 Found 1188 hits Enz. Inhib. hit(s) with Target = 'Prostaglandin E2 receptor EP1 subtype'
Found 1188 hits Enz. Inhib. hit(s) with Target = 'Prostaglandin E2 receptor EP1 subtype' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917
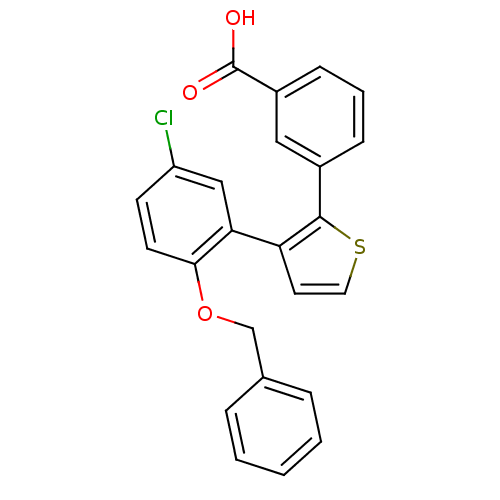
(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR |
Bioorg Med Chem Lett 16: 2666-71 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.014
BindingDB Entry DOI: 10.7270/Q2J102RM |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50419411

(CHEMBL1915012)Show InChI InChI=1S/C17H17ClN2O3/c1-9(2)15-7-11-5-13(18)6-12(16(11)23-15)8-20-10(3)4-14(19-20)17(21)22/h4-7,9H,8H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO-K1 cells assessed as inhibition of PGE2-mediated intracellular calcium mobiliz... |
Bioorg Med Chem Lett 21: 4343-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.047
BindingDB Entry DOI: 10.7270/Q2RN394N |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50376788
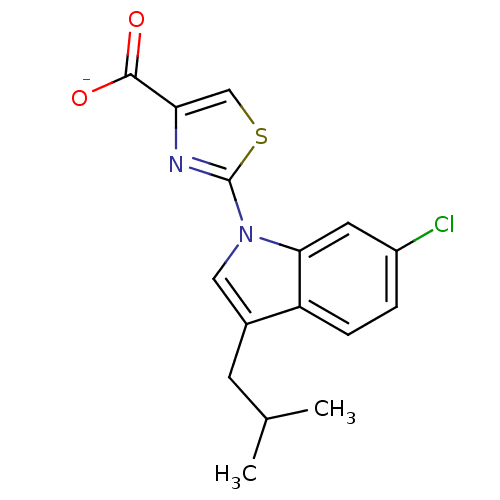
(CHEMBL257997)Show SMILES CC(C)Cc1cn(-c2nc(cs2)C([O-])=O)c2cc(Cl)ccc12 Show InChI InChI=1S/C16H15ClN2O2S/c1-9(2)5-10-7-19(14-6-11(17)3-4-12(10)14)16-18-13(8-22-16)15(20)21/h3-4,6-9H,5H2,1-2H3,(H,20,21)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human EP1 receptor expressed in CHOK1 cells assessed as inhibition of PGE2-induced intracellular calcium mobilization by ... |
Bioorg Med Chem Lett 18: 2684-90 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.018
BindingDB Entry DOI: 10.7270/Q2PC338K |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85603
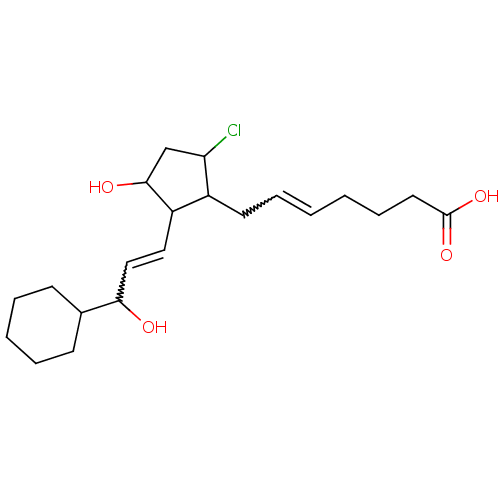
(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259548

(6-(5'-chloro-2'-isobutoxy-biphenyl-2-yl)-pyridine-...)Show SMILES CC(C)COc1ccc(Cl)cc1-c1ccccc1-c1cccc(n1)C([O-])=O Show InChI InChI=1S/C22H20ClNO3/c1-14(2)13-27-21-11-10-15(23)12-18(21)16-6-3-4-7-17(16)19-8-5-9-20(24-19)22(25)26/h3-12,14H,13H2,1-2H3,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from EP1 receptor |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160912
