 Found 3239 hits with Last Name = 'de jongh' and Initial = 's'
Found 3239 hits with Last Name = 'de jongh' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514737
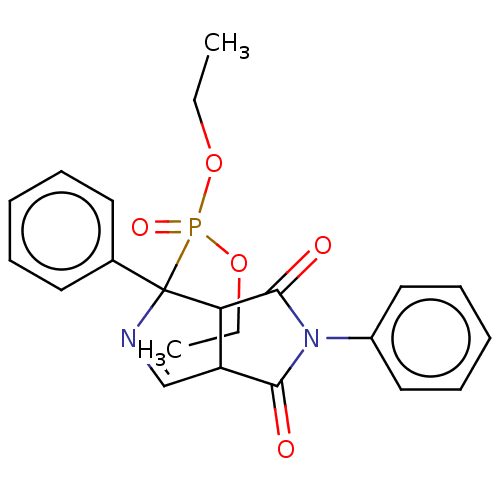
(CHEMBL4482861)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccccc1)c1ccccc1 |c:9| Show InChI InChI=1S/C22H23N2O5P/c1-3-28-30(27,29-4-2)22(16-11-7-5-8-12-16)19-18(15-23-22)20(25)24(21(19)26)17-13-9-6-10-14-17/h5-15,18-19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0417 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514738
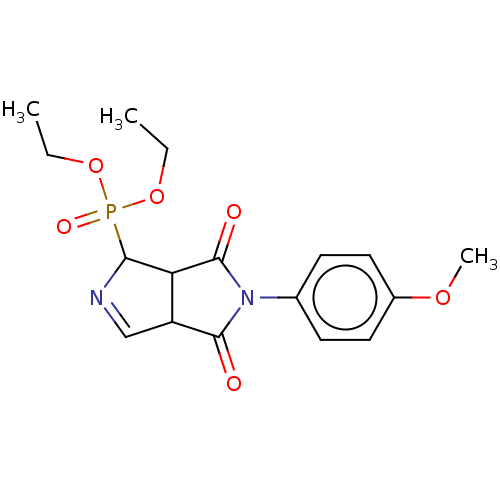
(CHEMBL4536304)Show SMILES CCOP(=O)(OCC)C1N=CC2C1C(=O)N(C2=O)c1ccc(OC)cc1 |c:9| Show InChI InChI=1S/C17H21N2O6P/c1-4-24-26(22,25-5-2)15-14-13(10-18-15)16(20)19(17(14)21)11-6-8-12(23-3)9-7-11/h6-10,13-15H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514722
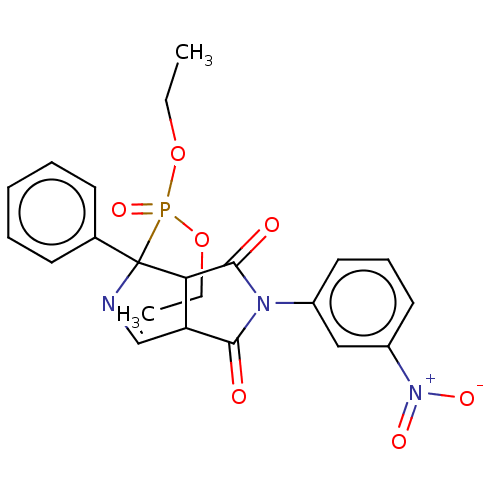
(CHEMBL4438801)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1cccc(c1)[N+]([O-])=O)c1ccccc1 |c:9| Show InChI InChI=1S/C22H22N3O7P/c1-3-31-33(30,32-4-2)22(15-9-6-5-7-10-15)19-18(14-23-22)20(26)24(21(19)27)16-11-8-12-17(13-16)25(28)29/h5-14,18-19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0661 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514727
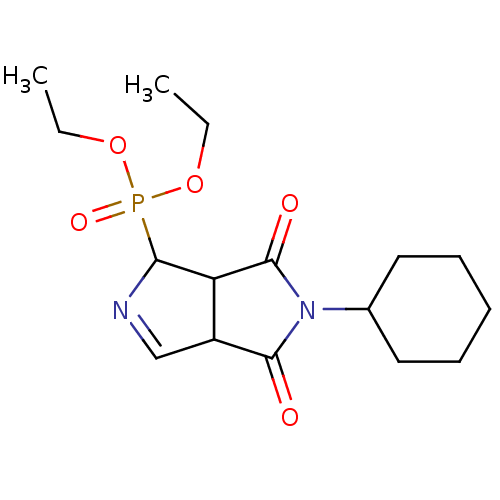
(CHEMBL4483022)Show SMILES CCOP(=O)(OCC)C1N=CC2C1C(=O)N(C1CCCCC1)C2=O |c:9| Show InChI InChI=1S/C16H25N2O5P/c1-3-22-24(21,23-4-2)14-13-12(10-17-14)15(19)18(16(13)20)11-8-6-5-7-9-11/h10-14H,3-9H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514724
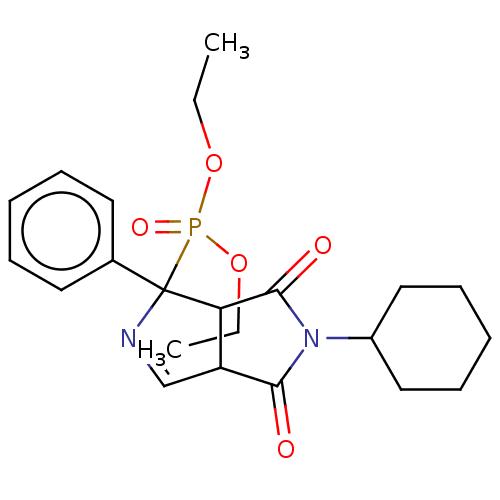
(CHEMBL4535472)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C1CCCCC1)C2=O)c1ccccc1 |c:9| Show InChI InChI=1S/C22H29N2O5P/c1-3-28-30(27,29-4-2)22(16-11-7-5-8-12-16)19-18(15-23-22)20(25)24(21(19)26)17-13-9-6-10-14-17/h5,7-8,11-12,15,17-19H,3-4,6,9-10,13-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514745

