 Found 87 hits with Last Name = 'de voogd' and Initial = 'nj'
Found 87 hits with Last Name = 'de voogd' and Initial = 'nj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50239948
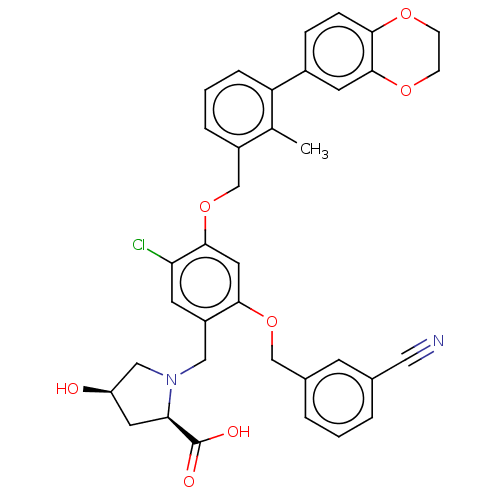
(CHEMBL4071326 | US9850225, Example 1166)Show SMILES Cc1c(COc2cc(OCc3cccc(c3)C#N)c(CN3C[C@H](O)C[C@@H]3C(O)=O)cc2Cl)cccc1-c1ccc2OCCOc2c1 |r| Show InChI InChI=1S/C36H33ClN2O7/c1-22-26(6-3-7-29(22)25-8-9-32-35(14-25)44-11-10-43-32)21-46-34-16-33(45-20-24-5-2-4-23(12-24)17-38)27(13-30(34)37)18-39-19-28(40)15-31(39)36(41)42/h2-9,12-14,16,28,31,40H,10-11,15,18-21H2,1H3,(H,41,42)/t28-,31-/m1/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50436124
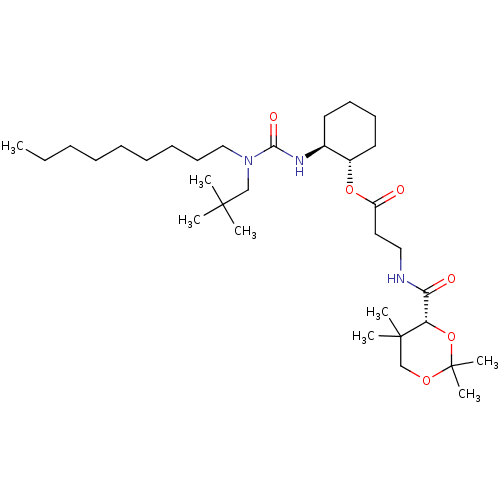
(CHEMBL2397474)Show SMILES CCCCCCCCCN(CC(C)(C)C)C(=O)N[C@H]1CCCC[C@@H]1OC(=O)CCNC(=O)[C@@H]1OC(C)(C)OCC1(C)C |r| Show InChI InChI=1S/C33H61N3O6/c1-9-10-11-12-13-14-17-22-36(23-31(2,3)4)30(39)35-25-18-15-16-19-26(25)41-27(37)20-21-34-29(38)28-32(5,6)24-40-33(7,8)42-28/h25-26,28H,9-24H2,1-8H3,(H,34,38)(H,35,39)/t25-,26-,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of macrophage ACAT (unknown origin) |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50362641
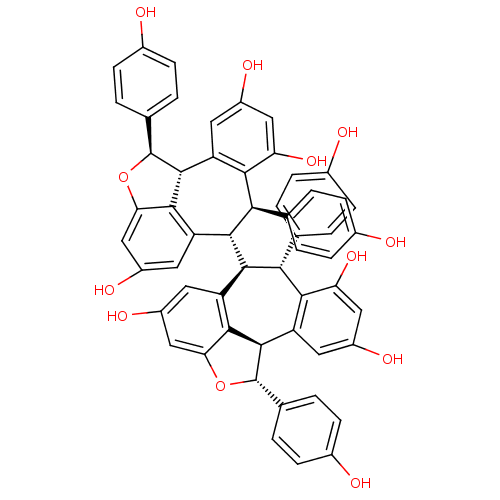
(CHEMBL384120)Show SMILES Oc1ccc(cc1)[C@@H]1Oc2cc(O)cc3[C@@H]([C@H]4[C@H](c5ccc(O)cc5)c5c(O)cc(O)cc5[C@H]5[C@@H](Oc6cc(O)cc4c56)c4ccc(O)cc4)[C@H](c4ccc(O)cc4)c4c(O)cc(O)cc4[C@@H]1c23 Show InChI InChI=1S/C56H42O12/c57-29-9-1-25(2-10-29)45-47-37(17-33(61)21-41(47)65)53-49-39(19-35(63)23-43(49)67-55(53)27-5-13-31(59)14-6-27)51(45)52-40-20-36(64)24-44-50(40)54(56(68-44)28-7-15-32(60)16-8-28)38-18-34(62)22-42(66)48(38)46(52)26-3-11-30(58)12-4-26/h1-24,45-46,51-66H/t45-,46-,51-,52-,53-,54-,55+,56+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50436151