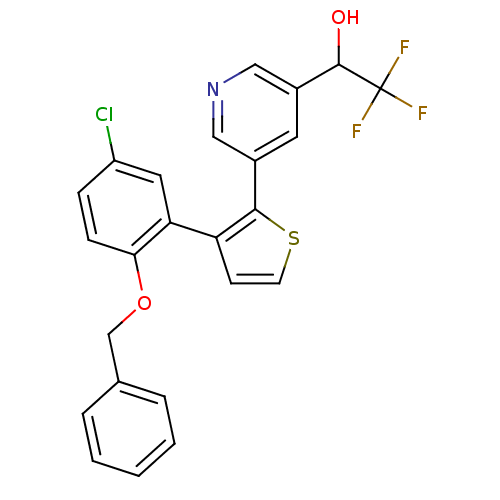
(1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...)Show SMILES OC(c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C24H17ClF3NO2S/c25-18-6-7-21(31-14-15-4-2-1-3-5-15)20(11-18)19-8-9-32-22(19)16-10-17(13-29-12-16)23(30)24(26,27)28/h1-13,23,30H,14H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160907
(5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...)Show SMILES OC(=O)c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C23H16ClNO3S/c24-18-6-7-21(28-14-15-4-2-1-3-5-15)20(11-18)19-8-9-29-22(19)16-10-17(23(26)27)13-25-12-16/h1-13H,14H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
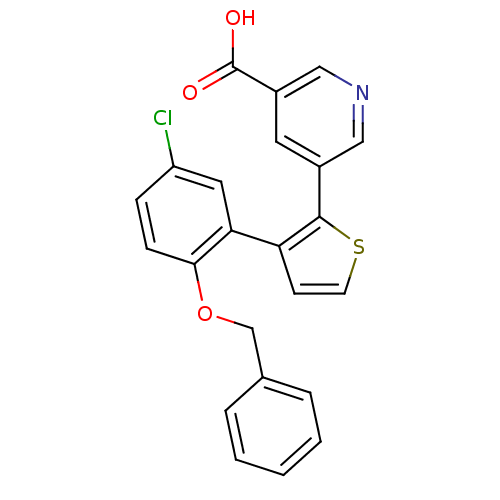
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160917

(3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...)Show SMILES OC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClO3S/c25-19-9-10-22(28-15-16-5-2-1-3-6-16)21(14-19)20-11-12-29-23(20)17-7-4-8-18(13-17)24(26)27/h1-14H,15H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160915
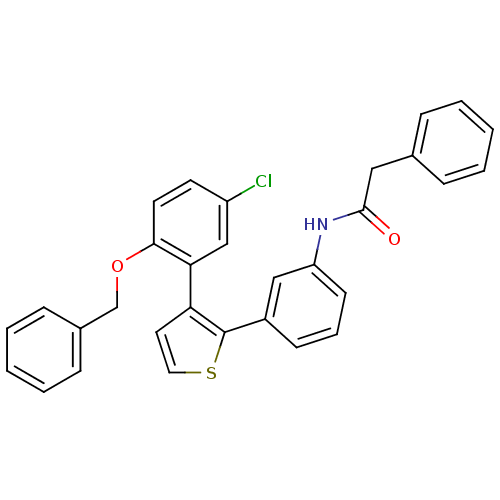
(CHEMBL180046 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...)Show SMILES Clc1ccc(OCc2ccccc2)c(c1)-c1ccsc1-c1cccc(NC(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C31H24ClNO2S/c32-25-14-15-29(35-21-23-10-5-2-6-11-23)28(20-25)27-16-17-36-31(27)24-12-7-13-26(19-24)33-30(34)18-22-8-3-1-4-9-22/h1-17,19-20H,18,21H2,(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160909
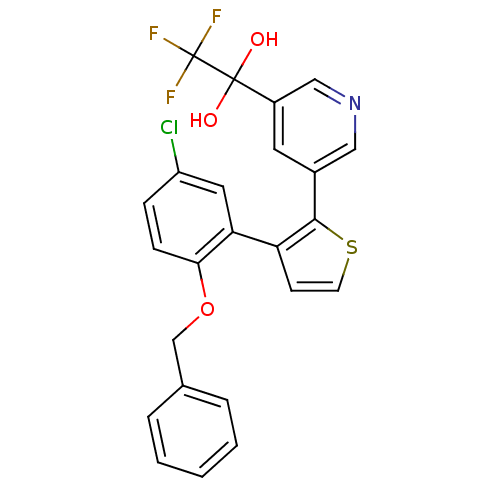
(1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...)Show SMILES OC(O)(c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C24H17ClF3NO3S/c25-18-6-7-21(32-14-15-4-2-1-3-5-15)20(11-18)19-8-9-33-22(19)16-10-17(13-29-12-16)23(30,31)24(26,27)28/h1-13,30-31H,14H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50142480

