 Found 822 hits Enz. Inhib. hit(s) with Target = 'Dual specificity protein kinase CLK2'
Found 822 hits Enz. Inhib. hit(s) with Target = 'Dual specificity protein kinase CLK2' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50224883
(7-chloro-3-oxo-8-[(thiazol-5-ylmethyl)-amino]-11,1...)Show SMILES Clc1cc2NC(=O)Nc3cnc(C#N)c(OCCCCOc2cc1NCc1cncs1)n3 Show InChI InChI=1S/C20H18ClN7O3S/c21-13-5-15-17(6-14(13)24-9-12-8-23-11-32-12)30-3-1-2-4-31-19-16(7-22)25-10-18(27-19)28-20(29)26-15/h5-6,8,10-11,24H,1-4,9H2,(H2,26,27,28,29) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CLK2 |
Bioorg Med Chem Lett 17: 6593-601 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.063
BindingDB Entry DOI: 10.7270/Q2X067WT |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50589394

(CHEMBL4797564)Show SMILES CNc1nc(NCc2ncccn2)c2c(c[nH]c2n1)-c1ccc2ncccc2c1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 0.0960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50512318
(CHEMBL4546504)Show SMILES C[C@@H](Oc1cc(ccc1Nc1nc(O[C@H]2CC[C@@](C)(O)CC2)c2c(Cl)c[nH]c2n1)C(=O)N(C)C)C(F)(F)F |r,wU:17.19,14.14,1.0,(59.2,-50.35,;60.54,-51.12,;61.87,-50.34,;61.86,-48.8,;60.53,-48.04,;60.52,-46.49,;61.86,-45.72,;63.19,-46.49,;63.18,-48.03,;64.52,-48.8,;65.85,-48.03,;65.85,-46.49,;67.18,-45.72,;67.18,-44.18,;65.84,-43.41,;64.51,-44.19,;63.18,-43.42,;63.17,-41.87,;62.4,-40.53,;61.63,-41.87,;64.51,-41.11,;65.84,-41.87,;68.52,-46.48,;69.98,-46,;70.45,-44.53,;70.89,-47.24,;70,-48.49,;68.53,-48.02,;67.18,-48.8,;59.19,-45.72,;59.19,-44.18,;57.86,-46.49,;56.52,-45.72,;57.86,-48.03,;60.54,-52.66,;59.21,-53.43,;61.88,-53.42,;60.53,-54.19,)| Show InChI InChI=1S/C25H29ClF3N5O4/c1-13(25(27,28)29)37-18-11-14(22(35)34(3)4)5-6-17(18)31-23-32-20-19(16(26)12-30-20)21(33-23)38-15-7-9-24(2,36)10-8-15/h5-6,11-13,15,36H,7-10H2,1-4H3,(H2,30,31,32,33)/t13-,15-,24+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.261 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged CLK2 catalytic domain (137 to 498 residues) expressed in baculovirus expression system by Z'-LYTE assay |
J Med Chem 62: 4401-4410 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01869
BindingDB Entry DOI: 10.7270/Q2KS6VVN |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 incubated for 2 hrs |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00131
BindingDB Entry DOI: 10.7270/Q2ZS319T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50613087

(CHEMBL5091238) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PDB
UniChem
| | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50609269

(CHEMBL5269810) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.822 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM445772

