 Found 592 hits Enz. Inhib. hit(s) with Target = 'Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1'
Found 592 hits Enz. Inhib. hit(s) with Target = 'Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184825
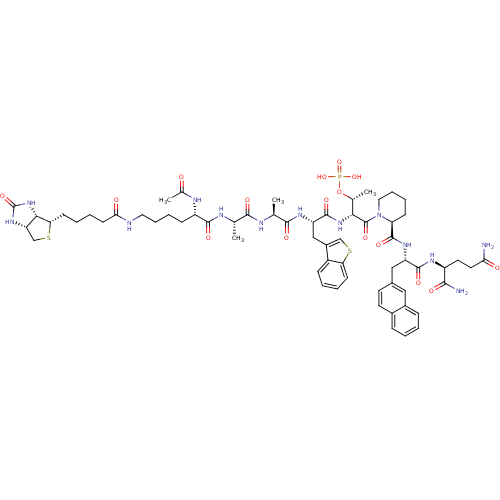
(Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-D-Thr(PO3H...)Show SMILES C[C@@H](OP(O)(O)=O)[C@@H](NC(=O)[C@H](Cc1csc2ccccc12)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(C)=O)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C63H86N13O16PS2/c1-34(68-58(83)44(69-37(4)77)18-11-13-27-66-52(79)22-10-9-21-50-54-47(33-95-50)73-63(88)75-54)56(81)67-35(2)57(82)71-46(31-41-32-94-49-20-8-7-17-42(41)49)60(85)74-53(36(3)92-93(89,90)91)62(87)76-28-14-12-19-48(76)61(86)72-45(59(84)70-43(55(65)80)25-26-51(64)78)30-38-23-24-39-15-5-6-16-40(39)29-38/h5-8,15-17,20,23-24,29,32,34-36,43-48,50,53-54H,9-14,18-19,21-22,25-28,30-31,33H2,1-4H3,(H2,64,78)(H2,65,80)(H,66,79)(H,67,81)(H,68,83)(H,69,77)(H,70,84)(H,71,82)(H,72,86)(H,74,85)(H2,73,75,88)(H2,89,90,91)/t34-,35-,36+,43-,44-,45-,46-,47-,48-,50-,53+,54-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184823
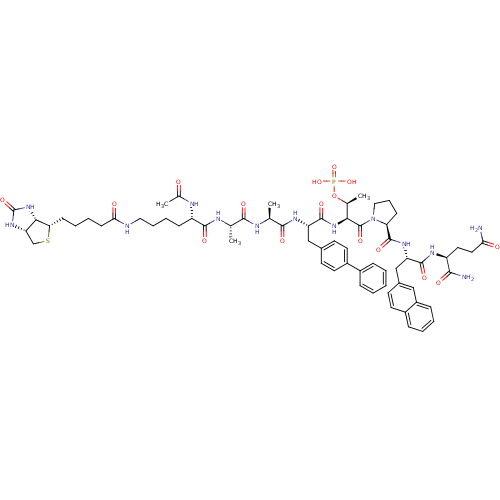
(Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bip-Thr(PO3H2)...)Show SMILES C[C@H](NC(=O)[C@H](CCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H]([C@H](C)OP(O)(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C66H88N13O16PS/c1-37(71-61(86)48(72-40(4)80)19-12-13-31-69-55(82)22-11-10-21-53-57-51(36-97-53)76-66(91)78-57)59(84)70-38(2)60(85)74-49(34-41-23-26-45(27-24-41)43-15-6-5-7-16-43)63(88)77-56(39(3)95-96(92,93)94)65(90)79-32-14-20-52(79)64(89)75-50(62(87)73-47(58(68)83)29-30-54(67)81)35-42-25-28-44-17-8-9-18-46(44)33-42/h5-9,15-18,23-28,33,37-39,47-53,56-57H,10-14,19-22,29-32,34-36H2,1-4H3,(H2,67,81)(H2,68,83)(H,69,82)(H,70,84)(H,71,86)(H,72,80)(H,73,87)(H,74,85)(H,75,89)(H,77,88)(H2,76,78,91)(H2,92,93,94)/t37-,38-,39-,47-,48-,49-,50-,51-,52-,53-,56-,57-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314714

((R)-N-(1-(3-fluorophenyl)-3-hydroxypropan-2-yl)ben...)Show SMILES OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H16FNO2S/c19-14-6-3-4-12(8-14)9-15(11-21)20-18(22)17-10-13-5-1-2-7-16(13)23-17/h1-8,10,15,21H,9,11H2,(H,20,22)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34012
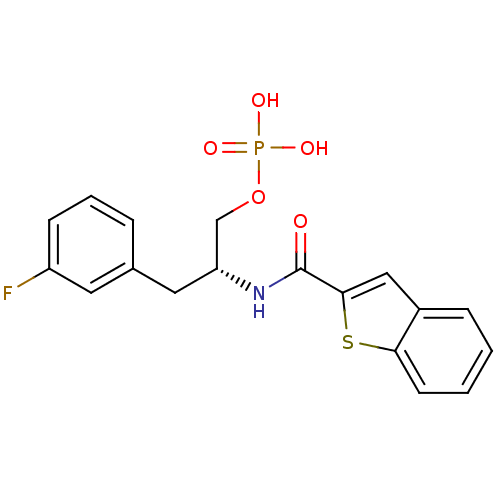
(3-fluorophenylalanine derivative, 21b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H17FNO5PS/c19-14-6-3-4-12(8-14)9-15(11-25-26(22,23)24)20-18(21)17-10-13-5-1-2-7-16(13)27-17/h1-8,10,15H,9,11H2,(H,20,21)(H2,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Pin1 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115878
BindingDB Entry DOI: 10.7270/Q21J9FDM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314706