((E)-7-[(1R,2S,3R)-3-Hydroxy-2-((E)-(S)-3-hydroxy-o...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)N1C\C=C\CCCC(O)=O Show InChI InChI=1S/C19H31NO5/c1-2-3-6-9-15(21)11-12-16-17(22)14-18(23)20(16)13-8-5-4-7-10-19(24)25/h5,8,11-12,15-17,21-22H,2-4,6-7,9-10,13-14H2,1H3,(H,24,25)/b8-5+,12-11+/t15-,16-,17+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto
Curated by ChEMBL
| Assay Description
Binding affinity was determined against prostanoid EP1 receptor |
Bioorg Med Chem Lett 14: 1655-9 (2004)
Article DOI: 10.1016/j.bmcl.2004.01.063
BindingDB Entry DOI: 10.7270/Q2MK6CBC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160909

(1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...)Show SMILES OC(O)(c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C24H17ClF3NO3S/c25-18-6-7-21(32-14-15-4-2-1-3-5-15)20(11-18)19-8-9-33-22(19)16-10-17(13-29-12-16)23(30,31)24(26,27)28/h1-13,30-31H,14H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160915

(CHEMBL180046 | N-{3-[3-(2-Benzyloxy-5-chloro-pheny...)Show SMILES Clc1ccc(OCc2ccccc2)c(c1)-c1ccsc1-c1cccc(NC(=O)Cc2ccccc2)c1 Show InChI InChI=1S/C31H24ClNO2S/c32-25-14-15-29(35-21-23-10-5-2-6-11-23)28(20-25)27-16-17-36-31(27)24-12-7-13-26(19-24)33-30(34)18-22-8-3-1-4-9-22/h1-17,19-20H,18,21H2,(H,33,34) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160913
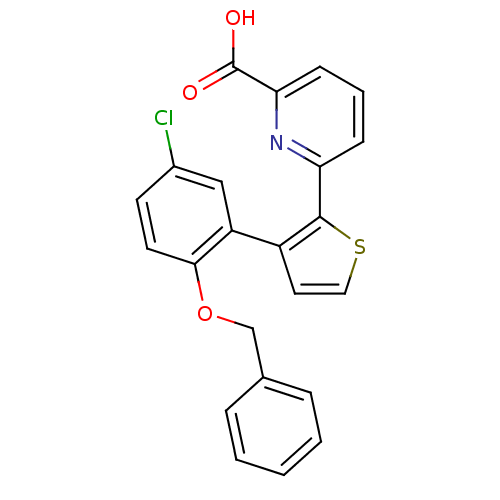
(6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...)Show SMILES OC(=O)c1cccc(n1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C23H16ClNO3S/c24-16-9-10-21(28-14-15-5-2-1-3-6-15)18(13-16)17-11-12-29-22(17)19-7-4-8-20(25-19)23(26)27/h1-13H,14H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP1 receptor expressed in CHO cells after 20 mins by liquid scintillation counting |
Bioorg Med Chem Lett 22: 396-401 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.109
BindingDB Entry DOI: 10.7270/Q27P8ZT2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from mouse EP1 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem 20: 2235-51 (2012)
Article DOI: 10.1016/j.bmc.2012.02.018
BindingDB Entry DOI: 10.7270/Q2542P2G |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160912