(US10669240, Compound 68 | US10669240, Compound 69)Show SMILES COc1ccc(NC(=O)c2n[nH]c3ccc(cc23)-c2cncc(CN3CCCCC3)c2)cn1 Show InChI InChI=1S/C25H26N6O2/c1-33-23-8-6-20(15-27-23)28-25(32)24-21-12-18(5-7-22(21)29-30-24)19-11-17(13-26-14-19)16-31-9-3-2-4-10-31/h5-8,11-15H,2-4,9-10,16H2,1H3,(H,28,32)(H,29,30) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.822 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116921
BindingDB Entry DOI: 10.7270/Q25B06FX |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM25036
(CHEMBL393525 | N'-[(1E)-{6-bromoimidazo[1,2-a]pyri...)Show SMILES CN(\N=C\c1cnc2ccc(Br)cn12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-5-13(22(23)24)7-15(11)27(25,26)20(2)19-9-14-8-18-16-6-4-12(17)10-21(14)16/h3-10H,1-2H3/b19-9+ | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50566490
(CHEMBL4848224)Show SMILES FCCOC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 incubated for 2 hrs |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00131
BindingDB Entry DOI: 10.7270/Q2ZS319T |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50589393
(CHEMBL5202010)Show SMILES COc1ncnn2ccc(-c3cnc4nc(C)n(Cc5nnc(o5)[C@@H](C)F)c4c3)c12 |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116921
BindingDB Entry DOI: 10.7270/Q25B06FX |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317482
(4-(4-((1s,4s)-4-hydroxy-4- methylcyclohexyloxy)-5-...)Show SMILES CNC(=O)c1ccc(cc1)-c1c[nH]c2nc(Nc3ccc(cc3OC)C(=O)NC3CN(C)C3)nc(OC3CC[C@@](C)(O)CC3)c12 |r,wU:39.43,wD:39.42,(6.47,11.4,;6.08,9.92,;4.59,9.52,;3.5,10.61,;4.19,8.03,;5.28,6.94,;4.88,5.45,;3.39,5.06,;2.3,6.15,;2.7,7.63,;2.99,3.57,;3.9,2.32,;2.99,1.08,;1.53,1.55,;.19,.78,;-1.14,1.55,;-2.47,.78,;-2.47,-.76,;-1.14,-1.53,;-1.14,-3.07,;-2.47,-3.84,;-3.81,-3.07,;-3.81,-1.53,;-5.14,-.76,;-6.47,-1.53,;-2.47,-5.38,;-1.14,-6.15,;-3.81,-6.15,;-3.81,-7.69,;-4.9,-8.78,;-3.81,-9.86,;-3.81,-11.4,;-2.72,-8.78,;-1.14,3.09,;.19,3.86,;.19,5.4,;-1.14,6.17,;-2.47,5.4,;-3.81,6.17,;-3.81,7.71,;-3.81,9.25,;-5.14,6.94,;-2.47,8.48,;-1.14,7.71,;1.53,3.09,)| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50566492

(CHEMBL4872225)Show SMILES FC(F)COC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 incubated for 2 hrs |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00131
BindingDB Entry DOI: 10.7270/Q2ZS319T |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50566489

(CHEMBL4848072)Show SMILES Clc1cccc(Nc2nc3cc(ccc3c3cnccc23)C(=O)OCCI)c1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 incubated for 2 hrs |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00131
BindingDB Entry DOI: 10.7270/Q2ZS319T |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317465
(4-(4-(cyclopentyloxy)-5-(4- (methylcarbamoyl)pheny...)Show SMILES CNC(=O)c1ccc(cc1)-c1c[nH]c2nc(Nc3ccc(cc3OC)C(=O)NC)nc(OC3CCCC3)c12 Show InChI InChI=1S/C28H30N6O4/c1-29-25(35)17-10-8-16(9-11-17)20-15-31-24-23(20)27(38-19-6-4-5-7-19)34-28(33-24)32-21-13-12-18(26(36)30-2)14-22(21)37-3/h8-15,19H,4-7H2,1-3H3,(H,29,35)(H,30,36)(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.91 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM293424
(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM293424

(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116914
BindingDB Entry DOI: 10.7270/Q2HH6Q1V |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM293424

(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116921
BindingDB Entry DOI: 10.7270/Q25B06FX |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50609268
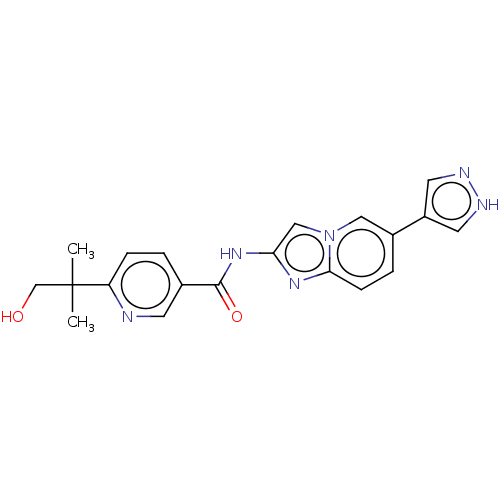
(CHEMBL5286791) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317483

