

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312096 (4-bromo-6,8-dihydroxy-2-(4-hydroxyphenyl)isoquinol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312133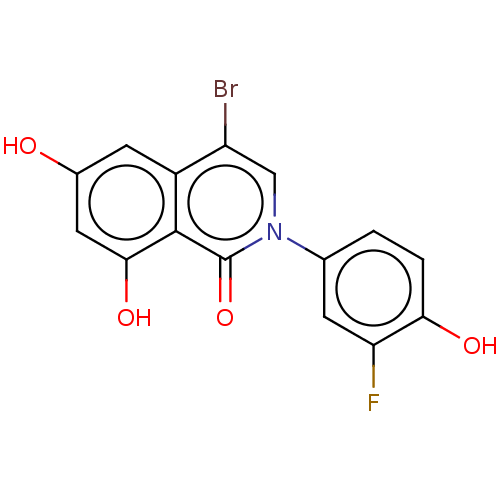 (US9604931, 12z) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312140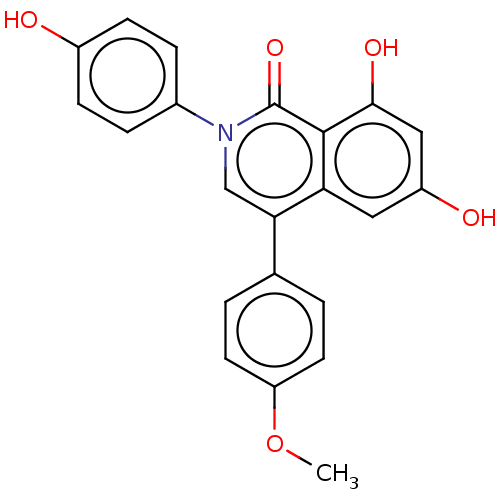 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312134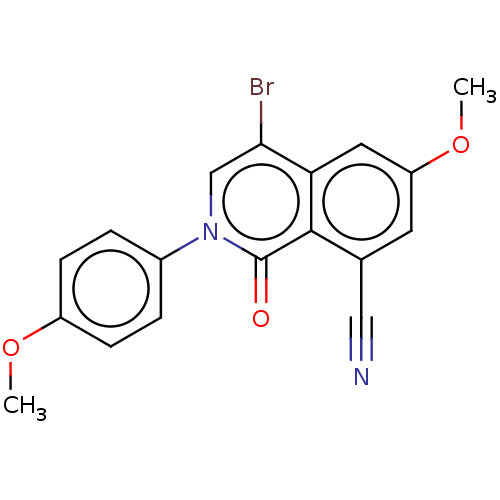 (4-bromo-6-methoxy-2-(4-methoxyphenyl)-1-oxo-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312135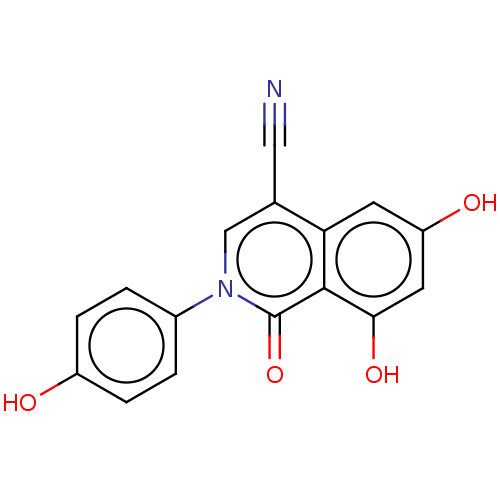 (6,8-dihydroxy-2-(4-hydroxyphenyl)-1-oxo-1,2-dihydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312151 ((E)-6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(prop-1-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312149 (6,8-dihydroxy-2-(4-hydroxyphenyl)-4-(4-methoxyphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312139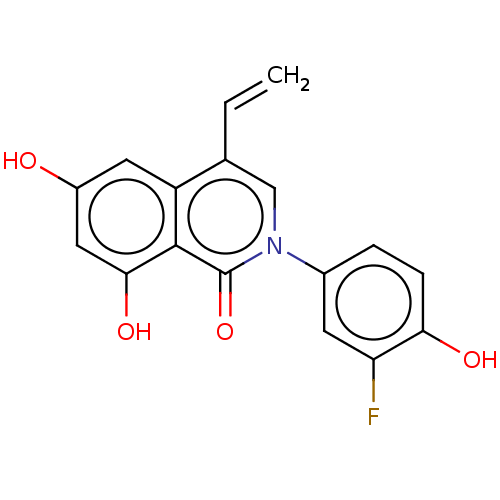 (2-(3-fluoro-4-hydroxyphenyl)-6,8-dihydroxy-4-vinyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312136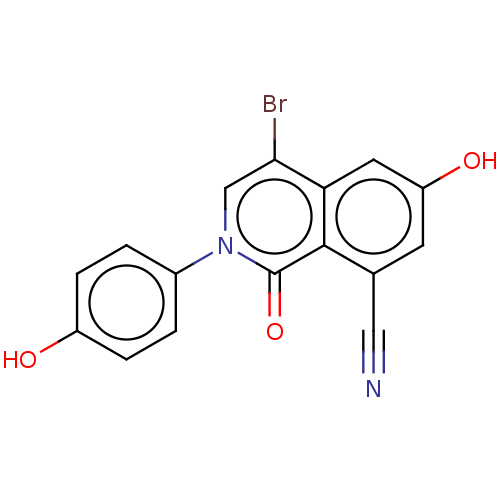 (4-bromo-6-hydroxy-2-(4-hydroxyphenyl)-1-oxo-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | >394 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM310198 (4-bromo-6-hydroxy-2-(4-hydroxyphenyl)isoquinolin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 998 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312137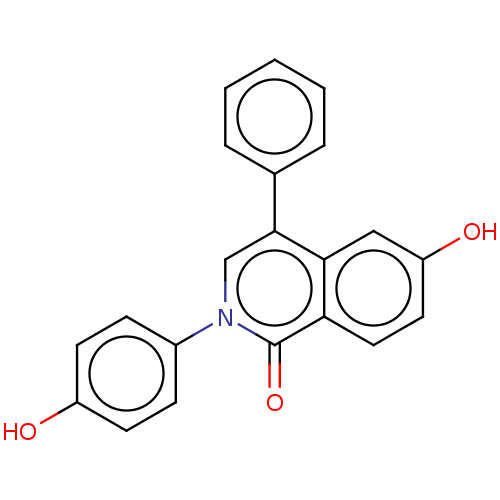 (6-hydroxy-2-(4-hydroxyphenyl)-4-phenylisoquinolin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM312138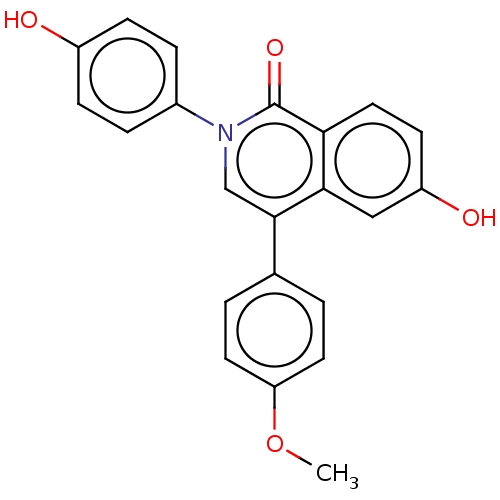 (6-hydroxy-2-(4-hydroxyphenol)-4-(4-methoxyphenyl)i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GTx, Inc.; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPARTMENT OF VETERANS AFFAIRS US Patent | Assay Description Estrogen receptor (ERβ) binding affinity of the NRBAs was also determined using an in vitro competitive radioligand-binding assay with [3H]-estr... | US Patent US9604931 (2017) BindingDB Entry DOI: 10.7270/Q23N25FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid hormone receptor ERR1 (Homo sapiens (Human)) | BDBM50091046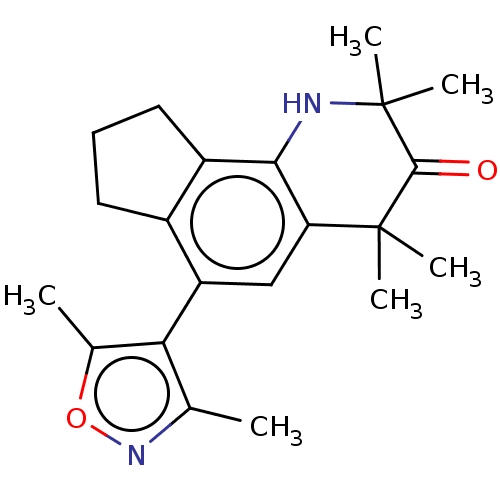 (CHEMBL3582112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of fluormone ES2 from human recombinant estrogen receptor-alpha expressed in insect Sf9 cells after 120 mins by fluorescence polarizatio... | J Med Chem 58: 4918-26 (2015) Article DOI: 10.1021/jm501758q BindingDB Entry DOI: 10.7270/Q2WM1G4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296480 (CHEMBL553249) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296473 (CHEMBL558940) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296481 (CHEMBL562618) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296477 (CHEMBL563460) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296478 (CHEMBL557433) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296476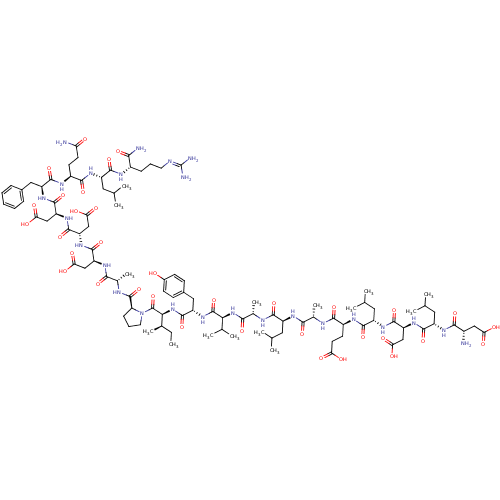 (CHEMBL557420) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296479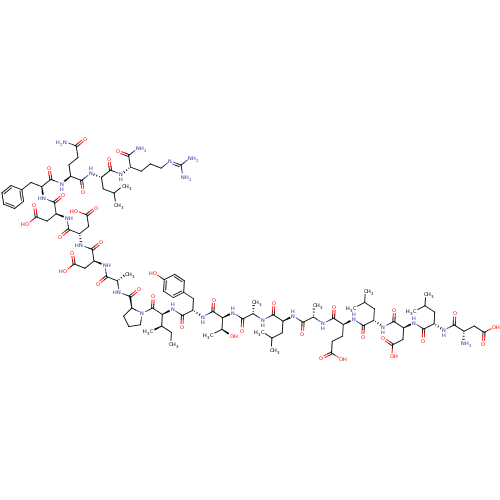 (CHEMBL562806) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296475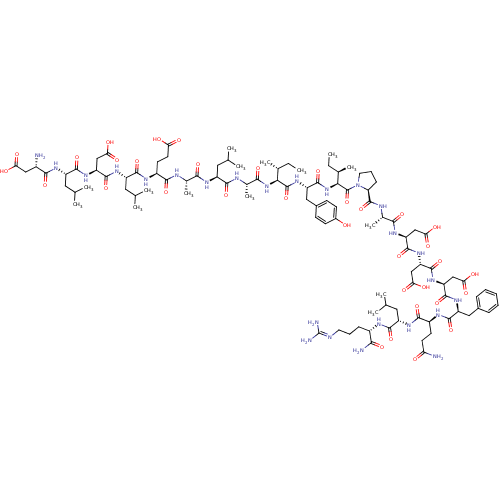 (CHEMBL557344) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296474 (CHEMBL558009) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296482 (CHEMBL541769) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296483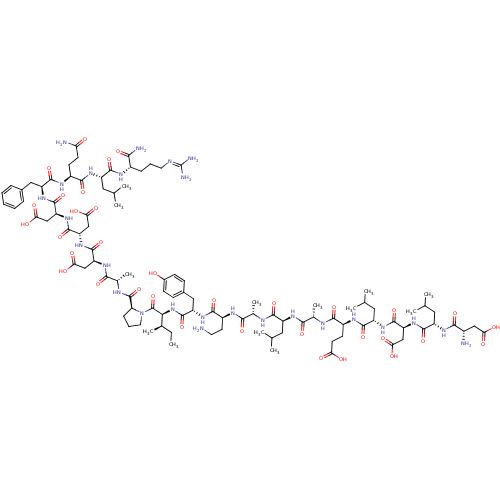 (CHEMBL538372) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.09E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50296472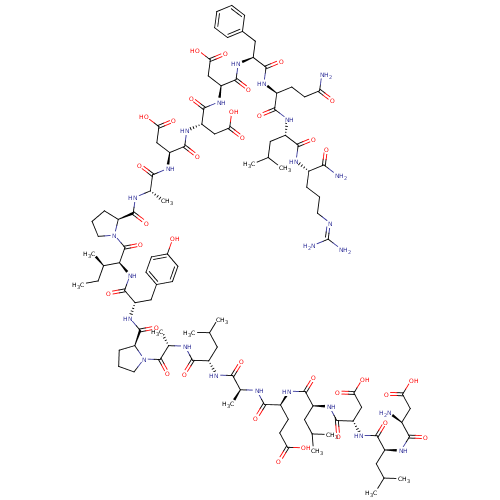 (CHEMBL558824) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology Curated by ChEMBL | Assay Description Inhibition of interaction between fluorescein-labeled proline hydroxylated human HIFalpha (556 to 575) and human VHL-human elongin B-human elongin C ... | Bioorg Med Chem Lett 19: 4403-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.083 BindingDB Entry DOI: 10.7270/Q2DJ5FP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50179013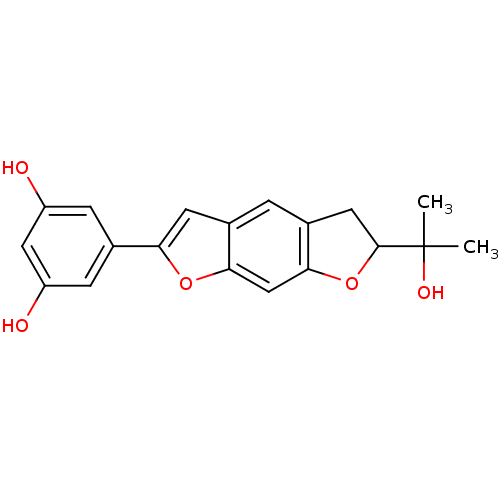 (CHEMBL205924 | moracin O) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis | J Nat Prod 72: 39-43 (2009) Article DOI: 10.1021/np800491u BindingDB Entry DOI: 10.7270/Q27947GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.199 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human IKKepsilon using casein as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276896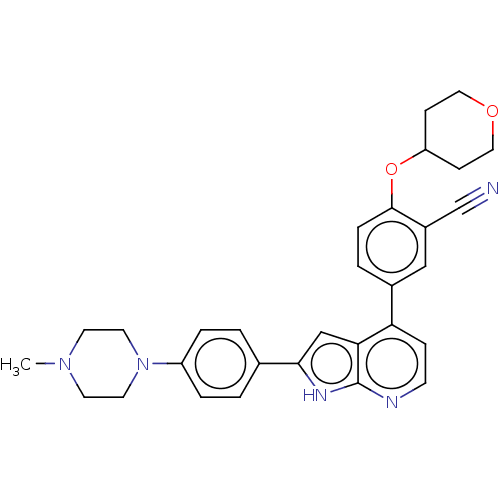 (US10072001, Example 82 | US10259811, Example 82) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276896 (US10072001, Example 82 | US10259811, Example 82) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.439 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human IKKepsilon using casein as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276969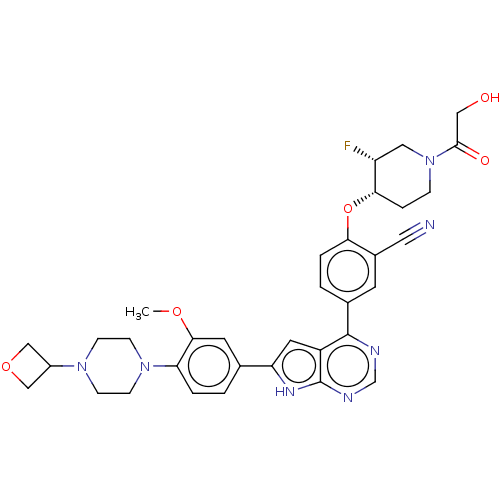 (US10072001, Example 145 | US10259811, Example 145) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276969 (US10072001, Example 145 | US10259811, Example 145) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.481 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50480476 ((-)-Moracin P | Moracin P) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Korean Research Institute of Biosciences and Biotechnology Curated by ChEMBL | Assay Description Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis | J Nat Prod 72: 39-43 (2009) Article DOI: 10.1021/np800491u BindingDB Entry DOI: 10.7270/Q27947GK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50179011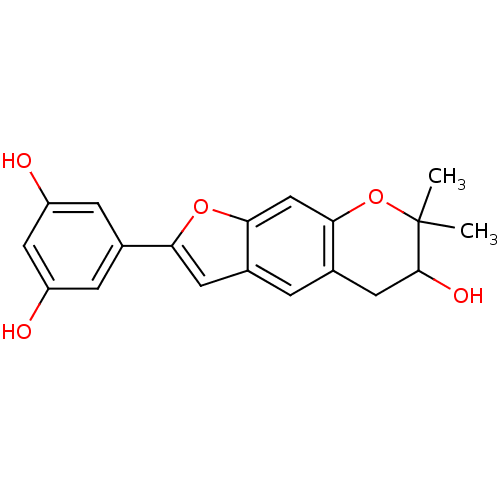 (CHEMBL380456 | Moracin P) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Dongguk University-Seoul Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus FabI-mediated trans-2-octenoyl N-acetylcysteamine (t-o-NAC thioester) substrate reduction assessed as decrease in... | Eur J Med Chem 90: 379-93 (2015) Article DOI: 10.1016/j.ejmech.2014.11.047 BindingDB Entry DOI: 