(1-{5-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-y...)Show SMILES OC(c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)C(F)(F)F Show InChI InChI=1S/C24H17ClF3NO2S/c25-18-6-7-21(31-14-15-4-2-1-3-5-15)20(11-18)19-8-9-32-22(19)16-10-17(13-29-12-16)23(30)24(26,27)28/h1-13,23,30H,14H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259611
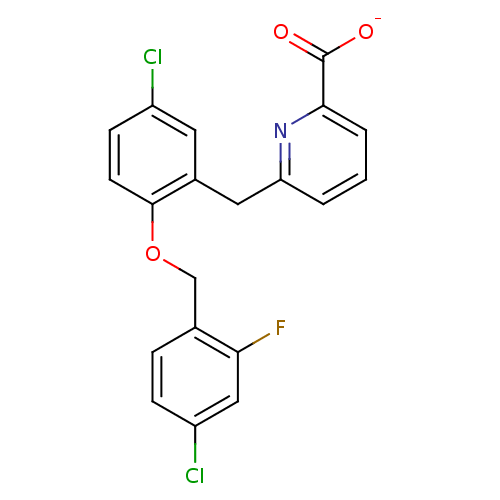
(CHEMBL467114 | sodium 6-(5-chloro-2-(4-chloro-2-fl...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2F)n1 Show InChI InChI=1S/C20H14Cl2FNO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50410087
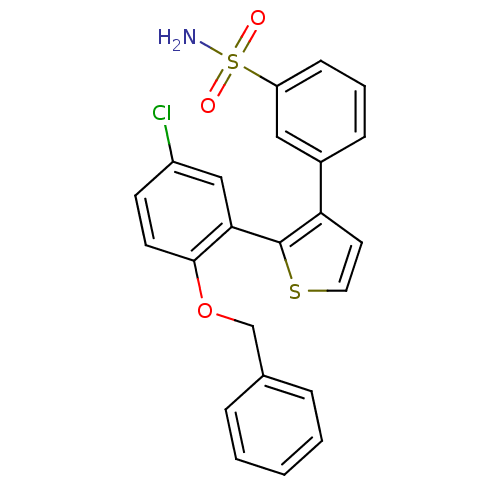
(CHEMBL2113029)Show SMILES NS(=O)(=O)c1cccc(c1)-c1ccsc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C23H18ClNO3S2/c24-18-9-10-22(28-15-16-5-2-1-3-6-16)21(14-18)23-20(11-12-29-23)17-7-4-8-19(13-17)30(25,26)27/h1-14H,15H2,(H2,25,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262
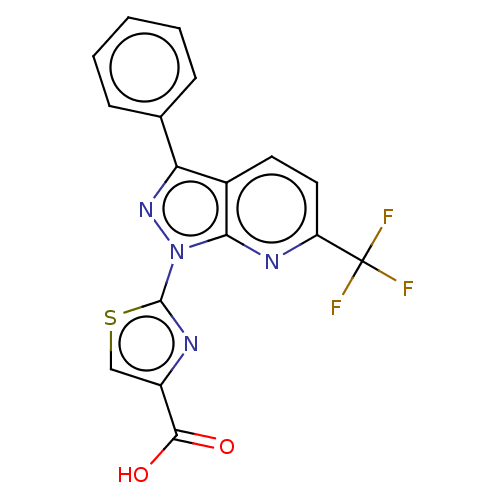
(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160914
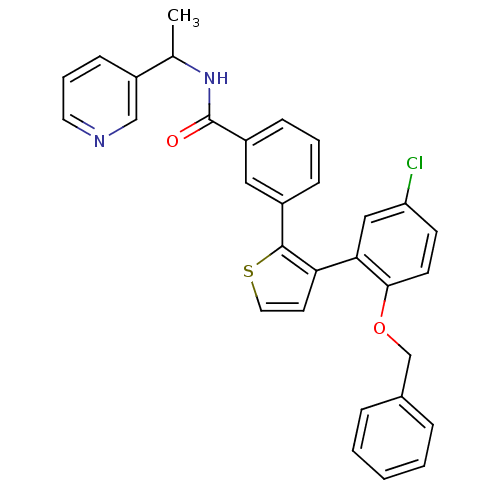
(3-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...)Show SMILES CC(NC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)c1cccnc1 Show InChI InChI=1S/C31H25ClN2O2S/c1-21(25-11-6-15-33-19-25)34-31(35)24-10-5-9-23(17-24)30-27(14-16-37-30)28-18-26(32)12-13-29(28)36-20-22-7-3-2-4-8-22/h2-19,21H,20H2,1H3,(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081444
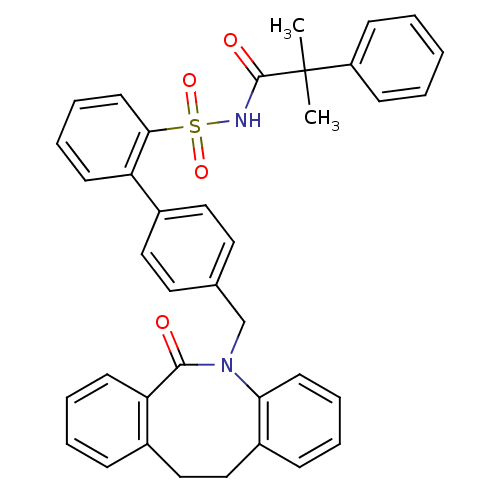
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C)(C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C38H34N2O4S/c1-38(2,31-14-4-3-5-15-31)37(42)39-45(43,44)35-19-11-9-16-32(35)29-22-20-27(21-23-29)26-40-34-18-10-7-13-30(34)25-24-28-12-6-8-17-33(28)36(40)41/h3-23H,24-26H2,1-2H3,(H,39,42) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101848
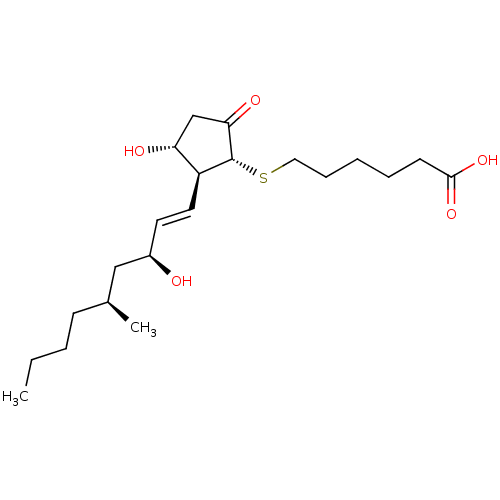