(CHEMBL2398212)Show InChI InChI=1S/C13H3ClN4O/c14-6-1-2-7-8(3-6)13(19)12-11(7)17-9(4-15)10(5-16)18-12/h1-3H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of USP7 (unknown origin) using ubiquitin-EKL as substrate |
Bioorg Med Chem Lett 23: 3884-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.066
BindingDB Entry DOI: 10.7270/Q29P332K |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50503685
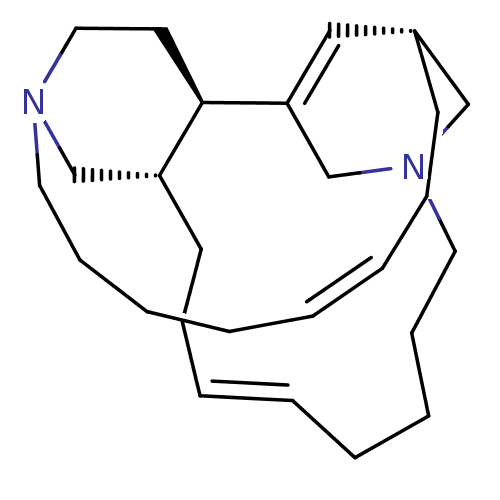
(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s constitutive proteasome beta5 chymotrypsin-like activity in human erythrocytes using Suc-Leu-Leu-ValTyr-MCA as substrate preincubat... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50503685

(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s immunoproteasome LMP2 in human erythrocytes using Ac-Pro-Ala-Leu-MCA as substrate preincubated for 10 mins followed by substrate ad... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50503685

(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s constitutive proteasome beta1 caspase-like activity in human erythrocytes using Boc-Leu-Arg-Arg-MCA as substrate preincubated for 1... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50503685

(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s immunoproteasome LMP7 in human erythrocytes using Suc-Leu-Leu-ValTyr-MCA as substrate preincubated for 10 mins followed by substrat... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50446566
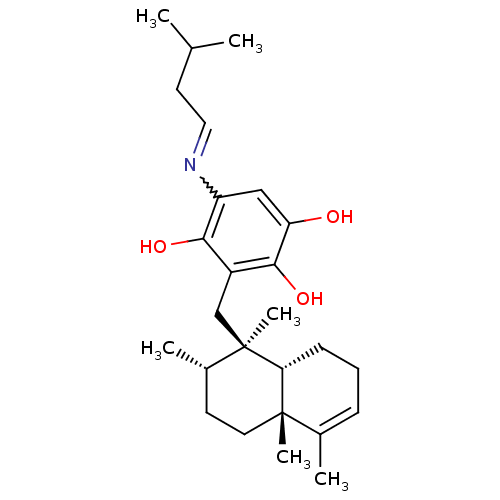
(CHEMBL3109401)Show SMILES CC(C)CC=Nc1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,w:5.5,c:26| Show InChI InChI=1S/C26H39NO3/c1-16(2)11-13-27-20-14-21(28)24(30)19(23(20)29)15-26(6)18(4)10-12-25(5)17(3)8-7-9-22(25)26/h8,13-14,16,18,22,28-30H,7,9-12,15H2,1-6H3/t18-,22+,25-,26+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ALK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613798
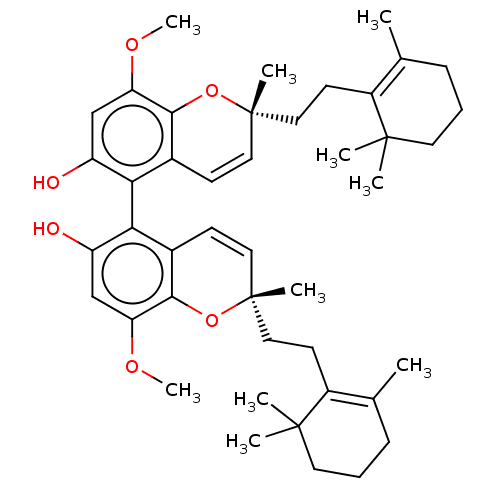
(CHEMBL5267016)Show SMILES COc1cc(O)c(c2C=C[C@](C)(CCC3=C(C)CCCC3(C)C)Oc12)-c1c(O)cc(OC)c2O[C@](C)(CCC3=C(C)CCCC3(C)C)C=Cc12 |r,wD:10.10,34.37,c:8,14,41,51,(8.67,2.69,;7.33,1.92,;6,2.69,;6,4.23,;4.67,5,;4.67,6.54,;3.33,4.23,;3.33,2.69,;2.01,1.91,;2.01,.37,;3.35,-.39,;4.69,-1.16,;3.35,-1.93,;2.02,-2.7,;2.02,-4.24,;3.35,-5.01,;4.69,-4.24,;3.35,-6.55,;2.02,-7.32,;.68,-6.55,;.68,-5.01,;-.09,-3.68,;-.86,-5.01,;4.68,.39,;4.67,1.92,;2,5,;2,6.55,;3.33,7.32,;.66,7.31,;-.66,6.55,;-2,7.32,;-3.33,6.55,;-.66,5,;-2,4.24,;-2,2.7,;-3.33,3.47,;-3.33,1.93,;-4.66,2.7,;-6,1.93,;-7.33,2.7,;-7.33,4.24,;-8.67,1.93,;-8.67,.39,;-7.33,-.38,;-6,.39,;-5.23,-.95,;-4.46,.39,;-.67,1.93,;.66,2.7,;.67,4.23,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50503684
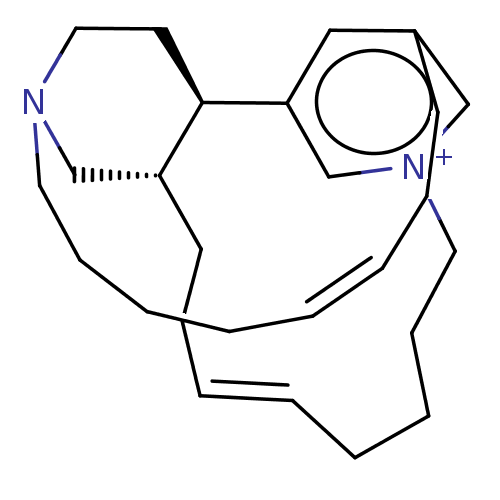
(CHEMBL4458350)Show SMILES [H][C@@]12CN3CC[C@@]1([H])c1cc(CC\C=C/CCCC3)c[n+](CCCC\C=C/CC2)c1 |r,c:14,27| Show InChI InChI=1S/C26H39N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19-20,22,24,26H,3-4,7-18,21H2/q+1/b5-1-,6-2-/t24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s constitutive proteasome beta5 chymotrypsin-like activity in human erythrocytes using Suc-Leu-Leu-ValTyr-MCA as substrate preincubat... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50446566