(CHEMBL4439953)Show SMILES CCOP(=O)(OCC)C1N=CC2C1C(=O)N(C2=O)c1ccc(F)c(Cl)c1 |c:9| Show InChI InChI=1S/C16H17ClFN2O5P/c1-3-24-26(23,25-4-2)14-13-10(8-19-14)15(21)20(16(13)22)9-5-6-12(18)11(17)7-9/h5-8,10,13-14H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514739
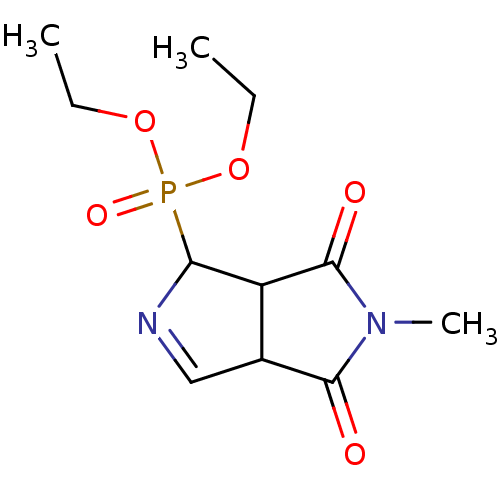
(CHEMBL4467833)Show InChI InChI=1S/C11H17N2O5P/c1-4-17-19(16,18-5-2)9-8-7(6-12-9)10(14)13(3)11(8)15/h6-9H,4-5H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50019848
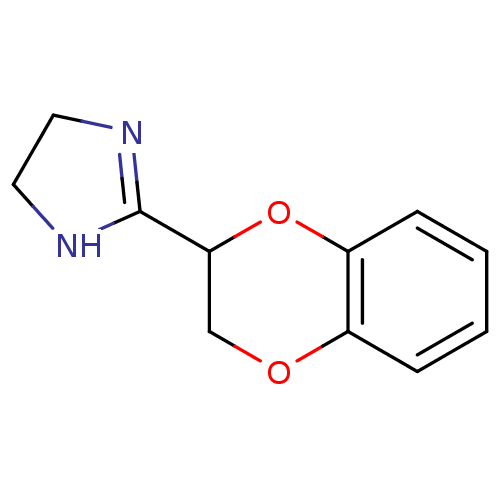
(2-(2,3-dihydro-1,4-benzodioxin-2-yl)-4,5-dihydro-1...)Show InChI InChI=1S/C11H12N2O2/c1-2-4-9-8(3-1)14-7-10(15-9)11-12-5-6-13-11/h1-4,10H,5-7H2,(H,12,13) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-RX821002 from adrenergic alpha 2 receptor in post-mortem human brain frontal cortex membrane measured after 30 mins by liquid sc... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113540
BindingDB Entry DOI: 10.7270/Q2X06BVM |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031825
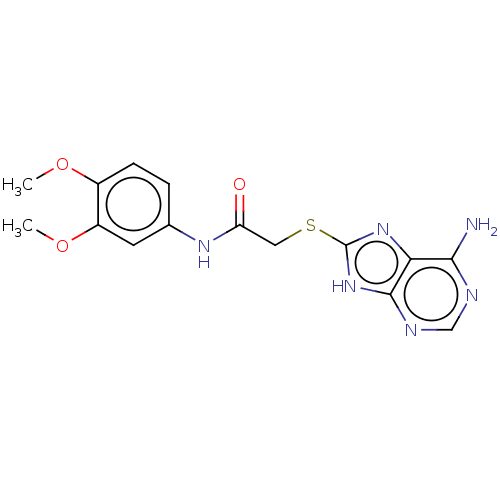
(CHEMBL3360892)Show InChI InChI=1S/C15H16N6O3S/c1-23-9-4-3-8(5-10(9)24-2)19-11(22)6-25-15-20-12-13(16)17-7-18-14(12)21-15/h3-5,7H,6H2,1-2H3,(H,19,22)(H3,16,17,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031825