(6-[(1R,2S,3R)-3-Hydroxy-2-((E)-(3S,5S)-3-hydroxy-5...)Show SMILES CCCC[C@H](C)C[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1SCCCCCC(O)=O Show InChI InChI=1S/C21H36O5S/c1-3-4-8-15(2)13-16(22)10-11-17-18(23)14-19(24)21(17)27-12-7-5-6-9-20(25)26/h10-11,15-18,21-23H,3-9,12-14H2,1-2H3,(H,25,26)/b11-10+/t15-,16+,17-,18+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Evaluated for its competitive binding affinity towards mouse Prostanoid EP1 receptor in CHO cells expressing prostanoid receptor |
Bioorg Med Chem Lett 11: 2029-31 (2001)
BindingDB Entry DOI: 10.7270/Q2833R9D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081440

(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CO[C@](C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)(c1ccccc1)C(F)(F)F Show InChI InChI=1S/C38H31F3N2O5S/c1-48-37(38(39,40)41,30-13-3-2-4-14-30)36(45)42-49(46,47)34-18-10-8-15-31(34)28-21-19-26(20-22-28)25-43-33-17-9-6-12-29(33)24-23-27-11-5-7-16-32(27)35(43)44/h2-22H,23-25H2,1H3,(H,42,45)/t37-/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Rattus norvegicus (Rat)) | BDBM50160913

(6-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...)Show SMILES OC(=O)c1cccc(n1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C23H16ClNO3S/c24-16-9-10-21(28-14-15-5-2-1-3-6-15)18(13-16)17-11-12-29-22(17)19-7-4-8-20(25-19)23(26)27/h1-13H,14H2,(H,26,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity towards Prostaglandin E receptor was determined in rat |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85599
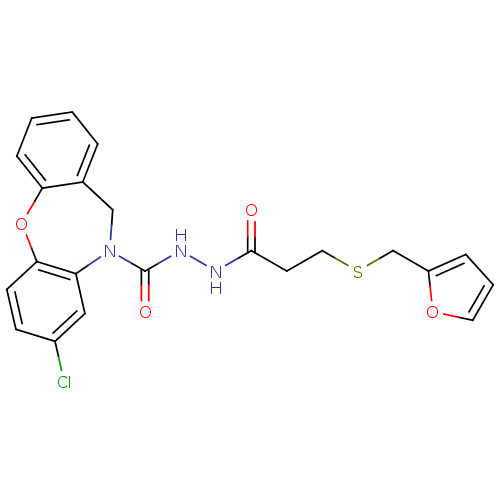
(CAS_146032-79-3 | SC-51322)Show SMILES Clc1ccc2Oc3ccccc3CN(C(=O)NNC(=O)CCSCc3ccco3)c2c1 Show InChI InChI=1S/C22H20ClN3O4S/c23-16-7-8-20-18(12-16)26(13-15-4-1-2-6-19(15)30-20)22(28)25-24-21(27)9-11-31-14-17-5-3-10-29-17/h1-8,10,12H,9,11,13-14H2,(H,24,27)(H,25,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM82094
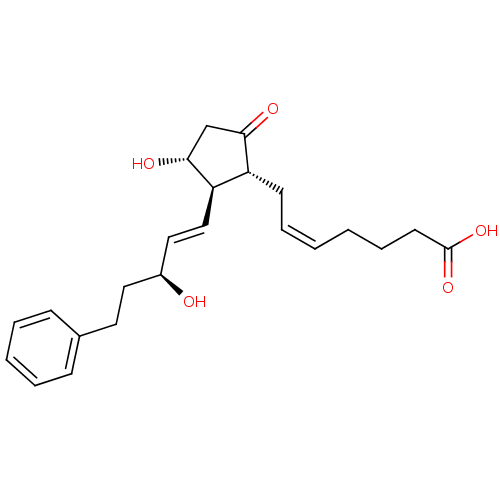
(17-PHENYL TRINOR PROSTAGLANDIN E2 | CAS_38315-43-4...)Show SMILES O[C@@H](CCc1ccccc1)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O |r| Show InChI InChI=1S/C23H30O5/c24-18(13-12-17-8-4-3-5-9-17)14-15-20-19(21(25)16-22(20)26)10-6-1-2-7-11-23(27)28/h1,3-6,8-9,14-15,18-20,22,24,26H,2,7,10-13,16H2,(H,27,28)/b6-1-,15-14+/t18-,19+,20+,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160910