10.7270/Q2GF0W5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276965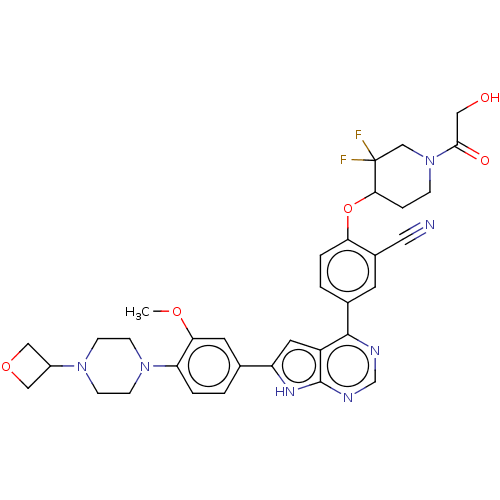 (US10072001, Example 141 | US10259811, Example 141) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.687 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276965 (US10072001, Example 141 | US10259811, Example 141) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.687 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276877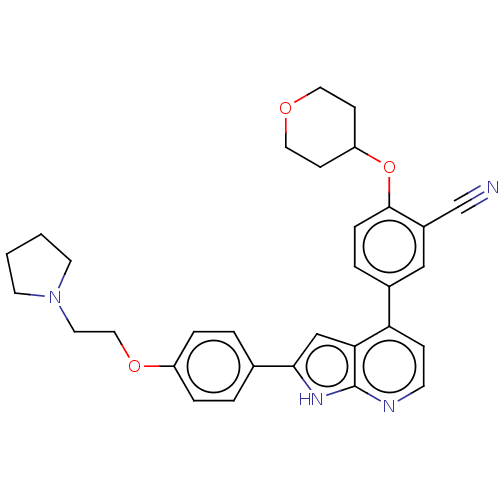 (US10072001, Example 63 | US10259811, Example 63) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276877 (US10072001, Example 63 | US10259811, Example 63) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276879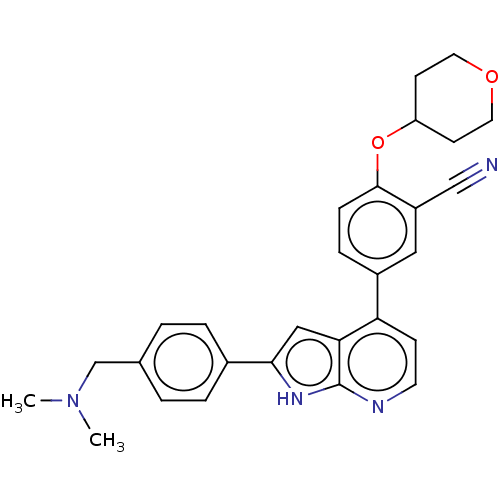 (US10072001, Example 65 | US10259811, Example 65) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276879 (US10072001, Example 65 | US10259811, Example 65) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276868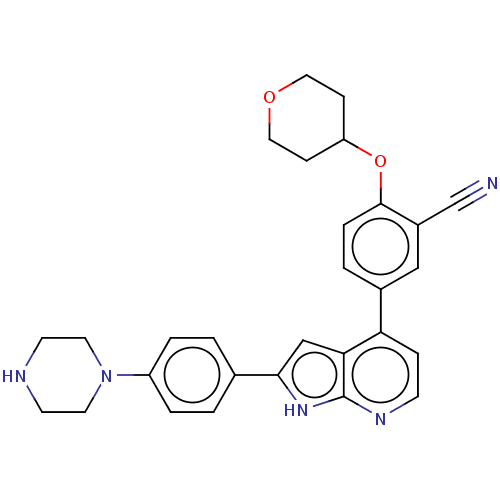 (US10072001, Example 54 | US10259811, Example 54) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM276868 (US10072001, Example 54 | US10259811, Example 54) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM277059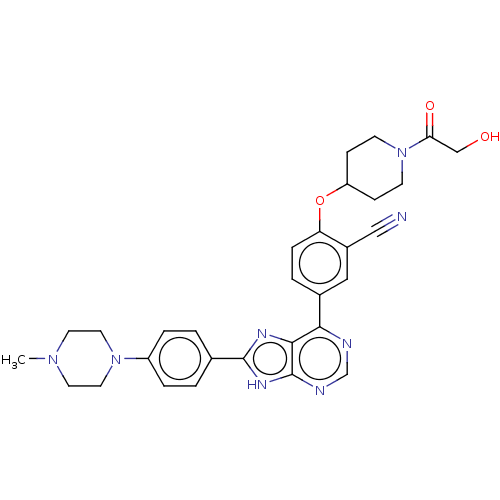 (US10072001, Example 251 | US10259811, Example 251) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.815 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM277059 (US10072001, Example 251 | US10259811, Example 251) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.815 