(3-chloro-N-methyl-4-(5-(4- (methylcarbamoyl)phenyl...)Show SMILES CNC(=O)c1ccc(cc1)-c1c[nH]c2nc(Nc3ccc(cc3Cl)C(=O)NC)nc(OC3CCOCC3)c12 Show InChI InChI=1S/C27H27ClN6O4/c1-29-24(35)16-5-3-15(4-6-16)19-14-31-23-22(19)26(38-18-9-11-37-12-10-18)34-27(33-23)32-21-8-7-17(13-20(21)28)25(36)30-2/h3-8,13-14,18H,9-12H2,1-2H3,(H,29,35)(H,30,36)(H2,31,32,33,34) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317471
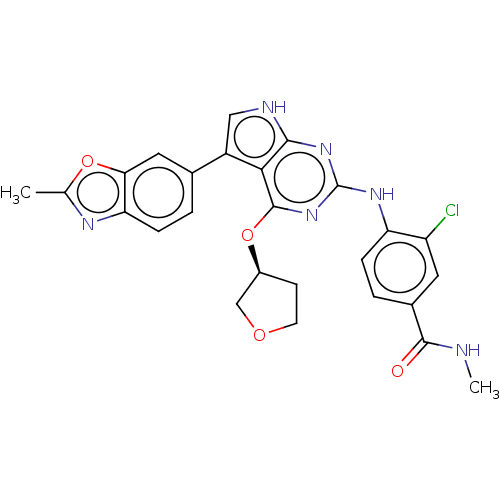
((S)-3-chloro-N-methyl-4-(5-(2- methylbenzo[d]oxazo...)Show SMILES CNC(=O)c1ccc(Nc2nc(O[C@H]3CCOC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(Cl)c1 |r| | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317469
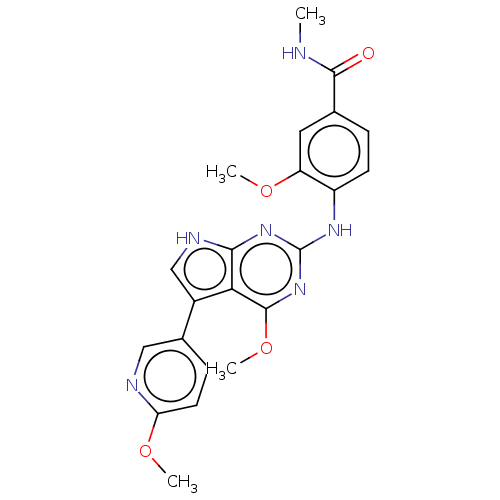
(3-methoxy-4-(4-methoxy-5-(6- methoxypyridin-3-yl)-...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC)c3c(c[nH]c3n2)-c2ccc(OC)nc2)c(OC)c1 Show InChI InChI=1S/C22H22N6O4/c1-23-20(29)12-5-7-15(16(9-12)30-2)26-22-27-19-18(21(28-22)32-4)14(11-25-19)13-6-8-17(31-3)24-10-13/h5-11H,1-4H3,(H,23,29)(H2,25,26,27,28) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317475
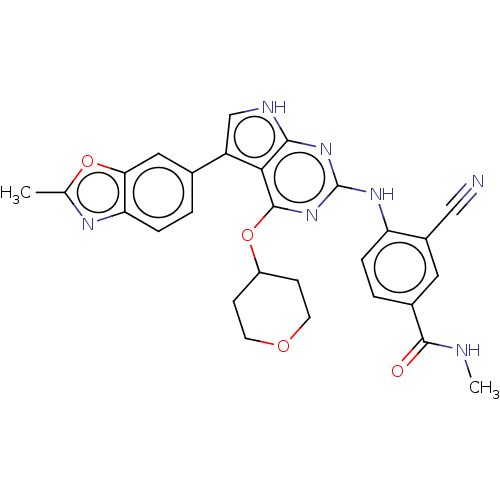
(3-cyano-N-methyl-4-(5-(2- methylbenzo[d]oxazol-6-y...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCOCC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(c1)C#N Show InChI InChI=1S/C28H25N7O4/c1-15-32-22-6-3-16(12-23(22)38-15)20-14-31-25-24(20)27(39-19-7-9-37-10-8-19)35-28(34-25)33-21-5-4-17(26(36)30-2)11-18(21)13-29/h3-6,11-12,14,19H,7-10H2,1-2H3,(H,30,36)(H2,31,33,34,35) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50566488
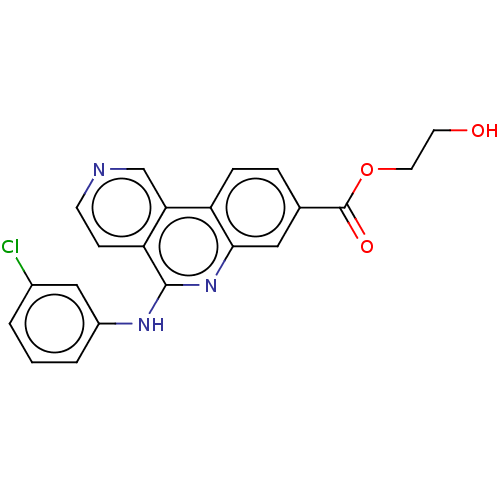
(CHEMBL4875513)Show SMILES OCCOC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 incubated for 2 hrs |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00131
BindingDB Entry DOI: 10.7270/Q2ZS319T |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50592247
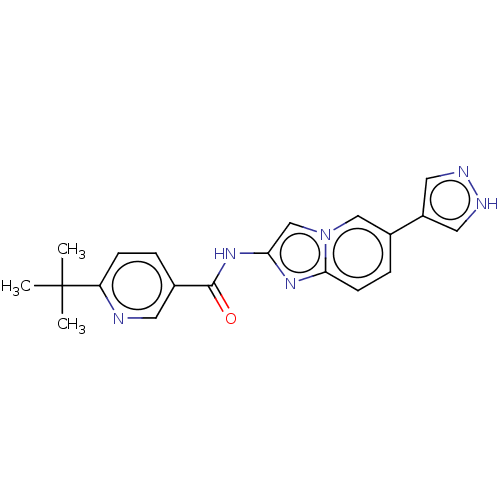
(CHEMBL5200367)Show SMILES CC(C)(C)c1ccc(cn1)C(=O)Nc1cn2cc(ccc2n1)-c1cn[nH]c1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114411
BindingDB Entry DOI: 10.7270/Q29Z98V0 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50609267