((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-eno...)Show SMILES OC(=O)[C@@H](C\C=C\c1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H18FNO3/c23-19-9-3-5-15(13-19)6-4-10-20(22(26)27)24-21(25)18-12-11-16-7-1-2-8-17(16)14-18/h1-9,11-14,20H,10H2,(H,24,25)(H,26,27)/b6-4+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of N-terminal His tagged human Pin1 expressed in Escherichia coli BL21 (DE3) using Suc-Ala-Glu-Pro-Phe-4-nitroanilide as substrate preincu... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115878
BindingDB Entry DOI: 10.7270/Q21J9FDM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34012

(3-fluorophenylalanine derivative, 21b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H17FNO5PS/c19-14-6-3-4-12(8-14)9-15(11-25-26(22,23)24)20-18(21)17-10-13-5-1-2-7-16(13)27-17/h1-8,10,15H,9,11H2,(H,20,21)(H2,22,23,24)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34011
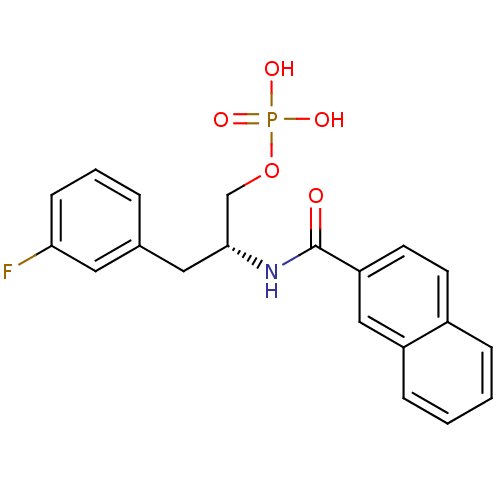
(3-fluorophenylalanine derivative, 21a)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H19FNO5P/c21-18-7-3-4-14(10-18)11-19(13-27-28(24,25)26)22-20(23)17-9-8-15-5-1-2-6-16(15)12-17/h1-10,12,19H,11,13H2,(H,22,23)(H2,24,25,26)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056217
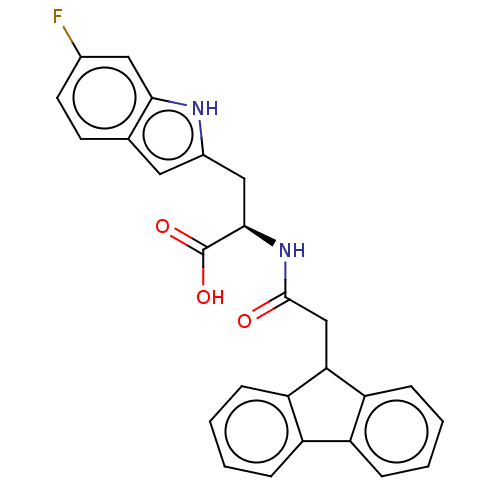
(CHEMBL3353369)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)CC1c2ccccc2-c2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184841
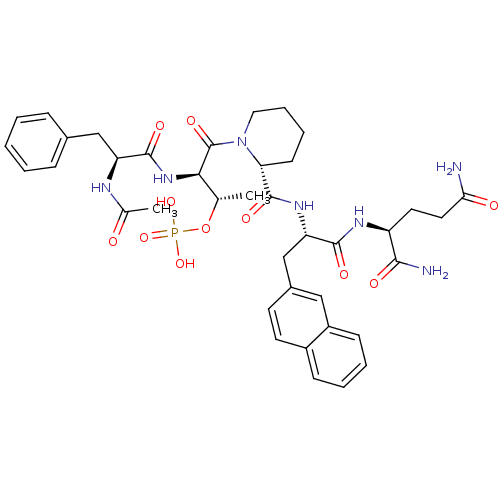
(Ac-Phe-D-Thr(PO3H2)-Pip-Nal-Gln-NH2 | CHEMBL436759)Show SMILES C[C@H](OP(O)(O)=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C39H50N7O11P/c1-23(57-58(54,55)56)34(45-37(51)30(42-24(2)47)21-25-10-4-3-5-11-25)39(53)46-19-9-8-14-32(46)38(52)44-31(36(50)43-29(35(41)49)17-18-33(40)48)22-26-15-16-27-12-6-7-13-28(27)20-26/h3-7,10-13,15-16,20,23,29-32,34H,8-9,14,17-19,21-22H2,1-2H3,(H2,40,48)(H2,41,49)(H,42,47)(H,43,50)(H,44,52)(H,45,51)(H2,54,55,56)/t23-,29-,30-,31-,32+,34+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50552327
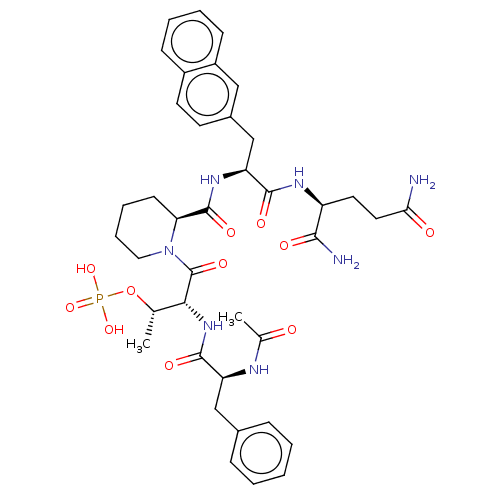
(CHEMBL4751817)Show SMILES C[C@H](OP(O)(O)=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA as substrate preincubated with enzyme for 12 hrs followed by substrate ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115878
BindingDB Entry DOI: 10.7270/Q21J9FDM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34013
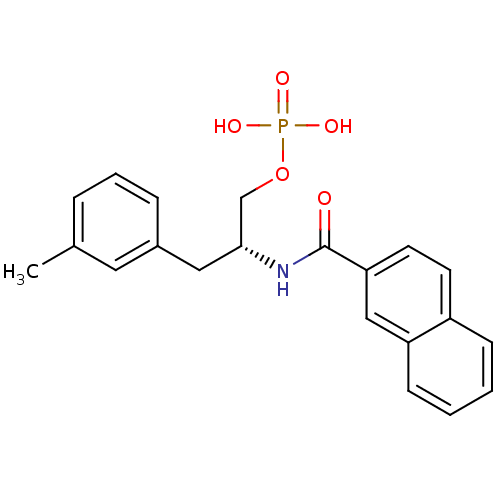
(3-methylphenylalanine derivative, 22a)Show SMILES Cc1cccc(C[C@H](COP(O)(O)=O)NC(=O)c2ccc3ccccc3c2)c1 |r| Show InChI InChI=1S/C21H22NO5P/c1-15-5-4-6-16(11-15)12-20(14-27-28(24,25)26)22-21(23)19-10-9-17-7-2-3-8-18(17)13-19/h2-11,13,20H,12,14H2,1H3,(H,22,23)(H2,24,25,26)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 32 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50552328