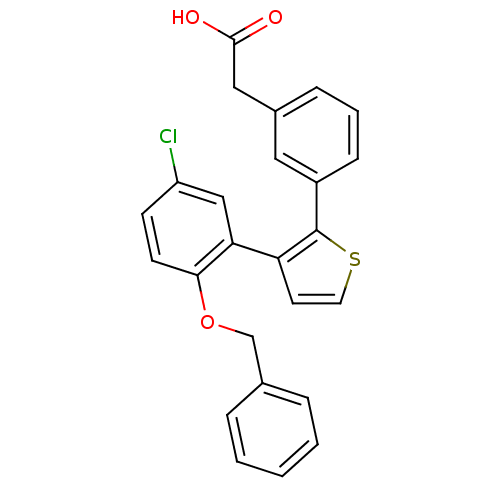
(CHEMBL184779 | {3-[3-(2-Benzyloxy-5-chloro-phenyl)...)Show SMILES OC(=O)Cc1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C25H19ClO3S/c26-20-9-10-23(29-16-17-5-2-1-3-6-17)22(15-20)21-11-12-30-25(21)19-8-4-7-18(13-19)14-24(27)28/h1-13,15H,14,16H2,(H,27,28) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
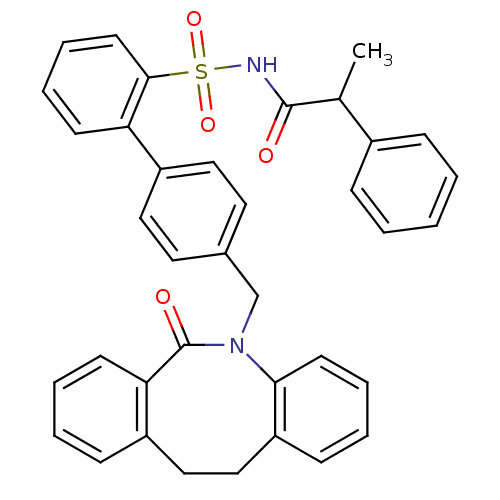
(Homo sapiens (Human)) | BDBM50081453
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1)c1ccccc1 Show InChI InChI=1S/C37H32N2O4S/c1-26(28-11-3-2-4-12-28)36(40)38-44(42,43)35-18-10-8-15-32(35)30-21-19-27(20-22-30)25-39-34-17-9-6-14-31(34)24-23-29-13-5-7-16-33(29)37(39)41/h2-22,26H,23-25H2,1H3,(H,38,40) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
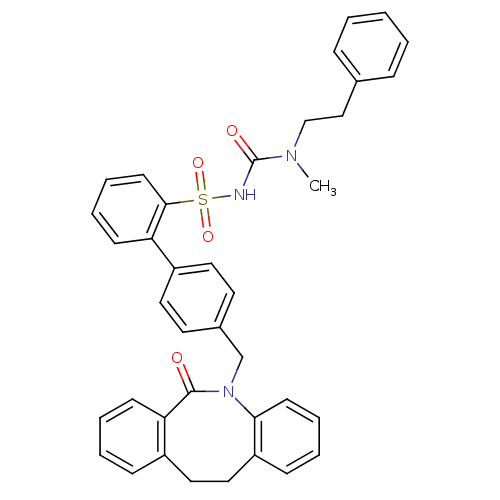
(Homo sapiens (Human)) | BDBM50081439
(CHEMBL92539 | sulfonylurea analogue)Show SMILES CN(CCc1ccccc1)C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C38H35N3O4S/c1-40(26-25-28-11-3-2-4-12-28)38(43)39-46(44,45)36-18-10-8-15-33(36)31-21-19-29(20-22-31)27-41-35-17-9-6-14-32(35)24-23-30-13-5-7-16-34(30)37(41)42/h2-22H,23-27H2,1H3,(H,39,43) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160914

(3-[3-(2-Benzyloxy-5-chloro-phenyl)-thiophen-2-yl]-...)Show SMILES CC(NC(=O)c1cccc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1)c1cccnc1 Show InChI InChI=1S/C31H25ClN2O2S/c1-21(25-11-6-15-33-19-25)34-31(35)24-10-5-9-23(17-24)30-27(14-16-37-30)28-18-26(32)12-13-29(28)36-20-22-7-3-2-4-8-22/h2-19,21H,20H2,1H3,(H,34,35) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
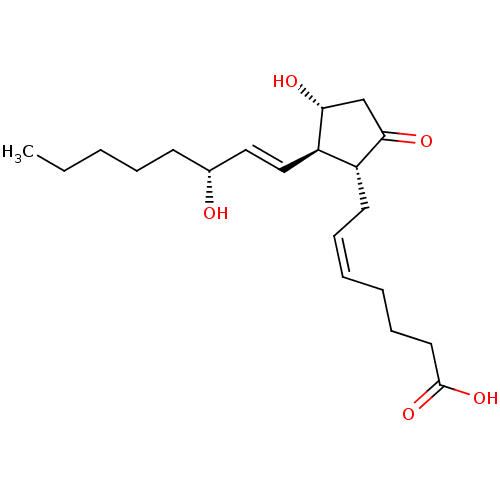
(Mus musculus (Mouse)) | BDBM50101822
((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...)Show SMILES CCCCC[C@@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16-,17-,19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity towards mouse Prostanoid EP1 receptor in CHO cells. |