(CHEMBL5283453) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CLK2 using MBP as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317485
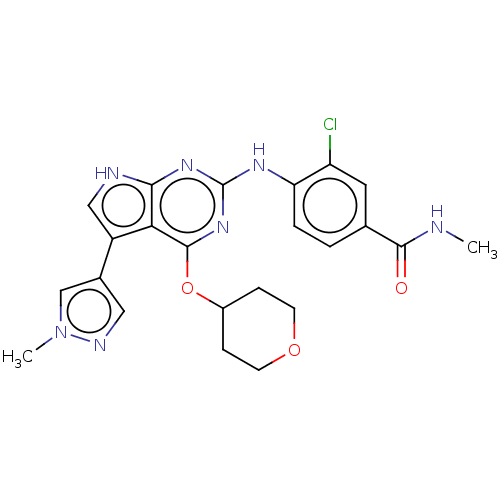
(3-chloro-N-methyl-4-(5-(1- methyl-1H-pyrazol-4-yl)...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCOCC3)c3c(c[nH]c3n2)-c2cnn(C)c2)c(Cl)c1 Show InChI InChI=1S/C23H24ClN7O3/c1-25-21(32)13-3-4-18(17(24)9-13)28-23-29-20-19(16(11-26-20)14-10-27-31(2)12-14)22(30-23)34-15-5-7-33-8-6-15/h3-4,9-12,15H,5-8H2,1-2H3,(H,25,32)(H2,26,28,29,30) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.29 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317468
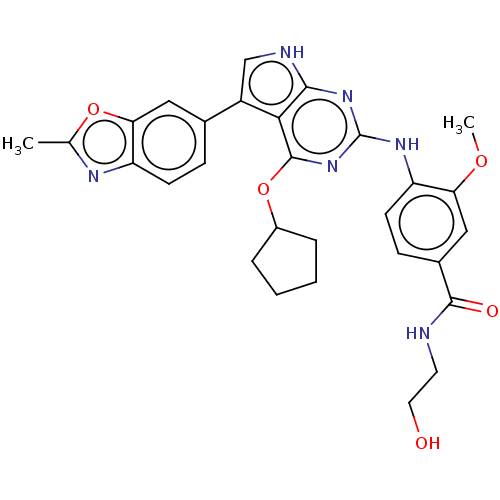
(4-(4-(cyclopentyloxy)-5-(2- methylbenzo[d]oxazol-6...)Show SMILES COc1cc(ccc1Nc1nc(OC2CCCC2)c2c(c[nH]c2n1)-c1ccc2nc(C)oc2c1)C(=O)NCCO Show InChI InChI=1S/C29H30N6O5/c1-16-32-22-9-7-17(13-24(22)39-16)20-15-31-26-25(20)28(40-19-5-3-4-6-19)35-29(34-26)33-21-10-8-18(14-23(21)38-2)27(37)30-11-12-36/h7-10,13-15,19,36H,3-6,11-12H2,1-2H3,(H,30,37)(H2,31,33,34,35) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.38 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317467
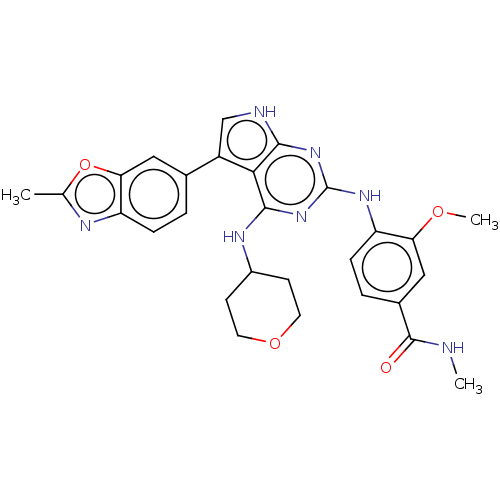
(3-methoxy-N-methyl-4-(5-(2- methylbenzo[d]oxazol-6...)Show SMILES CNC(=O)c1ccc(Nc2nc(NC3CCOCC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(OC)c1 Show InChI InChI=1S/C28H29N7O4/c1-15-31-21-6-4-16(12-23(21)39-15)19-14-30-25-24(19)26(32-18-8-10-38-11-9-18)35-28(34-25)33-20-7-5-17(27(36)29-2)13-22(20)37-3/h4-7,12-14,18H,8-11H2,1-3H3,(H,29,36)(H3,30,32,33,34,35) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM293424