(CHEMBL3360892)Show InChI InChI=1S/C15H16N6O3S/c1-23-9-4-3-8(5-10(9)24-2)19-11(22)6-25-15-20-12-13(16)17-7-18-14(12)21-15/h3-5,7H,6H2,1-2H3,(H,19,22)(H3,16,17,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514718
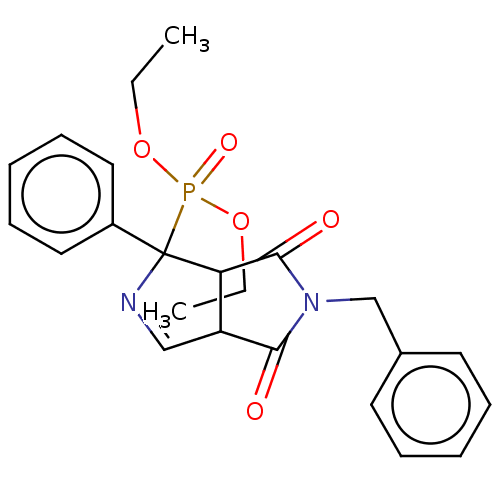
(CHEMBL4438158)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(Cc1ccccc1)C2=O)c1ccccc1 |c:9| Show InChI InChI=1S/C23H25N2O5P/c1-3-29-31(28,30-4-2)23(18-13-9-6-10-14-18)20-19(15-24-23)21(26)25(22(20)27)16-17-11-7-5-8-12-17/h5-15,19-20H,3-4,16H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031824
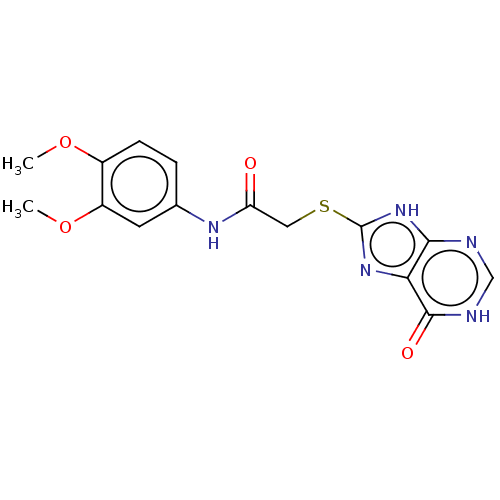
(CHEMBL3360893)Show SMILES COc1ccc(NC(=O)CSc2nc3c(nc[nH]c3=O)[nH]2)cc1OC Show InChI InChI=1S/C15H15N5O4S/c1-23-9-4-3-8(5-10(9)24-2)18-11(21)6-25-15-19-12-13(20-15)16-7-17-14(12)22/h3-5,7H,6H2,1-2H3,(H,18,21)(H2,16,17,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031824

(CHEMBL3360893)Show SMILES COc1ccc(NC(=O)CSc2nc3c(nc[nH]c3=O)[nH]2)cc1OC Show InChI InChI=1S/C15H15N5O4S/c1-23-9-4-3-8(5-10(9)24-2)18-11(21)6-25-15-19-12-13(20-15)16-7-17-14(12)22/h3-5,7H,6H2,1-2H3,(H,18,21)(H2,16,17,19,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50398211
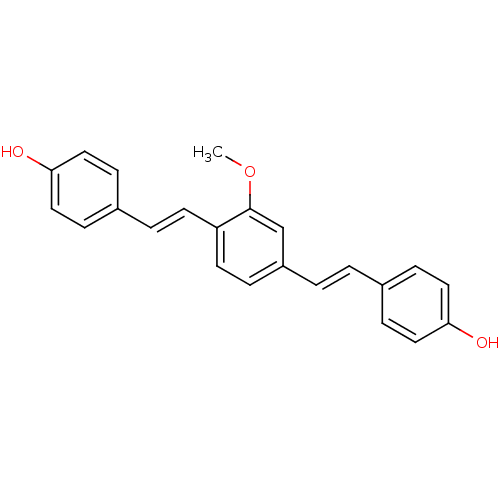
(CHEMBL2181036)Show SMILES COc1cc(\C=C\c2ccc(O)cc2)ccc1\C=C\c1ccc(O)cc1 Show InChI InChI=1S/C23H20O3/c1-26-23-16-19(3-2-17-6-12-21(24)13-7-17)5-11-20(23)10-4-18-8-14-22(25)15-9-18/h2-16,24-25H,1H3/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031827
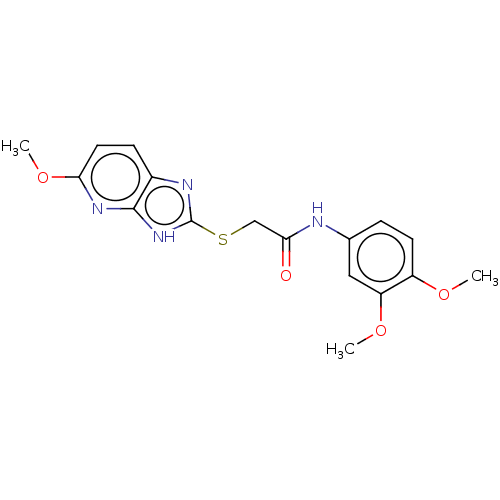
(CHEMBL3360884)Show SMILES COc1ccc2nc(SCC(=O)Nc3ccc(OC)c(OC)c3)[nH]c2n1 Show InChI InChI=1S/C17H18N4O4S/c1-23-12-6-4-10(8-13(12)24-2)18-14(22)9-26-17-19-11-5-7-15(25-3)20-16(11)21-17/h4-8H,9H2,1-3H3,(H,18,22)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031827

(CHEMBL3360884)Show SMILES COc1ccc2nc(SCC(=O)Nc3ccc(OC)c(OC)c3)[nH]c2n1 Show InChI InChI=1S/C17H18N4O4S/c1-23-12-6-4-10(8-13(12)24-2)18-14(22)9-26-17-19-11-5-7-15(25-3)20-16(11)21-17/h4-8H,9H2,1-3H3,(H,18,22)(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031817
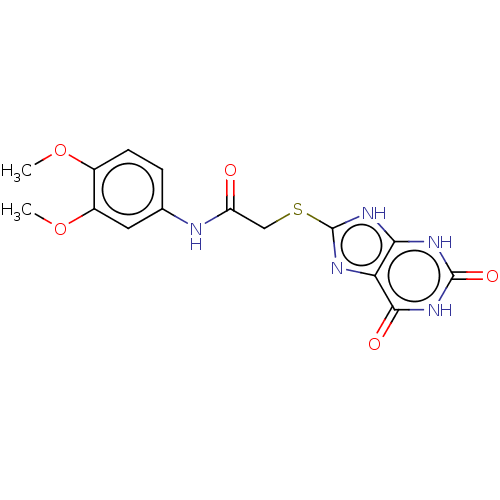
(CHEMBL3360901)Show SMILES COc1ccc(NC(=O)CSc2nc3c([nH]2)[nH]c(=O)[nH]c3=O)cc1OC Show InChI InChI=1S/C15H15N5O5S/c1-24-8-4-3-7(5-9(8)25-2)16-10(21)6-26-15-17-11-12(19-15)18-14(23)20-13(11)22/h3-5H,6H2,1-2H3,(H,16,21)(H3,17,18,19,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031817