(CHEMBL4750217)Show SMILES CCOC(=O)[C@H](CCCNC(N)=O)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCCN1C(=O)[C@H](Cc1ccccc1)N(C)C(=O)CCl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA as substrate preincubated with enzyme for 12 hrs followed by substrate ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115878
BindingDB Entry DOI: 10.7270/Q21J9FDM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50437704

(CHEMBL2409078)Show InChI InChI=1S/C12H20N2S3/c15-11(13-7-3-1-4-8-13)17-12(16)14-9-5-2-6-10-14/h1-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Horizon Discovery
Curated by ChEMBL
| Assay Description
Competitive inhibition of Pin1 (unknown origin) by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 23: 4283-91 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.088
BindingDB Entry DOI: 10.7270/Q2DV1M9T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34014
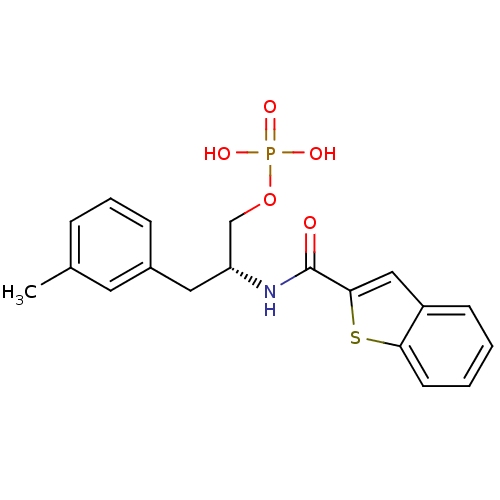
(3-methylphenylalanine derivative, 22b)Show SMILES Cc1cccc(C[C@H](COP(O)(O)=O)NC(=O)c2cc3ccccc3s2)c1 |r| Show InChI InChI=1S/C19H20NO5PS/c1-13-5-4-6-14(9-13)10-16(12-25-26(22,23)24)20-19(21)18-11-15-7-2-3-8-17(15)27-18/h2-9,11,16H,10,12H2,1H3,(H,20,21)(H2,22,23,24)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 57 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056216

(CHEMBL3353368)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)CCc1c(Cl)cccc1Cl |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056208