(CHEMBL3109401)Show SMILES CC(C)CC=Nc1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,w:5.5,c:26| Show InChI InChI=1S/C26H39NO3/c1-16(2)11-13-27-20-14-21(28)24(30)19(23(20)29)15-26(6)18(4)10-12-25(5)17(3)8-7-9-22(25)26/h8,13-14,16,18,22,28-30H,7,9-12,15H2,1-6H3/t18-,22+,25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IGF1R (unknown origin) using Poly (Glu,Tyr) 4:1 as substrate assessed as incorporation of [gamma-33P]-ATP into substrate by... |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50446564
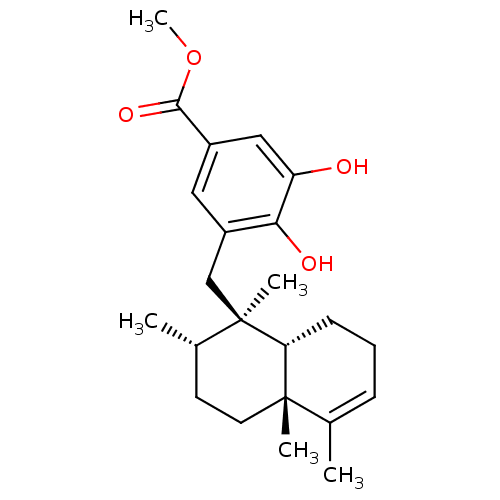
(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ALK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Ubiquitin-like modifier-activating enzyme 1
(Homo sapiens (Human)) | BDBM50387035
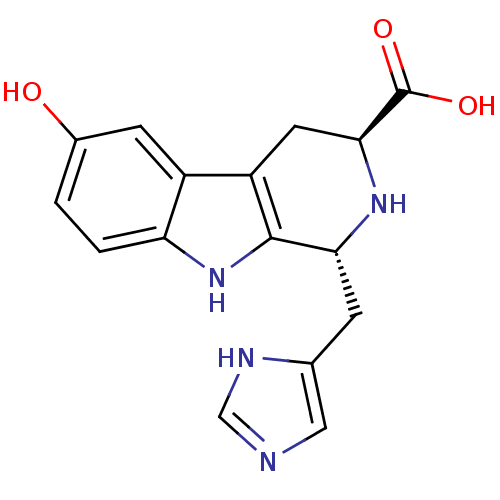
(CHEMBL2046768)Show SMILES OC(=O)[C@@H]1Cc2c([nH]c3ccc(O)cc23)[C@@H](Cc2cnc[nH]2)N1 |r| Show InChI InChI=1S/C16H16N4O3/c21-9-1-2-12-10(4-9)11-5-14(16(22)23)19-13(15(11)20-12)3-8-6-17-7-18-8/h1-2,4,6-7,13-14,19-21H,3,5H2,(H,17,18)(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of FLAG-tagged ubiquitin-activating enzyme E1 assessed as inhibition of GST-ubiquitin/FLAG-E1 intermediate formation by Western blot analy... |
Bioorg Med Chem 20: 4437-42 (2012)
Article DOI: 10.1016/j.bmc.2012.05.044
BindingDB Entry DOI: 10.7270/Q2WH2R2M |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50456399
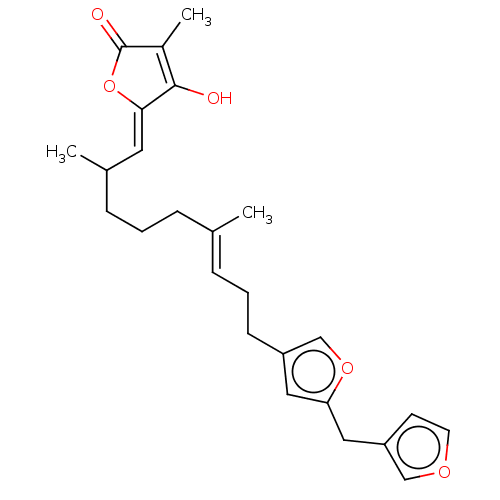
(CHEMBL465268)Show SMILES CC(CCC\C(C)=C\CCc1coc(Cc2ccoc2)c1)\C=C1/OC(=O)C(C)=C1O |c:29| Show InChI InChI=1S/C25H30O5/c1-17(6-4-8-18(2)12-23-24(26)19(3)25(27)30-23)7-5-9-20-13-22(29-16-20)14-21-10-11-28-15-21/h7,10-13,15-16,18,26H,4-6,8-9,14H2,1-3H3/b17-7+,23-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged human USP7 expressed in insect expression system using ubiquitin-Rh110 as substrate preincubated for 30 mins fo... |
J Nat Prod 80: 2045-2050 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00184
BindingDB Entry DOI: 10.7270/Q2W37ZX2 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50456397
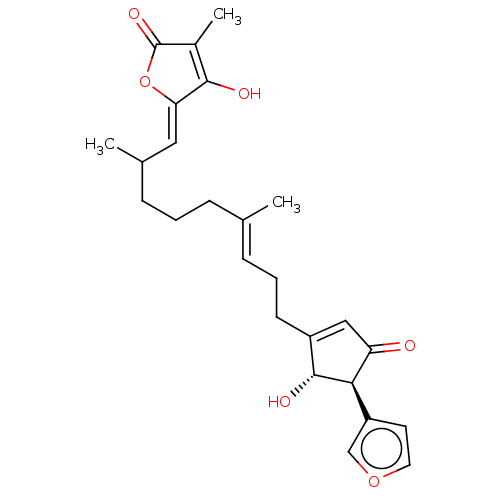
(CHEMBL4217374)Show SMILES CC(CCC\C(C)=C\CCC1=CC(=O)[C@H]([C@@H]1O)c1ccoc1)\C=C1/OC(=O)C(C)=C1O |r,c:30,t:10| Show InChI InChI=1S/C25H30O6/c1-15(6-4-8-16(2)12-21-23(27)17(3)25(29)31-21)7-5-9-18-13-20(26)22(24(18)28)19-10-11-30-14-19/h7,10-14,16,22,24,27-28H,4-6,8-9H2,1-3H3/b15-7+,21-12-/t16?,22-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged human USP7 expressed in insect expression system using ubiquitin-Rh110 as substrate preincubated for 30 mins fo... |
J Nat Prod 80: 2045-2050 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00184
BindingDB Entry DOI: 10.7270/Q2W37ZX2 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50263192
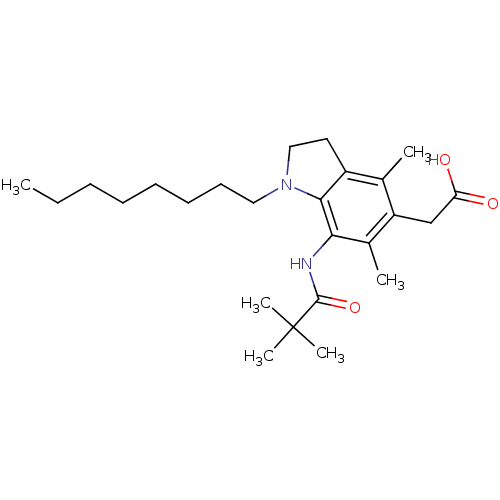
(CHEMBL478858 | CHEMBL564483 | Pactimibe | [7-(2,2-...)Show SMILES CCCCCCCCN1CCc2c1c(NC(=O)C(C)(C)C)c(C)c(CC(O)=O)c2C Show InChI InChI=1S/C25H40N2O3/c1-7-8-9-10-11-12-14-27-15-13-19-17(2)20(16-21(28)29)18(3)22(23(19)27)26-24(30)25(4,5)6/h7-16H2,1-6H3,(H,26,30)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of macrophage ACAT2 (unknown origin) |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50446566