(CHEMBL3360901)Show SMILES COc1ccc(NC(=O)CSc2nc3c([nH]2)[nH]c(=O)[nH]c3=O)cc1OC Show InChI InChI=1S/C15H15N5O5S/c1-24-8-4-3-7(5-9(8)25-2)16-10(21)6-26-15-17-11-12(19-15)18-14(23)20-13(11)22/h3-5H,6H2,1-2H3,(H,16,21)(H3,17,18,19,20,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002834
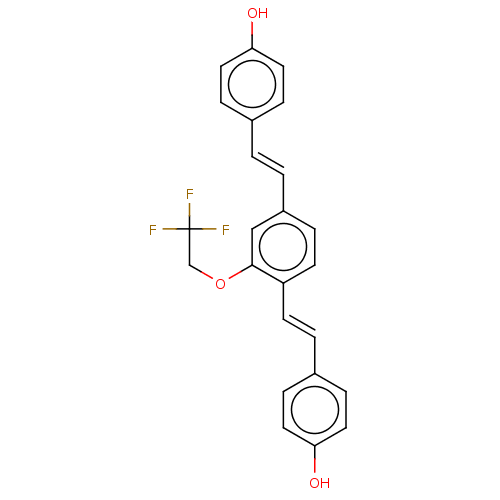
(CHEMBL3233658)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C24H19F3O3/c25-24(26,27)16-30-23-15-19(2-1-17-5-11-21(28)12-6-17)4-10-20(23)9-3-18-7-13-22(29)14-8-18/h1-15,28-29H,16H2/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031881
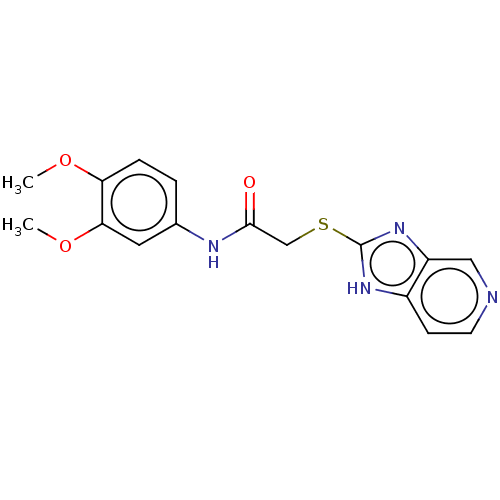
(CHEMBL3360878)Show InChI InChI=1S/C16H16N4O3S/c1-22-13-4-3-10(7-14(13)23-2)18-15(21)9-24-16-19-11-5-6-17-8-12(11)20-16/h3-8H,9H2,1-2H3,(H,18,21)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031881

(CHEMBL3360878)Show InChI InChI=1S/C16H16N4O3S/c1-22-13-4-3-10(7-14(13)23-2)18-15(21)9-24-16-19-11-5-6-17-8-12(11)20-16/h3-8H,9H2,1-2H3,(H,18,21)(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002835
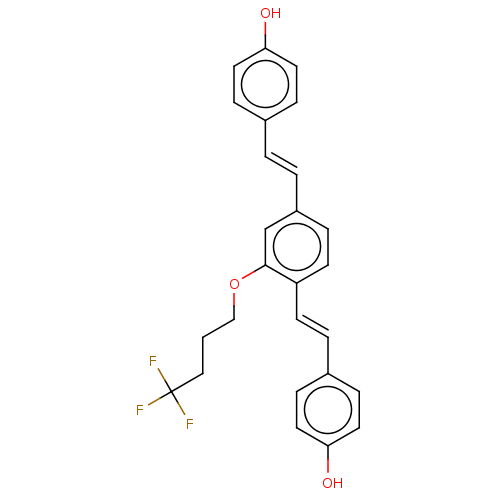
(CHEMBL3233659)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OCCCC(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H23F3O3/c27-26(28,29)16-1-17-32-25-18-21(3-2-19-6-12-23(30)13-7-19)5-11-22(25)10-4-20-8-14-24(31)15-9-20/h2-15,18,30-31H,1,16-17H2/b3-2+,10-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50179357
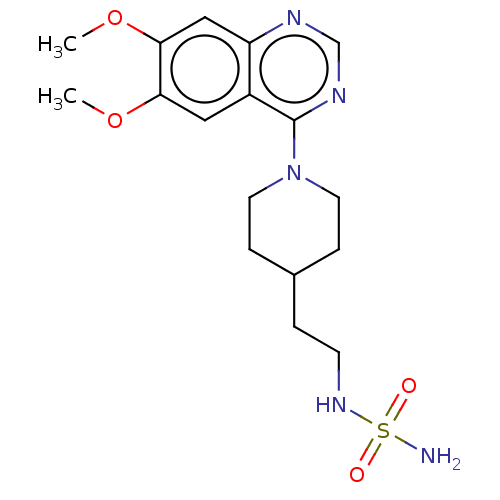
(CHEMBL1783609)Show SMILES COc1cc2ncnc(N3CCC(CCNS(N)(=O)=O)CC3)c2cc1OC Show InChI InChI=1S/C17H25N5O4S/c1-25-15-9-13-14(10-16(15)26-2)19-11-20-17(13)22-7-4-12(5-8-22)3-6-21-27(18,23)24/h9-12,21H,3-8H2,1-2H3,(H2,18,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human NPP1 expressed in African green monkey COS7 cells using p-Nph-5'-TMP as substrate after 60 mins by Dixon plot ana... |
Bioorg Med Chem 24: 3157-65 (2016)
Article DOI: 10.1016/j.bmc.2016.05.046
BindingDB Entry DOI: 10.7270/Q2SQ928T |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514734
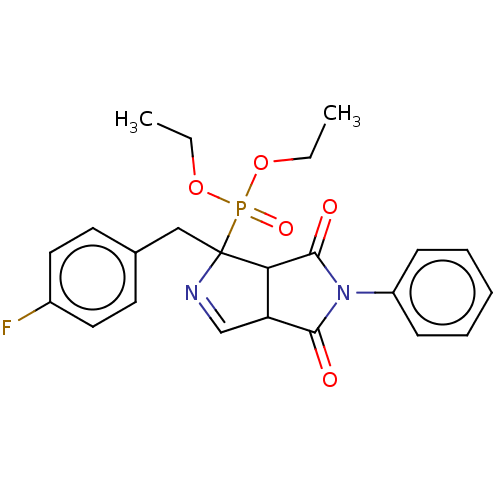
(CHEMBL4568994)Show SMILES CCOP(=O)(OCC)C1(Cc2ccc(F)cc2)N=CC2C1C(=O)N(C2=O)c1ccccc1 |c:18| Show InChI InChI=1S/C23H24FN2O5P/c1-3-30-32(29,31-4-2)23(14-16-10-12-17(24)13-11-16)20-19(15-25-23)21(27)26(22(20)28)18-8-6-5-7-9-18/h5-13,15,19-20H,3-4,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002836