(CHEMBL3322220)Show SMILES OC(=O)[C@@H](Cc1nc2cc(F)ccc2[nH]1)NC(=O)c1cc2ccccc2s1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34015
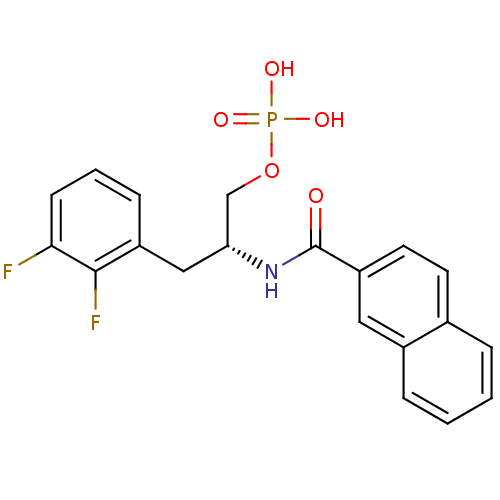
(2,3-difluorophenylalanine derivative, 23a)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1F)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H18F2NO5P/c21-18-7-3-6-15(19(18)22)11-17(12-28-29(25,26)27)23-20(24)16-9-8-13-4-1-2-5-14(13)10-16/h1-10,17H,11-12H2,(H,23,24)(H2,25,26,27)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056214
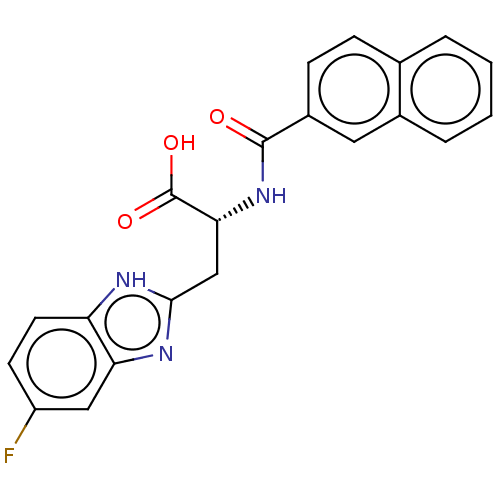
(CHEMBL3322219)Show SMILES OC(=O)[C@@H](Cc1nc2cc(F)ccc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34016
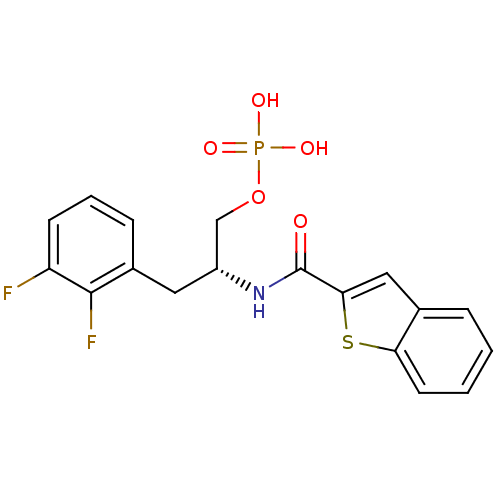
(2,3-difluorophenylalanine derivative, 23b)Show SMILES OP(O)(=O)OC[C@@H](Cc1cccc(F)c1F)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H16F2NO5PS/c19-14-6-3-5-12(17(14)20)8-13(10-26-27(23,24)25)21-18(22)16-9-11-4-1-2-7-15(11)28-16/h1-7,9,13H,8,10H2,(H,21,22)(H2,23,24,25)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34009
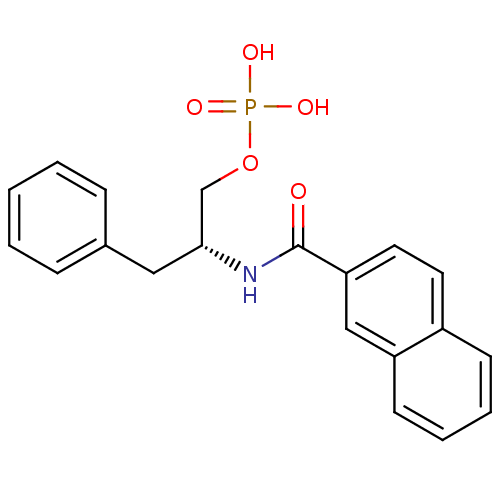
(naphthalene carboxamide, 18a)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C20H20NO5P/c22-20(18-11-10-16-8-4-5-9-17(16)13-18)21-19(14-26-27(23,24)25)12-15-6-2-1-3-7-15/h1-11,13,19H,12,14H2,(H,21,22)(H2,23,24,25)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 100 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056211
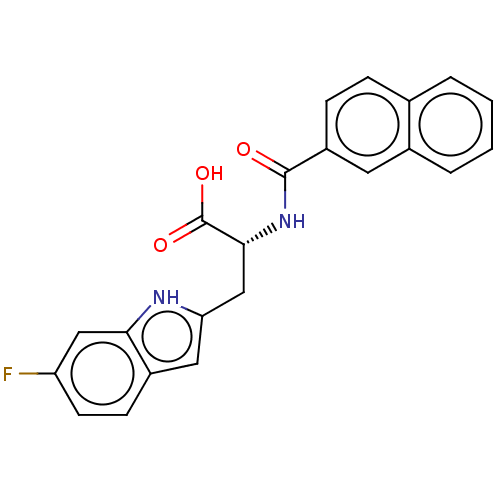
(CHEMBL3322225)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056204
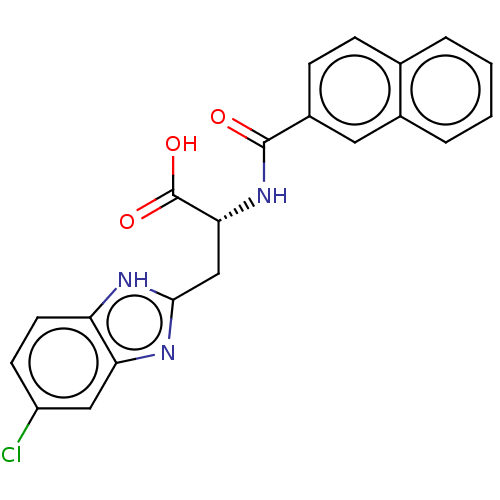
(CHEMBL3322215)Show SMILES OC(=O)[C@@H](Cc1nc2cc(Cl)ccc2[nH]1)NC(=O)c1ccc2ccccc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314715

((R)-N-(1-hydroxy-3-phenylpropan-2-yl)benzo[b]thiop...)Show InChI InChI=1S/C18H17NO2S/c20-12-15(10-13-6-2-1-3-7-13)19-18(21)17-11-14-8-4-5-9-16(14)22-17/h1-9,11,15,20H,10,12H2,(H,19,21)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 179 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34010