(US10106527, Compound 10 | US10106527, Compound 142...)Show SMILES CN1CCN(CC1)c1cc(ccn1)C(=O)Nc1cc2cc(ccc2cn1)-c1cnn(C)c1 Show InChI InChI=1S/C24H25N7O/c1-29-7-9-31(10-8-29)23-13-18(5-6-25-23)24(32)28-22-12-20-11-17(3-4-19(20)14-26-22)21-15-27-30(2)16-21/h3-6,11-16H,7-10H2,1-2H3,(H,26,28,32) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cambridge
Curated by ChEMBL
| Assay Description
Inhibition of CLK2 (unknown origin) |
Bioorg Med Chem 26: 3016-3020 (2018)
Article DOI: 10.1016/j.bmc.2018.05.011
BindingDB Entry DOI: 10.7270/Q2X3513P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK2 in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK2 in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human CLK2 (138 to end residues) using SR-rich substrate and [gamma-33P]ATP incubated for 40 mins by scintillatio... |
J Med Chem 62: 1817-1836 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01765
BindingDB Entry DOI: 10.7270/Q2SJ1Q27 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal GST-tagged human CLK2 (138 to end residues) using SR-rich substrate and [gamma-33P]ATP incubated for 40 mins by scintillatio... |
J Med Chem 62: 1817-1836 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01765
BindingDB Entry DOI: 10.7270/Q2SJ1Q27 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM8156
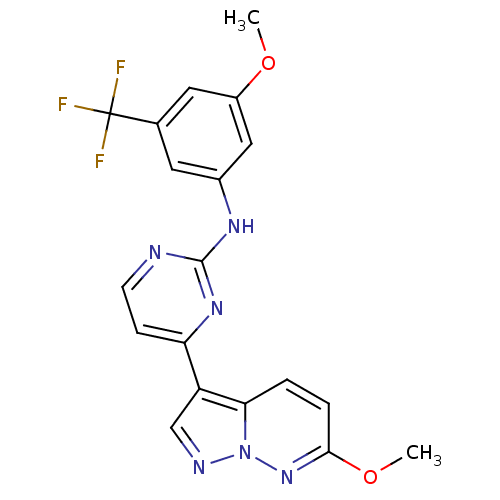
(N-[3-methoxy-5-(trifluoromethyl)phenyl]-4-{6-metho...)Show SMILES COc1cc(Nc2nccc(n2)-c2cnn3nc(OC)ccc23)cc(c1)C(F)(F)F Show InChI InChI=1S/C19H15F3N6O2/c1-29-13-8-11(19(20,21)22)7-12(9-13)25-18-23-6-5-15(26-18)14-10-24-28-16(14)3-4-17(27-28)30-2/h3-10H,1-2H3,(H,23,25,26) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317460
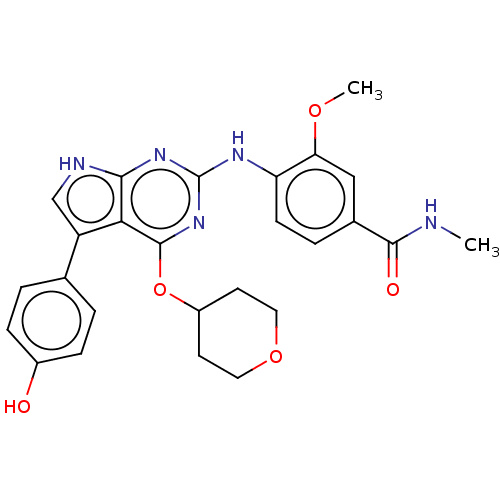
(4-(5-(4-hydroxyphenyl)-4- (tetrahydro-2H-pyran-4-y...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCOCC3)c3c(c[nH]c3n2)-c2ccc(O)cc2)c(OC)c1 Show InChI InChI=1S/C26H27N5O5/c1-27-24(33)16-5-8-20(21(13-16)34-2)29-26-30-23-22(25(31-26)36-18-9-11-35-12-10-18)19(14-28-23)15-3-6-17(32)7-4-15/h3-8,13-14,18,32H,9-12H2,1-2H3,(H,27,33)(H2,28,29,30,31) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317466
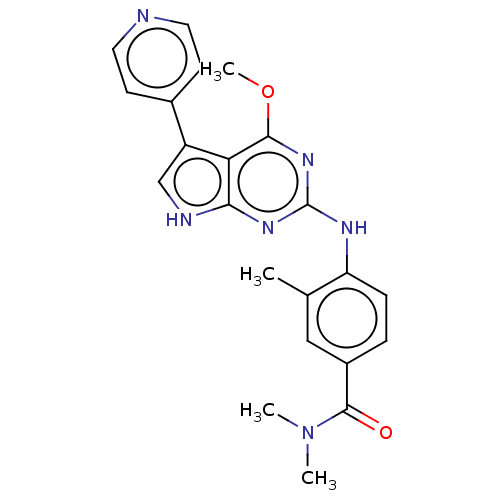
(4-(4-methoxy-5-(pyridin-4-yl)- 7H-pyrrolo[2,3-d]py...)Show SMILES COc1nc(Nc2ccc(cc2C)C(=O)N(C)C)nc2[nH]cc(-c3ccncc3)c12 Show InChI InChI=1S/C22H22N6O2/c1-13-11-15(21(29)28(2)3)5-6-17(13)25-22-26-19-18(20(27-22)30-4)16(12-24-19)14-7-9-23-10-8-14/h5-12H,1-4H3,(H2,24,25,26,27) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50246769