(CHEMBL3109401)Show SMILES CC(C)CC=Nc1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,w:5.5,c:26| Show InChI InChI=1S/C26H39NO3/c1-16(2)11-13-27-20-14-21(28)24(30)19(23(20)29)15-26(6)18(4)10-12-25(5)17(3)8-7-9-22(25)26/h8,13-14,16,18,22,28-30H,7,9-12,15H2,1-6H3/t18-,22+,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FAK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50503684

(CHEMBL4458350)Show SMILES [H][C@@]12CN3CC[C@@]1([H])c1cc(CC\C=C/CCCC3)c[n+](CCCC\C=C/CC2)c1 |r,c:14,27| Show InChI InChI=1S/C26H39N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19-20,22,24,26H,3-4,7-18,21H2/q+1/b5-1-,6-2-/t24-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s immunoproteasome LMP2 in human erythrocytes using Ac-Pro-Ala-Leu-MCA as substrate preincubated for 10 mins followed by substrate ad... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of wild type MET (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50446565

(CHEMBL3109402)Show SMILES COc1cc2nc(C)oc2c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,c:25| Show InChI InChI=1S/C24H33NO3/c1-14-8-7-9-20-23(14,4)11-10-15(2)24(20,5)13-17-21(26)19(27-6)12-18-22(17)28-16(3)25-18/h8,12,15,20,26H,7,9-11,13H2,1-6H3/t15-,20+,23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IGF1R (unknown origin) using Poly (Glu,Tyr) 4:1 as substrate assessed as incorporation of [gamma-33P]-ATP into substrate by... |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM50446565