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM277037 (US10072001, Example 229 | US10259811, Example 229) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | Bioorg Med Chem 16: 4272-85 (2008) BindingDB Entry DOI: 10.7270/Q2ZG6VKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM277037 (US10072001, Example 229 | US10259811, Example 229) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.851 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Gilead Sciences, Inc. US Patent | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | US Patent US10072001 (2018) BindingDB Entry DOI: 10.7270/Q27H1MMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278007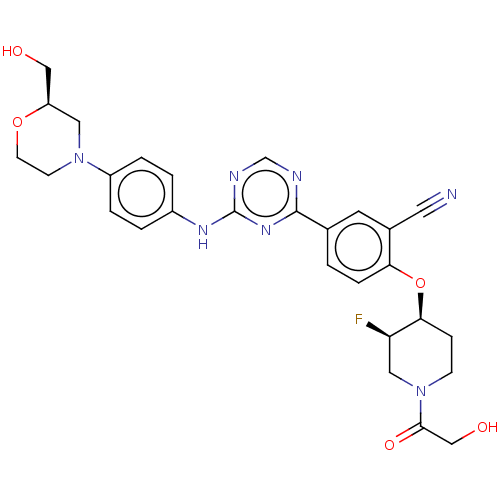 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278009 (2-(((3R,4S)-3-fluoro-1-((S)-2-hydroxypropanoyl)pip...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278010 (2-(((3R,4S)-3-fluoro-1-(2-hydroxyacetyl)piperidin-...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inhibitor of nuclear factor kappa-B kinase subunit epsilon (Homo sapiens (Human)) | BDBM278276 (2-(((3R,4S)-3-fluoro-1-((S)-2-hydroxypropanoyl)pip...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Rome La Sapienza | Assay Description Enzymatic activity of IKKε and TBK1 was measured using a homogeneous time resolved fluorescence resonance energy transfer (TR-FRET) assay that m... | J Med Chem 50: 6554-69 (2007) BindingDB Entry DOI: 10.7270/Q2Q52RXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4440 total ) | Next | Last >> |