(benzothiophene carboxamide, 18b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1cc2ccccc2s1 |r| Show InChI InChI=1S/C18H18NO5PS/c20-18(17-11-14-8-4-5-9-16(14)26-17)19-15(12-24-25(21,22)23)10-13-6-2-1-3-7-13/h1-9,11,15H,10,12H2,(H,19,20)(H2,21,22,23)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 179 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184830
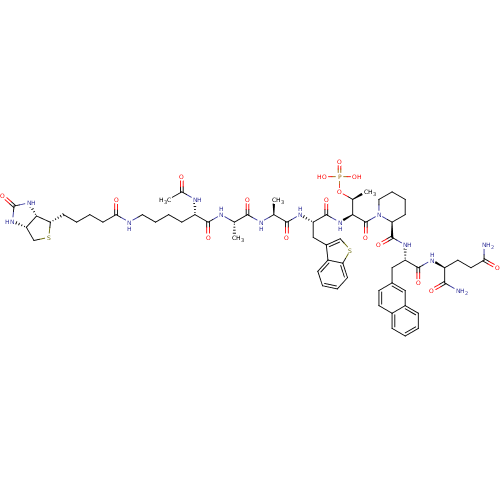
(Ac-Lys(N-epsilon-biotinoyl)-Ala-Ala-Bth-Thr(PO3H2)...)Show SMILES C[C@H](OP(O)(O)=O)[C@H](NC(=O)[C@H](Cc1csc2ccccc12)NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](CCCCNC(=O)CCCC[C@@H]1SC[C@@H]2NC(=O)N[C@H]12)NC(C)=O)C(=O)N1CCCC[C@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C63H86N13O16PS2/c1-34(68-58(83)44(69-37(4)77)18-11-13-27-66-52(79)22-10-9-21-50-54-47(33-95-50)73-63(88)75-54)56(81)67-35(2)57(82)71-46(31-41-32-94-49-20-8-7-17-42(41)49)60(85)74-53(36(3)92-93(89,90)91)62(87)76-28-14-12-19-48(76)61(86)72-45(59(84)70-43(55(65)80)25-26-51(64)78)30-38-23-24-39-15-5-6-16-40(39)29-38/h5-8,15-17,20,23-24,29,32,34-36,43-48,50,53-54H,9-14,18-19,21-22,25-28,30-31,33H2,1-4H3,(H2,64,78)(H2,65,80)(H,66,79)(H,67,81)(H,68,83)(H,69,77)(H,70,84)(H,71,82)(H,72,86)(H,74,85)(H2,73,75,88)(H2,89,90,91)/t34-,35-,36-,43-,44-,45-,46-,47-,48-,50-,53-,54-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056205
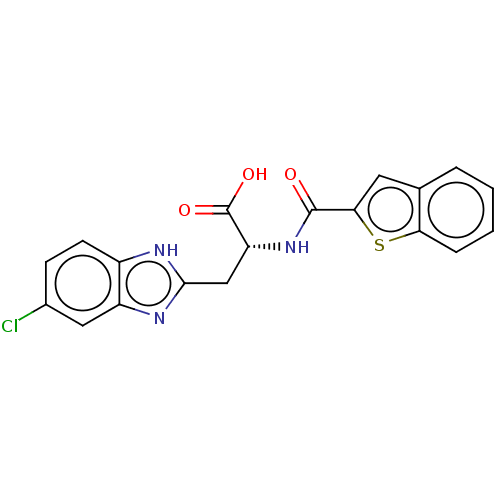
(CHEMBL3322216)Show SMILES OC(=O)[C@@H](Cc1nc2cc(Cl)ccc2[nH]1)NC(=O)c1cc2ccccc2s1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056219

(CHEMBL3322230)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(CCc2c(Cl)cccc2Cl)=N1 |c:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 196 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50381013

(CHEMBL2017129)Show SMILES Oc1c(cc(cc1[N+]([O-])=O)-c1cc(F)cc(c1O)[N+]([O-])=O)C(=O)C1CCc2ccccc12 Show InChI InChI=1S/C22H15FN2O7/c23-13-9-16(21(27)19(10-13)25(31)32)12-7-17(22(28)18(8-12)24(29)30)20(26)15-6-5-11-3-1-2-4-14(11)15/h1-4,7-10,15,27-28H,5-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences& Peking Union Medical College
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 |
Bioorg Med Chem 20: 2992-9 (2012)
Article DOI: 10.1016/j.bmc.2012.03.005
BindingDB Entry DOI: 10.7270/Q2CC11Q1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50345548

(CHEMBL1784538 | [13C,15N-Y,P,V]cyclic CRYPEVEIC | ...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] |r| Show InChI InChI=1S/C47H72N12O15S2/c1-5-24(4)37-44(71)56-32(46(73)74)22-76-75-21-27(48)38(65)52-28(8-6-18-51-47(49)50)39(66)55-31(20-25-10-12-26(60)13-11-25)45(72)59-19-7-9-33(59)42(69)53-29(14-16-34(61)62)40(67)57-36(23(2)3)43(70)54-30(41(68)58-37)15-17-35(63)64/h10-13,23-24,27-33,36-37,60H,5-9,14-22,48H2,1-4H3,(H,52,65)(H,53,69)(H,54,70)(H,55,66)(H,56,71)(H,57,67)(H,58,68)(H,61,62)(H,63,64)(H,73,74)(H4,49,50,51)/t24-,27-,28-,29-,30-,31-,32-,33-,36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Western Ontario
Curated by ChEMBL
| Assay Description
Competitive inhibition of human GST-tagged-Pin1 PPIase activity using WFYpSPR-pNA as substrate by Michaelis-Menton equation |
J Med Chem 54: 3854-65 (2011)
Article DOI: 10.1021/jm200156c
BindingDB Entry DOI: 10.7270/Q2DN45DV |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184834