(CHEMBL3233660)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(OC(C(F)(F)F)(C(F)(F)F)C(F)(F)F)c2)cc1 Show InChI InChI=1S/C26H17F9O3/c27-24(28,29)23(25(30,31)32,26(33,34)35)38-22-15-18(2-1-16-5-11-20(36)12-6-16)4-10-19(22)9-3-17-7-13-21(37)14-8-17/h1-15,36-37H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031826
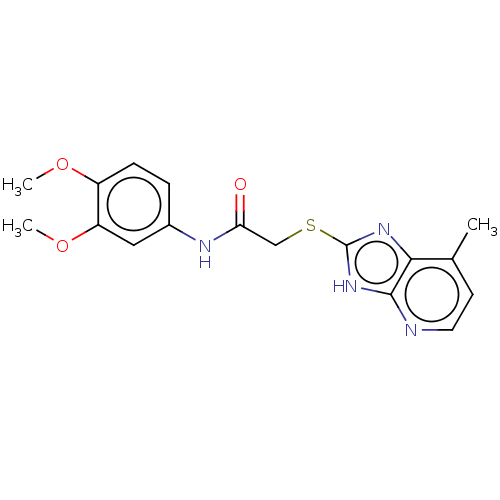
(CHEMBL3360885)Show InChI InChI=1S/C17H18N4O3S/c1-10-6-7-18-16-15(10)20-17(21-16)25-9-14(22)19-11-4-5-12(23-2)13(8-11)24-3/h4-8H,9H2,1-3H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031826

(CHEMBL3360885)Show InChI InChI=1S/C17H18N4O3S/c1-10-6-7-18-16-15(10)20-17(21-16)25-9-14(22)19-11-4-5-12(23-2)13(8-11)24-3/h4-8H,9H2,1-3H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031830
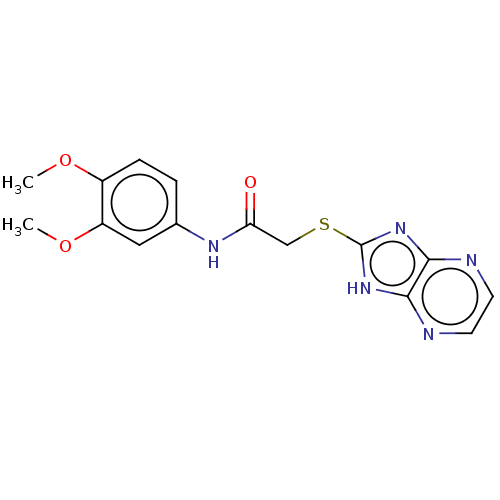
(CHEMBL3360880)Show InChI InChI=1S/C15H15N5O3S/c1-22-10-4-3-9(7-11(10)23-2)18-12(21)8-24-15-19-13-14(20-15)17-6-5-16-13/h3-7H,8H2,1-2H3,(H,18,21)(H,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031830