(CHEMBL3109402)Show SMILES COc1cc2nc(C)oc2c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,c:25| Show InChI InChI=1S/C24H33NO3/c1-14-8-7-9-20-23(14,4)11-10-15(2)24(20,5)13-17-21(26)19(27-6)12-18-22(17)28-16(3)25-18/h8,12,15,20,26H,7,9-11,13H2,1-6H3/t15-,20+,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant ALK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50456400
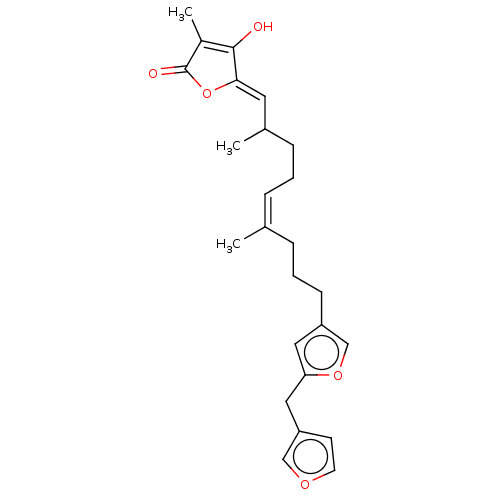
(CHEMBL464463)Show SMILES CC(CC\C=C(\C)CCCc1coc(Cc2ccoc2)c1)\C=C1/OC(=O)C(C)=C1O |c:29| Show InChI InChI=1S/C25H30O5/c1-17(6-4-8-18(2)12-23-24(26)19(3)25(27)30-23)7-5-9-20-13-22(29-16-20)14-21-10-11-28-15-21/h6,10-13,15-16,18,26H,4-5,7-9,14H2,1-3H3/b17-6-,23-12- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged human USP7 expressed in insect expression system using ubiquitin-Rh110 as substrate preincubated for 30 mins fo... |
J Nat Prod 80: 2045-2050 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00184
BindingDB Entry DOI: 10.7270/Q2W37ZX2 |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50436152
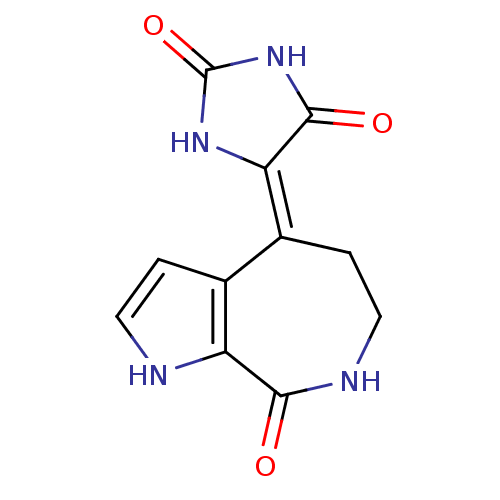
(SPONGIACIDIN C)Show InChI InChI=1S/C11H10N4O3/c16-9-7-5(1-3-12-7)6(2-4-13-9)8-10(17)15-11(18)14-8/h1,3,12H,2,4H2,(H,13,16)(H2,14,15,17,18)/b8-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of USP7 (unknown origin) using ubiquitin-EKL as substrate |
Bioorg Med Chem Lett 23: 3884-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.066
BindingDB Entry DOI: 10.7270/Q29P332K |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant aurora kinase B (unknown origin) using tetra(LRRWSLG) as substrate assessed as incorporation of [gamma-33P]-ATP into substr... |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50503684

(CHEMBL4458350)Show SMILES [H][C@@]12CN3CC[C@@]1([H])c1cc(CC\C=C/CCCC3)c[n+](CCCC\C=C/CC2)c1 |r,c:14,27| Show InChI InChI=1S/C26H39N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19-20,22,24,26H,3-4,7-18,21H2/q+1/b5-1-,6-2-/t24-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s immunoproteasome LMP7 in human erythrocytes using Suc-Leu-Leu-ValTyr-MCA as substrate preincubated for 10 mins followed by substrat... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FAK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50456398
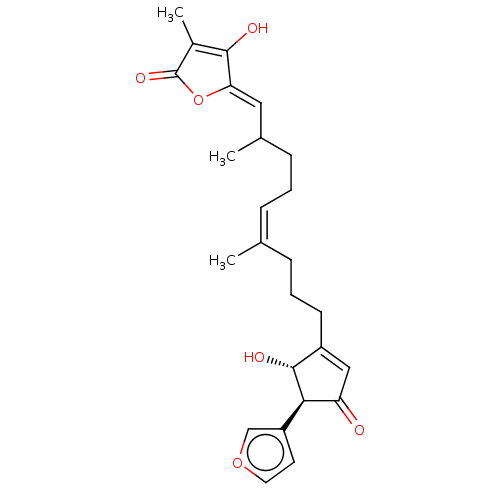
(CHEMBL4204537)Show SMILES CC(CC\C=C(\C)CCCC1=CC(=O)[C@H]([C@@H]1O)c1ccoc1)\C=C1/OC(=O)C(C)=C1O |r,c:30,t:10| Show InChI InChI=1S/C25H30O6/c1-15(6-4-8-16(2)12-21-23(27)17(3)25(29)31-21)7-5-9-18-13-20(26)22(24(18)28)19-10-11-30-14-19/h6,10-14,16,22,24,27-28H,4-5,7-9H2,1-3H3/b15-6-,21-12-/t16?,22-,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant His6-tagged human USP7 expressed in insect expression system using ubiquitin-Rh110 as substrate preincubated for 30 mins fo... |
J Nat Prod 80: 2045-2050 (2017)
Article DOI: 10.1021/acs.jnatprod.7b00184
BindingDB Entry DOI: 10.7270/Q2W37ZX2 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-1
(Homo sapiens (Human)) | BDBM50503684