(Ac-Bth-Thr(PO3H2)-Pip-Nal-Gln-NH2 | CHEMBL380685)Show SMILES C[C@@H](OP(O)(O)=O)[C@H](NC(=O)[C@H](Cc1csc2ccccc12)NC(C)=O)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C41H50N7O11PS/c1-23(59-60(56,57)58)36(47-39(53)32(44-24(2)49)21-28-22-61-34-13-6-5-11-29(28)34)41(55)48-18-8-7-12-33(48)40(54)46-31(38(52)45-30(37(43)51)16-17-35(42)50)20-25-14-15-26-9-3-4-10-27(26)19-25/h3-6,9-11,13-15,19,22-23,30-33,36H,7-8,12,16-18,20-21H2,1-2H3,(H2,42,50)(H2,43,51)(H,44,49)(H,45,52)(H,46,54)(H,47,53)(H2,56,57,58)/t23-,30+,31+,32+,33-,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056220
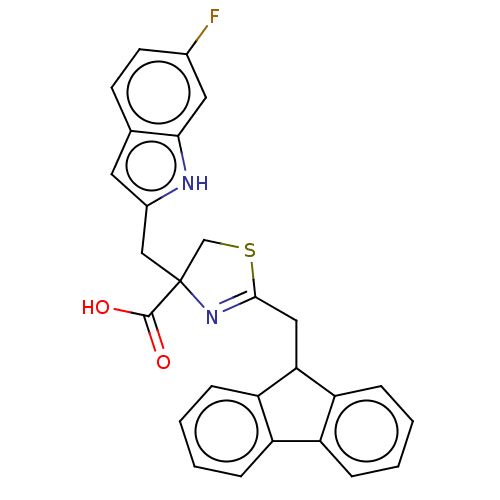
(CHEMBL3322295)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(CC2c3ccccc3-c3ccccc23)=N1 |c:36| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056215

(CHEMBL3353367)Show SMILES OC(=O)[C@@H](Cc1cc2ccc(F)cc2[nH]1)NC(=O)Cc1cccc2ccccc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056207

(CHEMBL3322218)Show SMILES Cc1ccc2[nH]c(C[C@@H](NC(=O)c3cc4ccccc4s3)C(O)=O)nc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50345548

(CHEMBL1784538 | [13C,15N-Y,P,V]cyclic CRYPEVEIC | ...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@@H]-1-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#16]-[#16]-[#6]-[#6@H](-[#7]-[#6]-1=O)-[#6](-[#8])=O)-[#6](-[#6])-[#6] |r| Show InChI InChI=1S/C47H72N12O15S2/c1-5-24(4)37-44(71)56-32(46(73)74)22-76-75-21-27(48)38(65)52-28(8-6-18-51-47(49)50)39(66)55-31(20-25-10-12-26(60)13-11-25)45(72)59-19-7-9-33(59)42(69)53-29(14-16-34(61)62)40(67)57-36(23(2)3)43(70)54-30(41(68)58-37)15-17-35(63)64/h10-13,23-24,27-33,36-37,60H,5-9,14-22,48H2,1-4H3,(H,52,65)(H,53,69)(H,54,70)(H,55,66)(H,56,71)(H,57,67)(H,58,68)(H,61,62)(H,63,64)(H,73,74)(H4,49,50,51)/t24-,27-,28-,29-,30-,31-,32-,33-,36-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Western Ontario
Curated by ChEMBL
| Assay Description
Inhibition of human GST-tagged-Pin1 PPIase activity using Suc-AEPF-pNA as substrate by Michaelis-Menton equation |
J Med Chem 54: 3854-65 (2011)
Article DOI: 10.1021/jm200156c
BindingDB Entry DOI: 10.7270/Q2DN45DV |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34008

(amide, 17c)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C16H18NO5P/c18-16(14-9-5-2-6-10-14)17-15(12-22-23(19,20)21)11-13-7-3-1-4-8-13/h1-10,15H,11-12H2,(H,17,18)(H2,19,20,21)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 525 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50184838
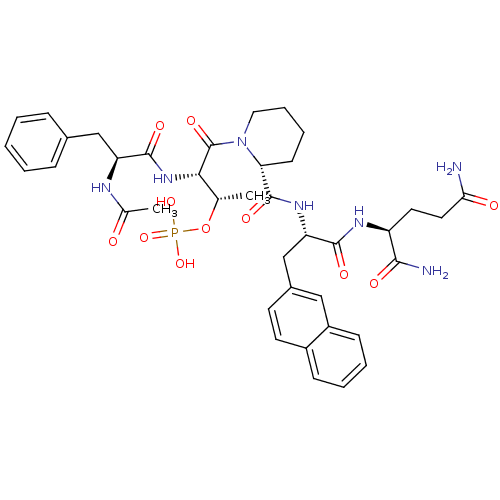
(Ac-Phe-Thr(PO3H2)-Pip-Nal-Gln-NH2 | CHEMBL383244)Show SMILES C[C@H](OP(O)(O)=O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(C)=O)C(=O)N1CCCC[C@@H]1C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(=O)N[C@@H](CCC(N)=O)C(N)=O Show InChI InChI=1S/C39H50N7O11P/c1-23(57-58(54,55)56)34(45-37(51)30(42-24(2)47)21-25-10-4-3-5-11-25)39(53)46-19-9-8-14-32(46)38(52)44-31(36(50)43-29(35(41)49)17-18-33(40)48)22-26-15-16-27-12-6-7-13-28(27)20-26/h3-7,10-13,15-16,20,23,29-32,34H,8-9,14,17-19,21-22H2,1-2H3,(H2,40,48)(H2,41,49)(H,42,47)(H,43,50)(H,44,52)(H,45,51)(H2,54,55,56)/t23-,29-,30-,31-,32+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 547 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max Planck Research Unit for Enzymology of Protein Folding
Curated by ChEMBL
| Assay Description
Inhibition of human Pin1 PPIase Activity by protease free PPIase assay |
J Med Chem 49: 2147-50 (2006)
Article DOI: 10.1021/jm060036n
BindingDB Entry DOI: 10.7270/Q2S46RJP |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056203

(CHEMBL3322296)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(\C=C\c2c(Cl)cccc2Cl)=N1 |c:30| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50518736