(CHEMBL3360880)Show InChI InChI=1S/C15H15N5O3S/c1-22-10-4-3-9(7-11(10)23-2)18-12(21)8-24-15-19-13-14(20-15)17-6-5-16-13/h3-7H,8H2,1-2H3,(H,18,21)(H,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031829
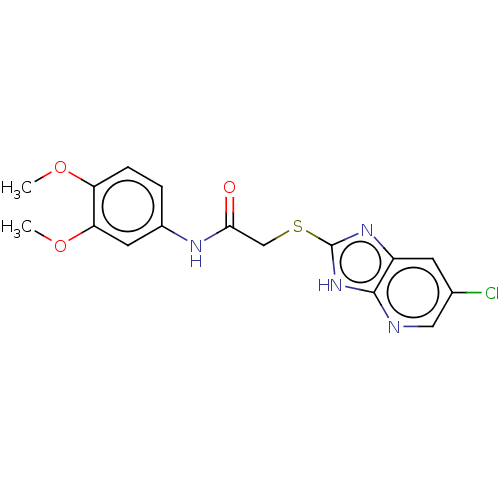
(CHEMBL3360882)Show InChI InChI=1S/C16H15ClN4O3S/c1-23-12-4-3-10(6-13(12)24-2)19-14(22)8-25-16-20-11-5-9(17)7-18-15(11)21-16/h3-7H,8H2,1-2H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031829

(CHEMBL3360882)Show InChI InChI=1S/C16H15ClN4O3S/c1-23-12-4-3-10(6-13(12)24-2)19-14(22)8-25-16-20-11-5-9(17)7-18-15(11)21-16/h3-7H,8H2,1-2H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031818
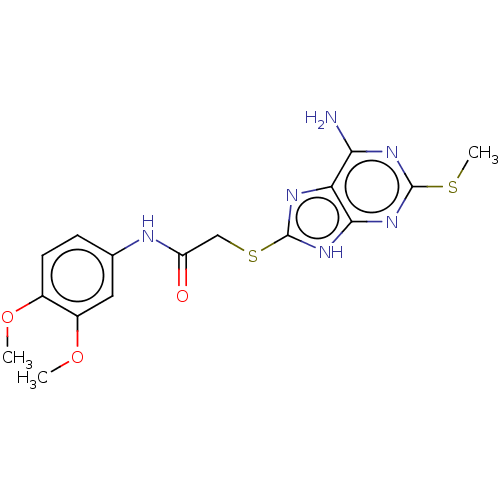
(CHEMBL3360899)Show SMILES COc1ccc(NC(=O)CSc2nc3c(N)nc(SC)nc3[nH]2)cc1OC Show InChI InChI=1S/C16H18N6O3S2/c1-24-9-5-4-8(6-10(9)25-2)18-11(23)7-27-16-19-12-13(17)20-15(26-3)21-14(12)22-16/h4-6H,7H2,1-3H3,(H,18,23)(H3,17,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031818

(CHEMBL3360899)Show SMILES COc1ccc(NC(=O)CSc2nc3c(N)nc(SC)nc3[nH]2)cc1OC Show InChI InChI=1S/C16H18N6O3S2/c1-24-9-5-4-8(6-10(9)25-2)18-11(23)7-27-16-19-12-13(17)20-15(26-3)21-14(12)22-16/h4-6H,7H2,1-3H3,(H,18,23)(H3,17,19,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514743

(CHEMBL4454147)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccccc1)c1ccc(F)cc1 |c:9| Show InChI InChI=1S/C22H22FN2O5P/c1-3-29-31(28,30-4-2)22(15-10-12-16(23)13-11-15)19-18(14-24-22)20(26)25(21(19)27)17-8-6-5-7-9-17/h5-14,18-19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514751

(CHEMBL4554450)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)C(C)(C)C)c1ccccc1 |c:9| Show InChI InChI=1S/C20H27N2O5P/c1-6-26-28(25,27-7-2)20(14-11-9-8-10-12-14)16-15(13-21-20)17(23)22(18(16)24)19(3,4)5/h8-13,15-16H,6-7H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
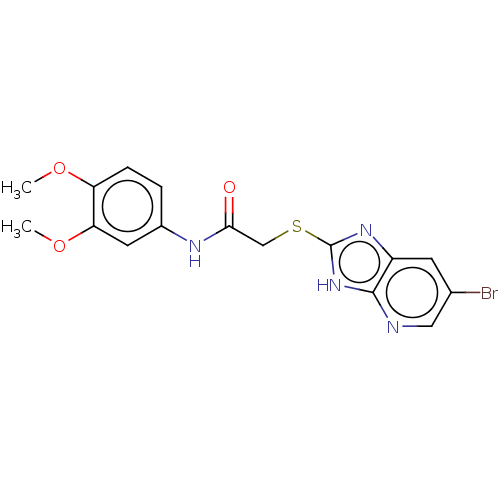
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031828
(CHEMBL3360883)Show InChI InChI=1S/C16H15BrN4O3S/c1-23-12-4-3-10(6-13(12)24-2)19-14(22)8-25-16-20-11-5-9(17)7-18-15(11)21-16/h3-7H,8H2,1-2H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031828

(CHEMBL3360883)Show InChI InChI=1S/C16H15BrN4O3S/c1-23-12-4-3-10(6-13(12)24-2)19-14(22)8-25-16-20-11-5-9(17)7-18-15(11)21-16/h3-7H,8H2,1-2H3,(H,19,22)(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 192 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
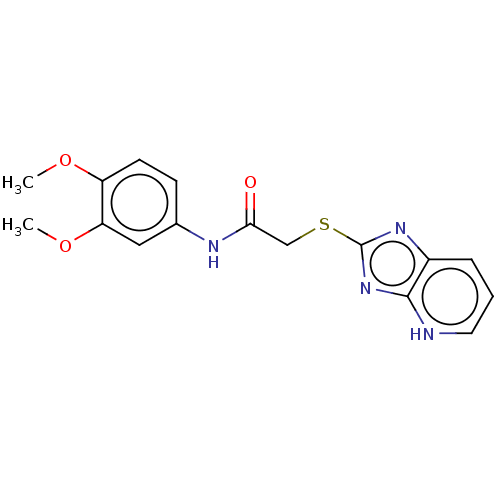
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031882
(CHEMBL1433061)Show InChI InChI=1S/C16H16N4O3S/c1-22-12-6-5-10(8-13(12)23-2)18-14(21)9-24-16-19-11-4-3-7-17-15(11)20-16/h3-8H,9H2,1-2H3,(H,18,21)(H,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031882