(CHEMBL4458350)Show SMILES [H][C@@]12CN3CC[C@@]1([H])c1cc(CC\C=C/CCCC3)c[n+](CCCC\C=C/CC2)c1 |r,c:14,27| Show InChI InChI=1S/C26H39N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19-20,22,24,26H,3-4,7-18,21H2/q+1/b5-1-,6-2-/t24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s constitutive proteasome beta1 caspase-like activity in human erythrocytes using Boc-Leu-Arg-Arg-MCA as substrate preincubated for 1... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50232478
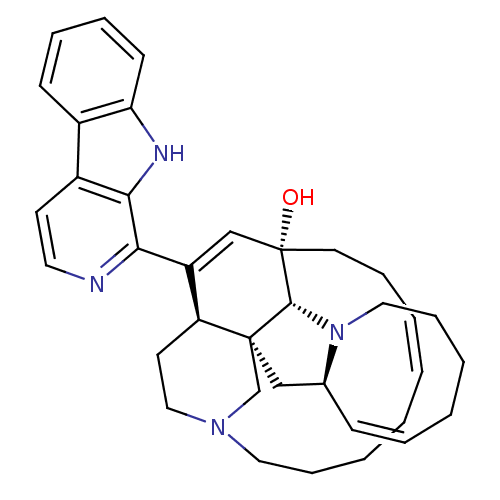
(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human microsomal ACAT2 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50263192

(CHEMBL478858 | CHEMBL564483 | Pactimibe | [7-(2,2-...)Show SMILES CCCCCCCCN1CCc2c1c(NC(=O)C(C)(C)C)c(C)c(CC(O)=O)c2C Show InChI InChI=1S/C25H40N2O3/c1-7-8-9-10-11-12-14-27-15-13-19-17(2)20(16-21(28)29)18(3)22(23(19)27)26-24(30)25(4,5)6/h7-16H2,1-6H3,(H,26,30)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT1 (unknown origin) |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50613798

(CHEMBL5267016)Show SMILES COc1cc(O)c(c2C=C[C@](C)(CCC3=C(C)CCCC3(C)C)Oc12)-c1c(O)cc(OC)c2O[C@](C)(CCC3=C(C)CCCC3(C)C)C=Cc12 |r,wD:10.10,34.37,c:8,14,41,51,(8.67,2.69,;7.33,1.92,;6,2.69,;6,4.23,;4.67,5,;4.67,6.54,;3.33,4.23,;3.33,2.69,;2.01,1.91,;2.01,.37,;3.35,-.39,;4.69,-1.16,;3.35,-1.93,;2.02,-2.7,;2.02,-4.24,;3.35,-5.01,;4.69,-4.24,;3.35,-6.55,;2.02,-7.32,;.68,-6.55,;.68,-5.01,;-.09,-3.68,;-.86,-5.01,;4.68,.39,;4.67,1.92,;2,5,;2,6.55,;3.33,7.32,;.66,7.31,;-.66,6.55,;-2,7.32,;-3.33,6.55,;-.66,5,;-2,4.24,;-2,2.7,;-3.33,3.47,;-3.33,1.93,;-4.66,2.7,;-6,1.93,;-7.33,2.7,;-7.33,4.24,;-8.67,1.93,;-8.67,.39,;-7.33,-.38,;-6,.39,;-5.23,-.95,;-4.46,.39,;-.67,1.93,;.66,2.7,;.67,4.23,)| | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of human microsomal ACAT1 overexpressed in CHO cells using [14C]oleoyl-CoA as substrate assessed as formation of cholester... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50503685

(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s constitutive proteasome beta2 trypsin-like activity in human erythrocytes using Boc-Leu-Arg-Arg-MCA as substrate preincubated for 1... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50232478

