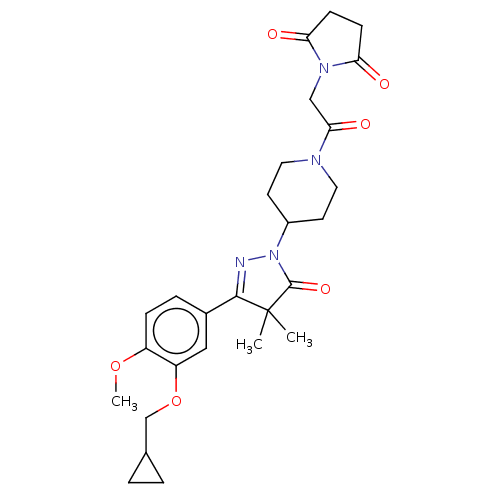 (US8865745, 5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70.8 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH US Patent | Assay Description PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... | US Patent US8865745 (2014) BindingDB Entry DOI: 10.7270/Q2XK8DBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 182 total ) | Next | Last >> |