(CHEMBL1433061)Show InChI InChI=1S/C16H16N4O3S/c1-22-12-6-5-10(8-13(12)23-2)18-14(21)9-24-16-19-11-4-3-7-17-15(11)20-16/h3-8H,9H2,1-2H3,(H,18,21)(H,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 217 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50086502
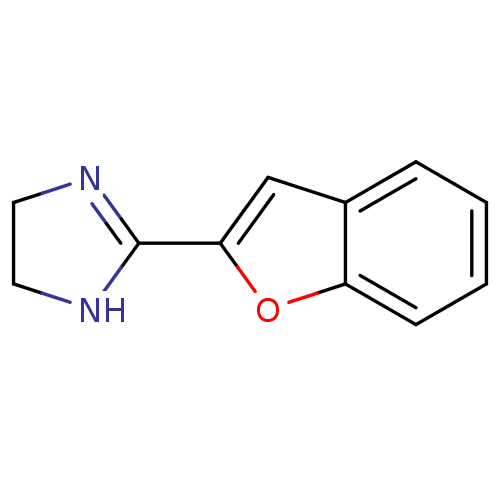
(2-BFi | 2-Benzofuran-2-yl-4,5-dihydro-1H-imidazole...)Show InChI InChI=1S/C11H10N2O/c1-2-4-9-8(3-1)7-10(14-9)11-12-5-6-13-11/h1-4,7H,5-6H2,(H,12,13) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50002833

(CHEMBL3233657)Show SMILES Oc1ccc(\C=C\c2ccc(\C=C\c3ccc(O)cc3)c(c2)C(F)(F)F)cc1 Show InChI InChI=1S/C23H17F3O2/c24-23(25,26)22-15-18(2-1-16-5-11-20(27)12-6-16)4-10-19(22)9-3-17-7-13-21(28)14-8-17/h1-15,27-28H/b2-1+,9-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Medical Center
Curated by ChEMBL
| Assay Description
Displacement of [3H]chrysamine G from fibrillar amyloid beta (1 to 40) (unknown origin) after 1 hr by liquid scintillation spectrophotometric analysi... |
Bioorg Med Chem 22: 2469-81 (2014)
Article DOI: 10.1016/j.bmc.2014.02.054
BindingDB Entry DOI: 10.7270/Q2DB83C0 |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50179360

(CHEMBL3040216)Show SMILES CC1=CCC(=C\C1=N\C(=O)C1=CC=C\C(C1)=N/C(=O)/N=C1/CC(=CC=C1)C(=O)\N=C1\CC(=CC=C1C)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O)C(=O)\N=C1/CC=C(c2cc(cc(c12)S(O)(=O)=O)S(O)(=O)=O)S(O)(=O)=O |c:4,13,24,26,34,36,45,72,t:1,11| Show InChI InChI=1S/C51H40N6O23S6/c1-25-9-11-29(49(60)54-37-13-15-41(83(69,70)71)35-21-33(81(63,64)65)23-43(45(35)37)85(75,76)77)19-39(25)56-47(58)27-5-3-7-31(17-27)52-51(62)53-32-8-4-6-28(18-32)48(59)57-40-20-30(12-10-26(40)2)50(61)55-38-14-16-42(84(72,73)74)36-22-34(82(66,67)68)24-44(46(36)38)86(78,79)80/h3-11,15-16,20-24H,12-14,17-19H2,1-2H3,(H,63,64,65)(H,66,67,68)(H,69,70,71)(H,72,73,74)(H,75,76,77)(H,78,79,80)/b52-31+,53-32+,54-37+,55-38+,56-39-,57-40- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 expressed in African green monkey COS7 cells using ATP as substrate after 20 mins by Michaelis-Menten plot analy... |
Bioorg Med Chem 24: 3157-65 (2016)
Article DOI: 10.1016/j.bmc.2016.05.046
BindingDB Entry DOI: 10.7270/Q2SQ928T |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031831

(CHEMBL3360879)Show InChI InChI=1S/C15H15N5O3S/c1-22-11-4-3-9(5-12(11)23-2)18-13(21)7-24-15-19-10-6-16-8-17-14(10)20-15/h3-6,8H,7H2,1-2H3,(H,18,21)(H,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Inhibition of human NPP1 using p-Nph-5'-TMP as substrate |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50031831

(CHEMBL3360879)Show InChI InChI=1S/C15H15N5O3S/c1-22-11-4-3-9(5-12(11)23-2)18-13(21)7-24-15-19-10-6-16-8-17-14(10)20-15/h3-6,8H,7H2,1-2H3,(H,18,21)(H,16,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 281 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 using p-Nph-5'-TMP as substrate by Lineweaver-Burk plot analysis |
J Med Chem 57: 10080-100 (2014)
Article DOI: 10.1021/jm501434y
BindingDB Entry DOI: 10.7270/Q2377B9S |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50424331