(CHEMBL403233)Show SMILES O[C@@]12CC\C=C/CCCCN3CC[C@@H](C(=C1)c1nccc4c5ccccc5[nH]c14)[C@]1(C[C@@H]4\C=C/CCCCN4[C@@H]21)C3 |r,c:4,14,36| Show InChI InChI=1S/C36H44N4O/c41-36-18-10-4-1-2-5-11-20-39-22-17-30(35(25-39)23-26-13-7-3-6-12-21-40(26)34(35)36)29(24-36)32-33-28(16-19-37-32)27-14-8-9-15-31(27)38-33/h1,4,7-9,13-16,19,24,26,30,34,38,41H,2-3,5-6,10-12,17-18,20-23,25H2/b4-1-,13-7-/t26-,30-,34+,35-,36-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of microsomal ACAT in human MDM using [14C]oleoyl-CoA as substrate assessed as formation of cholesteryl [14C]-oleate after... |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme 2
(Homo sapiens (Human)) | BDBM50613799
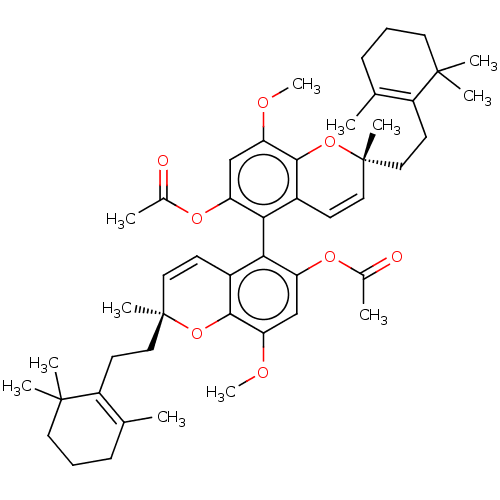
(CHEMBL5283695)Show SMILES COc1cc(OC(C)=O)c(c2C=C[C@](C)(CCC3=C(C)CCCC3(C)C)Oc12)-c1c(OC(C)=O)cc(OC)c2O[C@](C)(CCC3=C(C)CCCC3(C)C)C=Cc12 |r,wD:13.13,40.43,c:11,17,47,57,(8.67,1.54,;7.33,.77,;6,1.54,;6,3.08,;4.67,3.85,;4.67,5.39,;6,6.16,;7.34,5.39,;6,7.7,;3.33,3.08,;3.33,1.53,;2.01,.75,;2.01,-.79,;3.35,-1.55,;4.69,-2.31,;3.35,-3.09,;2.02,-3.86,;2.02,-5.4,;3.35,-6.17,;4.69,-5.4,;3.35,-7.71,;2.02,-8.48,;.68,-7.71,;.68,-6.17,;-.09,-4.83,;-.86,-6.17,;4.68,-.77,;4.67,.77,;2,3.85,;2,5.4,;3.33,6.17,;3.33,7.71,;2,8.48,;4.67,8.48,;.66,6.16,;-.66,5.39,;-2,6.16,;-3.33,5.39,;-.66,3.85,;-2,3.08,;-2,1.55,;-3.33,2.32,;-3.33,.78,;-4.66,1.55,;-6,.78,;-7.33,1.55,;-7.33,3.09,;-8.67,.78,;-8.67,-.76,;-7.33,-1.53,;-6,-.76,;-5.23,-2.1,;-4.46,-.76,;-.67,.78,;.66,1.54,;.67,3.08,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek6
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NEK6 (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50446565

(CHEMBL3109402)Show SMILES COc1cc2nc(C)oc2c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,c:25| Show InChI InChI=1S/C24H33NO3/c1-14-8-7-9-20-23(14,4)11-10-15(2)24(20,5)13-17-21(26)19(27-6)12-18-22(17)28-16(3)25-18/h8,12,15,20,26H,7,9-11,13H2,1-6H3/t15-,20+,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant FAK (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-10
(Homo sapiens (Human)) | BDBM50503685

(CHEMBL4092241)Show SMILES [H][C@]12CN3CC(=C1)[C@]1([H])CCN(C[C@@]1([H])CC\C=C/CCCC3)CCCC\C=C/CC2 |r,c:5,19,30| Show InChI InChI=1S/C26H42N2/c1-3-7-11-16-27-18-15-26-24(21-27)14-10-6-2-4-8-12-17-28-20-23(13-9-5-1)19-25(26)22-28/h1-2,5-6,19,23-24,26H,3-4,7-18,20-22H2/b5-1-,6-2-/t23-,24-,26-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of 20s immunoproteasome MECL1 in human erythrocytes using Suc-Leu-Leu-ValTyr-MCA as substrate preincubated for 10 mins followed by substra... |
Bioorg Med Chem Lett 29: 8-10 (2019)
Article DOI: 10.1016/j.bmcl.2018.11.028
BindingDB Entry DOI: 10.7270/Q2QN6B20 |
More data for this
Ligand-Target Pair | |
Sterol O-acyltransferase 2
(Homo sapiens (Human)) | BDBM50069900

(2,6-diisopropylphenyl 2-(2,4,6-triisopropylphenyl)...)Show SMILES CC(C)c1cc(C(C)C)c(CC(=O)NS(=O)(=O)Oc2c(cccc2C(C)C)C(C)C)c(c1)C(C)C Show InChI InChI=1S/C29H43NO4S/c1-17(2)22-14-25(20(7)8)27(26(15-22)21(9)10)16-28(31)30-35(32,33)34-29-23(18(3)4)12-11-13-24(29)19(5)6/h11-15,17-21H,16H2,1-10H3,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of ACAT2 in human MDM assessed as inhibition of acetylated LDL-induced cholesterol ester accumulation after 1 hr by HPLC analysis |
Bioorg Med Chem 21: 3831-8 (2013)
Article DOI: 10.1016/j.bmc.2013.04.025
BindingDB Entry DOI: 10.7270/Q2PV6MR1 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421038

(CHEMBL2087614)Show SMILES C[C@]12CC[C@H]3[C@@](C)(CC[C@@H]4[C@]3(C)CC[C@@H]3O[C@@]5(C)CCCC(C)(C)[C@@H]5CC[C@@]43C)[C@@H]1Cc1c2c(ccc1O)C(O)C(O)=O |r| Show InChI InChI=1S/C38H56O5/c1-33(2)15-8-16-38(7)25(33)11-18-36(5)27-12-17-35(4)26(34(27,3)20-14-29(36)43-38)13-19-37(6)28(35)21-23-24(39)10-9-22(30(23)37)31(40)32(41)42/h9-10,25-29,31,39-40H,8,11-21H2,1-7H3,(H,41,42)/t25-,26+,27+,28-,29-,31?,34+,35+,36-,37-,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50421039