Bioorg Med Chem Lett 11: 2025-8 (2001)
BindingDB Entry DOI: 10.7270/Q2CV4H1T |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University School of Medicine
Curated by ChEMBL
| Assay Description
Binding affinity to EP1 receptor (unknown origin) |
J Med Chem 57: 4454-65 (2014)
Article DOI: 10.1021/jm401431x
BindingDB Entry DOI: 10.7270/Q2CR5VXZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50081445
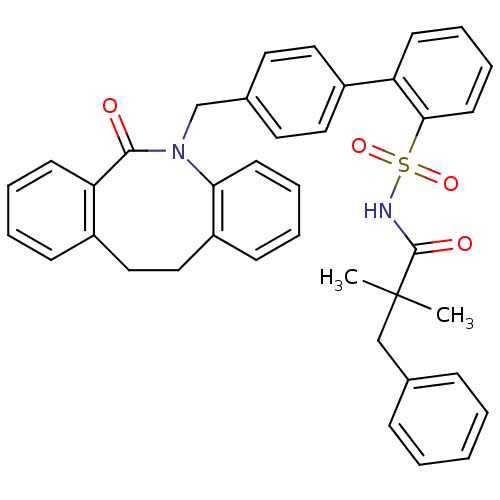
(4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...)Show SMILES CC(C)(Cc1ccccc1)C(=O)NS(=O)(=O)c1ccccc1-c1ccc(CN2c3ccccc3CCc3ccccc3C2=O)cc1 Show InChI InChI=1S/C39H36N2O4S/c1-39(2,26-28-12-4-3-5-13-28)38(43)40-46(44,45)36-19-11-9-16-33(36)31-22-20-29(21-23-31)27-41-35-18-10-7-15-32(35)25-24-30-14-6-8-17-34(30)37(41)42/h3-23H,24-27H2,1-2H3,(H,40,43) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Affinity at human EP1 receptor. |
Bioorg Med Chem Lett 9: 2699-704 (1999)
BindingDB Entry DOI: 10.7270/Q2HX1BV9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50410087

(CHEMBL2113029)Show SMILES NS(=O)(=O)c1cccc(c1)-c1ccsc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C23H18ClNO3S2/c24-18-9-10-22(28-15-16-5-2-1-3-6-16)21(14-18)23-20(11-12-29-23)17-7-4-8-19(13-17)30(25,26)27/h1-14H,15H2,(H2,25,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor in presence of 2% human serum albumin |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM23954

(5-[(2E,3aS,4R,5R,6aS)-5-hydroxy-4-[(1E,3S)-3-hydro...)Show SMILES [H][C@]12C[C@@H](O)[C@H](\C=C\[C@@H](O)C(C)CC#CC)[C@@]1([H])C\C(C2)=C\CCCC(O)=O Show InChI InChI=1S/C22H32O4/c1-3-4-7-15(2)20(23)11-10-18-19-13-16(8-5-6-9-22(25)26)12-17(19)14-21(18)24/h8,10-11,15,17-21,23-24H,5-7,9,12-14H2,1-2H3,(H,25,26)/b11-10+,16-8+/t15?,17-,18+,19-,20+,21+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM85183
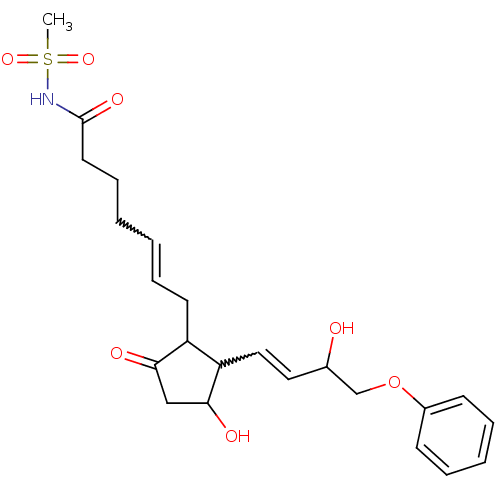
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University
Curated by PDSP Ki Database
| |
Br J Pharmacol 122: 217-24 (1997)