(CHEMBL2314497)Show InChI InChI=1S/C16H17FN6/c17-11-3-1-10(2-4-11)13-9-12-14(20-13)21-16(18)22-15(12)23-7-5-19-6-8-23/h1-4,9,19H,5-8H2,(H3,18,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Displacement of [3H]Histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai/o and Gbeta1gamma |
Bioorg Med Chem Lett 23: 132-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.139
BindingDB Entry DOI: 10.7270/Q28P61TF |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50179375
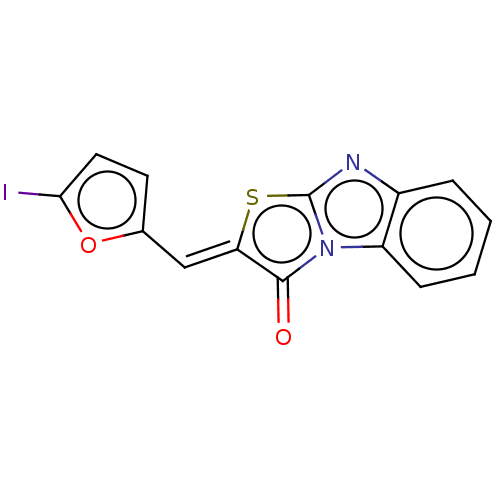
(CHEMBL3813806)Show InChI InChI=1S/C14H7IN2O2S/c15-12-6-5-8(19-12)7-11-13(18)17-10-4-2-1-3-9(10)16-14(17)20-11/h1-7H/b11-7- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 467 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NPP1 expressed in insect SF9 cells using ATP as substrate preincubated with substrate followed by protein addition an... |
Bioorg Med Chem 24: 3157-65 (2016)
Article DOI: 10.1016/j.bmc.2016.05.046
BindingDB Entry DOI: 10.7270/Q2SQ928T |
More data for this
Ligand-Target Pair | |
Ectonucleotide pyrophosphatase/phosphodiesterase family member 1
(Homo sapiens (Human)) | BDBM50179359

(CHEBI:34946 | Reactive Blue 2)Show SMILES Nc1c(cc(Nc2ccc(Nc3nc(Cl)nc(Nc4cccc(c4)S(O)(=O)=O)n3)c(c2)S(O)(=O)=O)c2C(=O)c3ccccc3C(=O)c12)S(O)(=O)=O Show InChI InChI=1S/C29H20ClN7O11S3/c30-27-35-28(33-13-4-3-5-15(10-13)49(40,41)42)37-29(36-27)34-18-9-8-14(11-20(18)50(43,44)45)32-19-12-21(51(46,47)48)24(31)23-22(19)25(38)16-6-1-2-7-17(16)26(23)39/h1-12,32H,31H2,(H,40,41,42)(H,43,44,45)(H,46,47,48)(H2,33,34,35,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Competitive inhibition of human NPP1 expressed in African green monkey COS7 cells using ATP as substrate after 20 mins by Michaelis-Menten plot analy... |
Bioorg Med Chem 24: 3157-65 (2016)
Article DOI: 10.1016/j.bmc.2016.05.046
BindingDB Entry DOI: 10.7270/Q2SQ928T |
More data for this
Ligand-Target Pair | |
Histamine H4 receptor
(Homo sapiens (Human)) | BDBM50304501
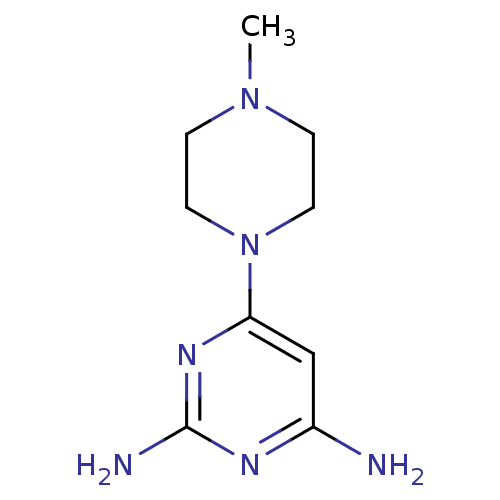
(6-(4-Methylpiperazin-1-yl)pyrimidine-2,4-diamine |...)Show InChI InChI=1S/C9H16N6/c1-14-2-4-15(5-3-14)8-6-7(10)12-9(11)13-8/h6H,2-5H2,1H3,(H4,10,11,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
KU Leuven
Curated by ChEMBL
| Assay Description
Displacement of [3H]Histamine from human histamine H4 receptor expressed in Sf9 cells co-expressing Galphai/o and Gbeta1gamma |
Bioorg Med Chem Lett 23: 132-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.139
BindingDB Entry DOI: 10.7270/Q28P61TF |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514721

(CHEMBL4450506)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccc(F)c(Cl)c1)c1ccccc1 |c:9| Show InChI InChI=1S/C22H21ClFN2O5P/c1-3-30-32(29,31-4-2)22(14-8-6-5-7-9-14)19-16(13-25-22)20(27)26(21(19)28)15-10-11-18(24)17(23)12-15/h5-13,16,19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50514721

(CHEMBL4450506)Show SMILES CCOP(=O)(OCC)C1(N=CC2C1C(=O)N(C2=O)c1ccc(F)c(Cl)c1)c1ccccc1 |c:9| Show InChI InChI=1S/C22H21ClFN2O5P/c1-3-30-32(29,31-4-2)22(14-8-6-5-7-9-14)19-16(13-25-22)20(27)26(21(19)28)15-10-11-18(24)17(23)12-15/h5-13,16,19H,3-4H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Barcelona
Curated by ChEMBL
| Assay Description
Displacement of [3H]RX821002 from alpha2-AR in human brain frontal cortex incubated for 30 mins by liquid scintillation spectrometry |
J Med Chem 63: 3610-3633 (2020)
Article DOI: 10.1021/acs.jmedchem.9b02080
BindingDB Entry DOI: 10.7270/Q2FB569H |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data