(CHEMBL4064608)Show SMILES C1CC(CCO1)c1c[nH]c2ncnc(N[C@H]3CC[C@@H](CC3)N3CCOCC3)c12 |r,wU:15.15,wD:18.22,(7.34,-15.64,;7.82,-17.1,;6.8,-18.25,;5.29,-17.94,;4.81,-16.48,;5.83,-15.33,;7.28,-19.71,;6.38,-20.97,;7.3,-22.21,;8.77,-21.73,;10.1,-22.5,;11.43,-21.72,;11.43,-20.18,;10.1,-19.41,;10.09,-17.87,;11.43,-17.1,;11.42,-15.56,;12.75,-14.8,;14.09,-15.57,;14.08,-17.11,;12.75,-17.87,;15.42,-14.8,;16.75,-15.58,;18.08,-14.82,;18.09,-13.28,;16.76,-12.5,;15.42,-13.27,;8.76,-20.18,)| Show InChI InChI=1S/C21H31N5O2/c1-3-17(26-7-11-28-12-8-26)4-2-16(1)25-21-19-18(15-5-9-27-10-6-15)13-22-20(19)23-14-24-21/h13-17H,1-12H2,(H2,22,23,24,25)/t16-,17- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal GST-tagged human CLK2 (138 end residues) expressed in baculovirus infected Sf21 cells |
J Med Chem 60: 10071-10091 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01290
BindingDB Entry DOI: 10.7270/Q2T72KWX |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50237672
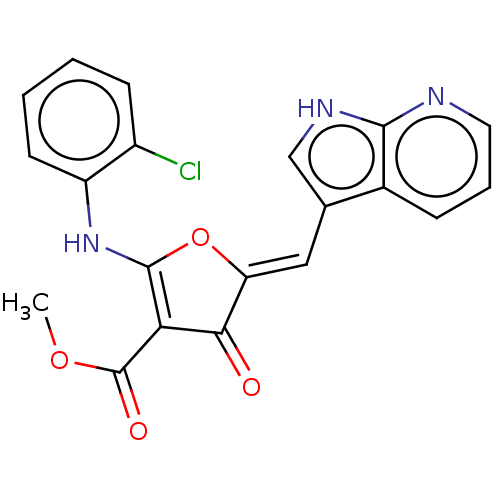
(CHEMBL4089159)Show SMILES COC(=O)C1=C(Nc2ccccc2Cl)O\C(=C/c2c[nH]c3ncccc23)C1=O |c:4| Show InChI InChI=1S/C20H14ClN3O4/c1-27-20(26)16-17(25)15(9-11-10-23-18-12(11)5-4-8-22-18)28-19(16)24-14-7-3-2-6-13(14)21/h2-10,24H,1H3,(H,22,23)/b15-9- | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Carna Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CLK2 (unknown origin) pretreated for 30 mins followed by substrate addition measured after 5 hrs in presence of 1 mM ATP |
Eur J Med Chem 130: 406-418 (2017)
Article DOI: 10.1016/j.ejmech.2017.02.030
BindingDB Entry DOI: 10.7270/Q2X63Q71 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human CLK2 using MBP as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CLK2 (unknown origin) assessed as transfer of radiolabelled phosphate group from ATP by reaction biology method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01579
BindingDB Entry DOI: 10.7270/Q2ST7TPK |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50538084