(CHEMBL2087615)Show SMILES C[C@@H]1CCC=C(C)[C@@]1(C)CC[C@]1(C)[C@@H](O)CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](Cc5c4c(ccc5O)C(O)C(O)=O)[C@]3(C)CC[C@@H]12 |r,t:4| Show InChI InChI=1S/C38H56O5/c1-22-9-8-10-23(2)34(22,3)19-20-37(6)28-13-16-36(5)27(35(28,4)18-15-30(37)40)14-17-38(7)29(36)21-25-26(39)12-11-24(31(25)38)32(41)33(42)43/h9,11-12,23,27-30,32,39-41H,8,10,13-21H2,1-7H3,(H,42,43)/t23-,27-,28-,29+,30+,32?,34-,35-,36-,37+,38+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human IDO expressed in Escherichia coli using L-tryptophan as substrate after 60 mins by spectrophotometry |
J Nat Prod 75: 1451-8 (2012)
Article DOI: 10.1021/np300345j
BindingDB Entry DOI: 10.7270/Q2SQ91PS |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant IGF1R (unknown origin) using Poly (Glu,Tyr) 4:1 as substrate assessed as incorporation of [gamma-33P]-ATP into substrate by... |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AXL (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Nek2
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant NEK2 (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase receptor UFO
(Homo sapiens (Human)) | BDBM50446565

(CHEMBL3109402)Show SMILES COc1cc2nc(C)oc2c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1O |r,c:25| Show InChI InChI=1S/C24H33NO3/c1-14-8-7-9-20-23(14,4)11-10-15(2)24(20,5)13-17-21(26)19(27-6)12-18-22(17)28-16(3)25-18/h8,12,15,20,26H,7,9-11,13H2,1-6H3/t15-,20+,23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant AXL (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 21
(Homo sapiens (Human)) | BDBM50436152

(SPONGIACIDIN C)Show InChI InChI=1S/C11H10N4O3/c16-9-7-5(1-3-12-7)6(2-4-13-9)8-10(17)15-11(18)14-8/h1,3,12H,2,4H2,(H,13,16)(H2,14,15,17,18)/b8-6- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of core catalytic domain of USP21 (unknown origin) using ubiquitin-Rh110 as substrate |
Bioorg Med Chem Lett 23: 3884-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.066
BindingDB Entry DOI: 10.7270/Q29P332K |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PIM1 (unknown origin) assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric assay |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50446564

(CHEMBL3109404)Show SMILES COC(=O)c1cc(O)c(O)c(C[C@]2(C)[C@@H](C)CC[C@]3(C)[C@H]2CCC=C3C)c1 |r,c:24| Show InChI InChI=1S/C23H32O4/c1-14-7-6-8-19-22(14,3)10-9-15(2)23(19,4)13-17-11-16(21(26)27-5)12-18(24)20(17)25/h7,11-12,15,19,24-25H,6,8-10,13H2,1-5H3/t15-,19+,22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Heinrich-Heine-Universit£t
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PLK1 (unknown origin) using casein as substrate assessed as incorporation of [gamma-33P]-ATP into substrate by radiometric ... |
J Nat Prod 77: 218-26 (2014)
Article DOI: 10.1021/np400633m
BindingDB Entry DOI: 10.7270/Q24F1S7C |
More data for this
Ligand-Target Pair | |
Ubiquitin carboxyl-terminal hydrolase 7
(Homo sapiens (Human)) | BDBM50436149
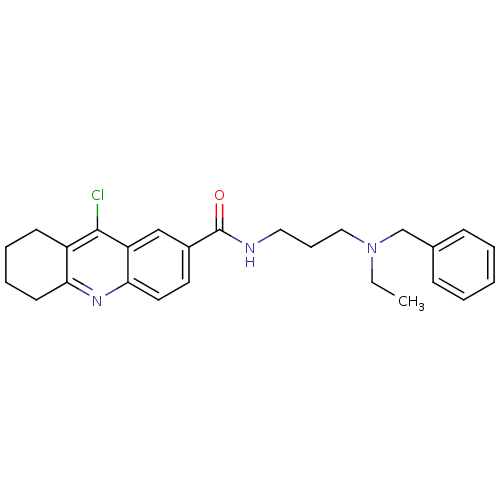
(CHEMBL2398214)Show SMILES CCN(CCCNC(=O)c1ccc2nc3CCCCc3c(Cl)c2c1)Cc1ccccc1 Show InChI InChI=1S/C26H30ClN3O/c1-2-30(18-19-9-4-3-5-10-19)16-8-15-28-26(31)20-13-14-24-22(17-20)25(27)21-11-6-7-12-23(21)29-24/h3-5,9-10,13-14,17H,2,6-8,11-12,15-16,18H2,1H3,(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of USP7 (unknown origin) using ubiquitin-EKL as substrate |
Bioorg Med Chem Lett 23: 3884-6 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.066
BindingDB Entry DOI: 10.7270/Q29P332K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data