(CHEMBL4526940)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)\N=C1/C=C(SCC(O)=O)C(=O)c2ccccc12 |t:16| Show InChI InChI=1S/C22H21NO5S2/c1-22(2,3)14-8-10-15(11-9-14)30(27,28)23-18-12-19(29-13-20(24)25)21(26)17-7-5-4-6-16(17)18/h4-12H,13H2,1-3H3,(H,24,25)/b23-18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya Citi University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Pin1 (unknown origin) using Suc-AEPF-MCA as substrate preincubated followed by substrate addition and measured after 60 to ... |
Bioorg Med Chem Lett 29: 353-356 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.044
BindingDB Entry DOI: 10.7270/Q27D2ZH0 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056206

(CHEMBL3322217)Show SMILES Cc1ccc2[nH]c(C[C@@H](NC(=O)c3ccc4ccccc4c3)C(O)=O)nc2c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34005
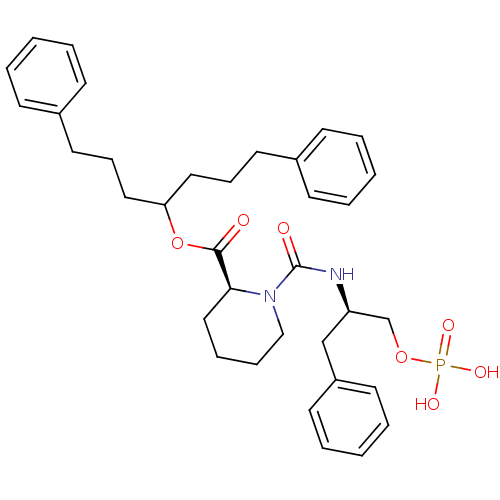
(pipecolate deriv., 12b)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)N1CCCC[C@H]1C(=O)OC(CCCc1ccccc1)CCCc1ccccc1 |r| Show InChI InChI=1S/C35H45N2O7P/c38-34(44-32(22-12-20-28-14-4-1-5-15-28)23-13-21-29-16-6-2-7-17-29)33-24-10-11-25-37(33)35(39)36-31(27-43-45(40,41)42)26-30-18-8-3-9-19-30/h1-9,14-19,31-33H,10-13,20-27H2,(H,36,39)(H2,40,41,42)/t31-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 800 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-tagged Pin1 (unknown origin) using Suc-Ala-pSer-Pro-Phe-pNA, Suc-Ala-Glu-Pro-Phe-pNA or Suc-Ala-Ala-Pro-Phe-pNA as substrate preinc... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115878
BindingDB Entry DOI: 10.7270/Q21J9FDM |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314706

((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-eno...)Show SMILES OC(=O)[C@@H](C\C=C\c1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H18FNO3/c23-19-9-3-5-15(13-19)6-4-10-20(22(26)27)24-21(25)18-12-11-16-7-1-2-8-17(16)14-18/h1-9,11-14,20H,10H2,(H,24,25)(H,26,27)/b6-4+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056210
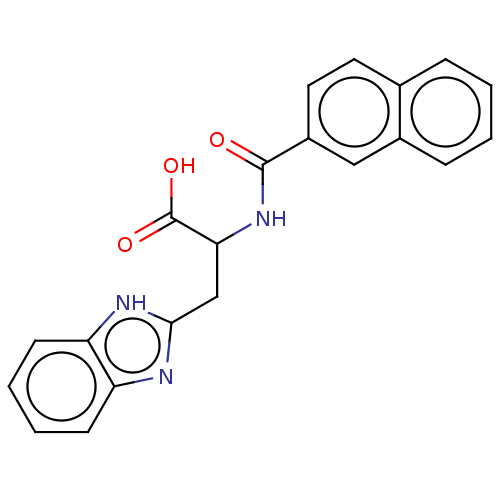
(CHEMBL3322222)Show SMILES OC(=O)C(Cc1nc2ccccc2[nH]1)NC(=O)c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056218

(CHEMBL3322229)Show SMILES OC(=O)C1(Cc2cc3ccc(F)cc3[nH]2)CSC(=N1)c1ccc2ccccc2c1 |c:19| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
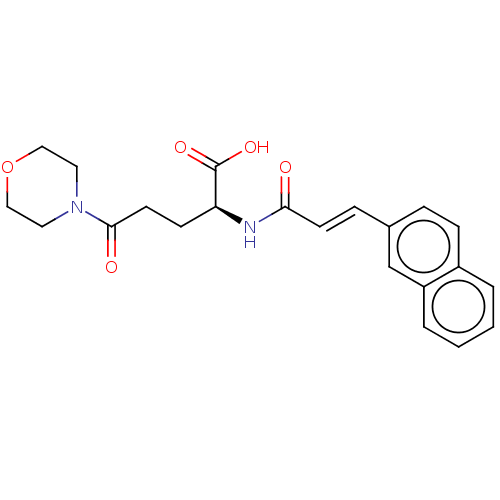
(Homo sapiens (Human)) | BDBM50518734
(CHEMBL4467081)Show SMILES OC(=O)[C@H](CCC(=O)N1CCOCC1)NC(=O)\C=C\c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H24N2O5/c25-20(9-6-16-5-7-17-3-1-2-4-18(17)15-16)23-19(22(27)28)8-10-21(26)24-11-13-29-14-12-24/h1-7,9,15,19H,8,10-14H2,(H,23,25)(H,27,28)/b9-6+/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nagoya Citi University
Curated by ChEMBL
| Assay Description
Irreversible inhibition of Pin1 (unknown origin) using suc-Ala-Glu-Pro-Phe-pNA as substrate preincubated for 10 mins followed by substrate addition a... |
Bioorg Med Chem Lett 29: 353-356 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.044
BindingDB Entry DOI: 10.7270/Q27D2ZH0 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM34003