(CHEMBL4647659)Show SMILES CC(C)Oc1ccc(F)c(c1)-c1cnc(N)c(n1)C(=O)Nc1cnccc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C25H27FN6O4/c1-14(2)36-16-5-6-18(26)17(10-16)19-12-29-23(27)22(30-19)24(33)31-20-11-28-8-7-21(20)32-9-3-4-15(13-32)25(34)35/h5-8,10-12,14-15H,3-4,9,13H2,1-2H3,(H2,27,29)(H,31,33)(H,34,35)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317481
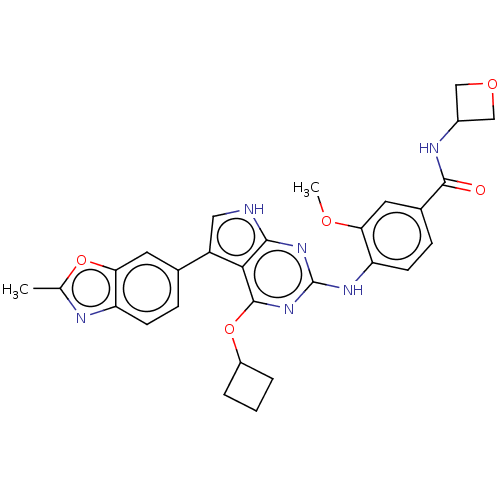
(4-(4-cyclobutoxy-5-(2- methylbenzo[d]oxazol-6-yl)-...)Show SMILES COc1cc(ccc1Nc1nc(OC2CCC2)c2c(c[nH]c2n1)-c1ccc2nc(C)oc2c1)C(=O)NC1COC1 Show InChI InChI=1S/C29H28N6O5/c1-15-31-22-8-6-16(10-24(22)39-15)20-12-30-26-25(20)28(40-19-4-3-5-19)35-29(34-26)33-21-9-7-17(11-23(21)37-2)27(36)32-18-13-38-14-18/h6-12,18-19H,3-5,13-14H2,1-2H3,(H,32,36)(H2,30,33,34,35) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.92 | n/a | n/a | n/a | n/a | n/a | n/a |
Signal Pharmaceuticals, LLC
US Patent
| Assay Description
The kinase activity is measured using a radioactivity based kinase assay, which measures the incorporation of a radioactively labeled phosphate moiet... |
US Patent US9623028 (2017)
BindingDB Entry DOI: 10.7270/Q2VQ34R8 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50609261
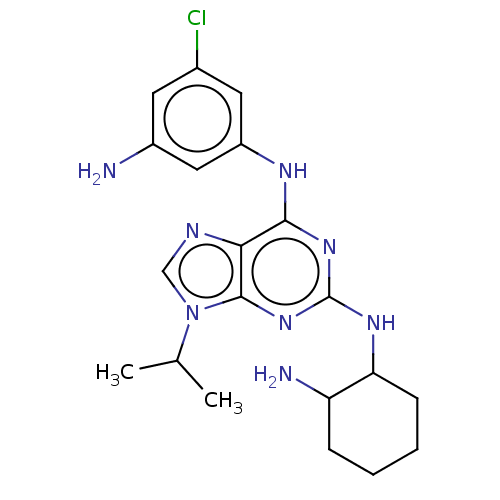
(CHEMBL5276465) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50235022
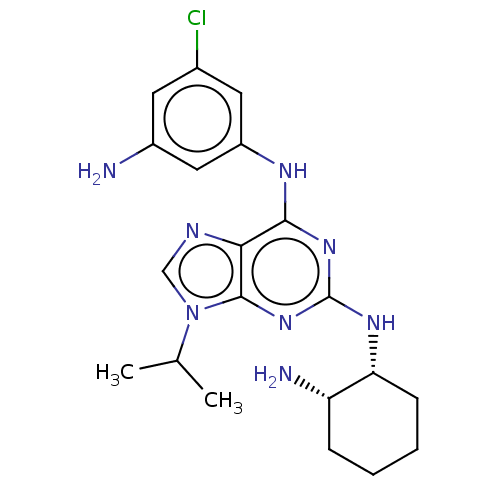
(CHEMBL4078898)Show SMILES CC(C)n1cnc2c(Nc3cc(N)cc(Cl)c3)nc(N[C@@H]3CCCC[C@@H]3N)nc12 |r| Show InChI InChI=1S/C20H27ClN8/c1-11(2)29-10-24-17-18(25-14-8-12(21)7-13(22)9-14)27-20(28-19(17)29)26-16-6-4-3-5-15(16)23/h7-11,15-16H,3-6,22-23H2,1-2H3,(H2,25,26,27,28)/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibition of human CLK2 using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method |
Bioorg Med Chem Lett 27: 406-412 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.056
BindingDB Entry DOI: 10.7270/Q27946ZF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50235022