(pipecolate deriv., 11)Show SMILES OP(O)(=O)OC[C@@H](Cc1ccccc1)NC(=O)N1CCCC[C@H]1C(=O)OCCCCc1ccccc1 |r| Show InChI InChI=1S/C26H35N2O7P/c29-25(34-18-10-8-13-21-11-3-1-4-12-21)24-16-7-9-17-28(24)26(30)27-23(20-35-36(31,32)33)19-22-14-5-2-6-15-22/h1-6,11-12,14-15,23-24H,7-10,13,16-20H2,(H,27,30)(H2,31,32,33)/t23-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 15 |
Pfizer
| Assay Description
The cis/trans conversion of cis-Suc-AEPFpNA peptide by the PPIase led to the cleavage of para nitroaniline by subtilisin which was monitored at 390 n... |
Bioorg Med Chem Lett 19: 5613-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.034
BindingDB Entry DOI: 10.7270/Q2XD100H |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
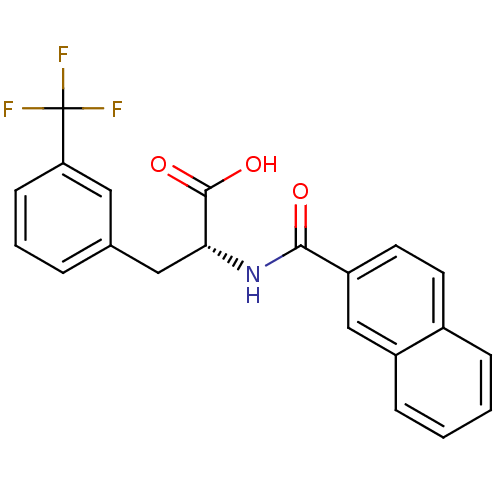
(Homo sapiens (Human)) | BDBM50314682
((R)-2-(2-naphthamido)-3-(3-(trifluoromethyl)phenyl...)Show SMILES OC(=O)[C@@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H16F3NO3/c22-21(23,24)17-7-3-4-13(10-17)11-18(20(27)28)25-19(26)16-9-8-14-5-1-2-6-15(14)12-16/h1-10,12,18H,11H2,(H,25,26)(H,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
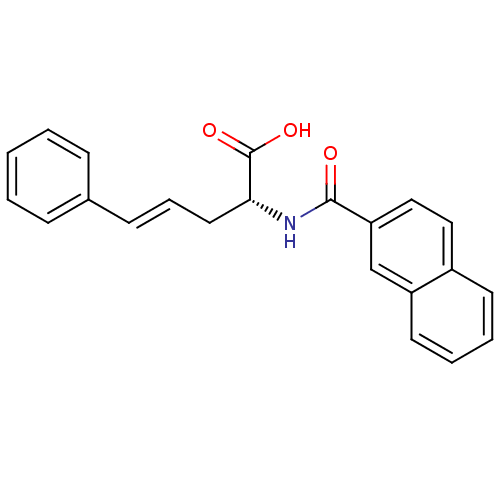
(Homo sapiens (Human)) | BDBM50314705
((2R,4E)-2-[(naphthalen-2-ylcarbonyl)amino]-5-pheny...)Show SMILES OC(=O)[C@@H](C\C=C\c1ccccc1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H19NO3/c24-21(19-14-13-17-10-4-5-11-18(17)15-19)23-20(22(25)26)12-6-9-16-7-2-1-3-8-16/h1-11,13-15,20H,12H2,(H,23,24)(H,25,26)/b9-6+/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50314701
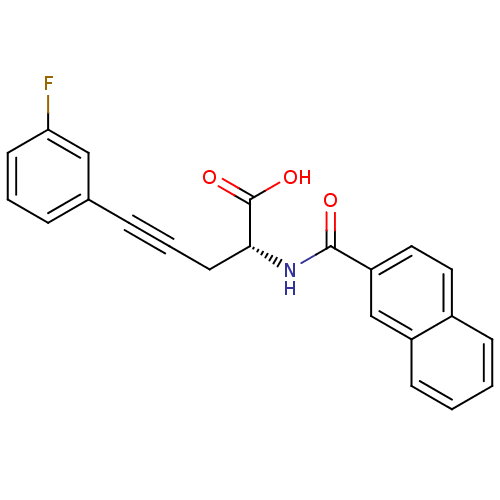
((R)-2-(2-naphthamido)-5-(3-fluorophenyl)pent-4-yno...)Show SMILES OC(=O)[C@@H](CC#Cc1cccc(F)c1)NC(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H16FNO3/c23-19-9-3-5-15(13-19)6-4-10-20(22(26)27)24-21(25)18-12-11-16-7-1-2-8-17(16)14-18/h1-3,5,7-9,11-14,20H,10H2,(H,24,25)(H,26,27)/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of WFYpSPFLE from human Pin1 catalytic domain after 10-20 mins by fluorescence polarization assay |
Bioorg Med Chem Lett 20: 2210-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.033
BindingDB Entry DOI: 10.7270/Q2GX4BPW |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1
(Homo sapiens (Human)) | BDBM50056212

(CHEMBL3322224)Show SMILES OC(=O)C(Cc1cc2ccccc2[nH]1)NC(=O)c1ccc2ccccc2c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Pin1 (unknown origin) |
Bioorg Med Chem Lett 24: 4187-91 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.044
BindingDB Entry DOI: 10.7270/Q2QJ7JX1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data