(CHEMBL4078898)Show SMILES CC(C)n1cnc2c(Nc3cc(N)cc(Cl)c3)nc(N[C@@H]3CCCC[C@@H]3N)nc12 |r| Show InChI InChI=1S/C20H27ClN8/c1-11(2)29-10-24-17-18(25-14-8-12(21)7-13(22)9-14)27-20(28-19(17)29)26-16-6-4-3-5-15(16)23/h7-11,15-16H,3-6,22-23H2,1-2H3,(H2,25,26,27,28)/t15-,16+/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Inhibition of human CLK2 using YRRAAVPPSPSLSRHSSPHQS(p)EDEEE as substrate in presence of [gamma-33P]ATP after 40 mins by scintillation counter method |
Bioorg Med Chem Lett 27: 406-412 (2017)
Article DOI: 10.1016/j.bmcl.2016.12.056
BindingDB Entry DOI: 10.7270/Q27946ZF |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317462
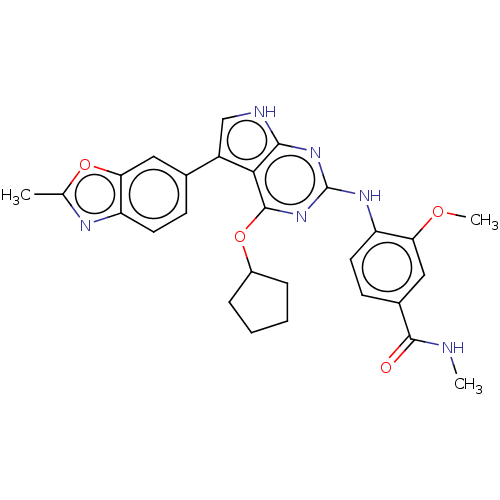
(4-(4-(cyclopentyloxy)-5-(2- methylbenzo[d]oxazol-6...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCCC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(OC)c1 Show InChI InChI=1S/C28H28N6O4/c1-15-31-21-10-8-16(12-23(21)37-15)19-14-30-25-24(19)27(38-18-6-4-5-7-18)34-28(33-25)32-20-11-9-17(26(35)29-2)13-22(20)36-3/h8-14,18H,4-7H2,1-3H3,(H,29,35)(H2,30,32,33,34) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged CLK2 expressed in baculovirus by Z'-LYTE assay |
J Med Chem 60: 8989-9002 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01223
BindingDB Entry DOI: 10.7270/Q2GF0WX9 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM317462

(4-(4-(cyclopentyloxy)-5-(2- methylbenzo[d]oxazol-6...)Show SMILES CNC(=O)c1ccc(Nc2nc(OC3CCCC3)c3c(c[nH]c3n2)-c2ccc3nc(C)oc3c2)c(OC)c1 Show InChI InChI=1S/C28H28N6O4/c1-15-31-21-10-8-16(12-23(21)37-15)19-14-30-25-24(19)27(38-18-6-4-5-7-18)34-28(33-25)32-20-11-9-17(26(35)29-2)13-22(20)36-3/h8-14,18H,4-7H2,1-3H3,(H,29,35)(H2,30,32,33,34) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data