Article DOI: 10.1038/sj.bjp.0701367
BindingDB Entry DOI: 10.7270/Q26M35CT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Affinity for mouse Prostanoid EP4 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 2033-5 (2001)
BindingDB Entry DOI: 10.7270/Q24B30K2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Evaluated for its competitive binding affinity towards mouse Prostanoid EP1 receptor in CHO cells expressing prostanoid receptor |
Bioorg Med Chem Lett 11: 2029-31 (2001)
BindingDB Entry DOI: 10.7270/Q2833R9D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101853

((11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCCCCC(O)=O |r| Show InChI InChI=1S/C20H34O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h12-13,15-17,19,21,23H,2-11,14H2,1H3,(H,24,25)/b13-12+/t15-,16+,17+,19+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 2033-5 (2001)
BindingDB Entry DOI: 10.7270/Q24B30K2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50109546
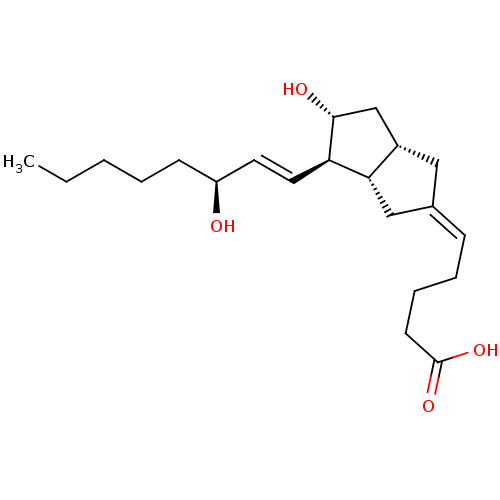
(5-[(3aS,4R,5R,6aS)-5-Hydroxy-4-((S)-3-hydroxy-oct-...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)C[C@@H]2C\C(C[C@H]12)=C\CCCC(O)=O Show InChI InChI=1S/C21H34O4/c1-2-3-4-8-17(22)10-11-18-19-13-15(7-5-6-9-21(24)25)12-16(19)14-20(18)23/h7,10-11,16-20,22-23H,2-6,8-9,12-14H2,1H3,(H,24,25)/b11-10+,15-7-/t16-,17-,18+,19-,20+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50160908
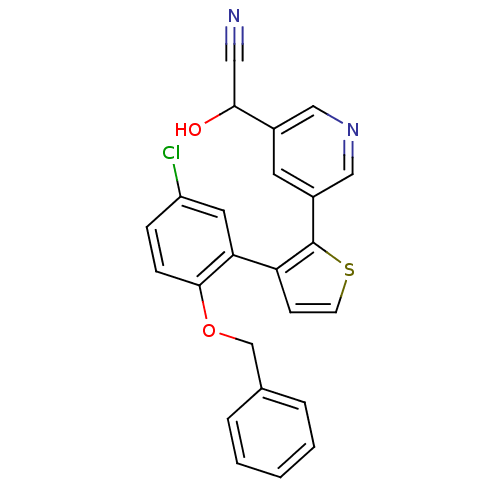
(CHEMBL182623 | {5-[3-(2-Benzyloxy-5-chloro-phenyl)...)Show SMILES OC(C#N)c1cncc(c1)-c1sccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C24H17ClN2O2S/c25-19-6-7-23(29-15-16-4-2-1-3-5-16)21(11-19)20-8-9-30-24(20)18-10-17(13-27-14-18)22(28)12-26/h1-11,13-14,22,28H,15H2 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity against Prostaglandin E receptor was determined in human |
Bioorg Med Chem Lett 15: 1155-60 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.005
BindingDB Entry DOI: 10.7270/Q2SF2VNV |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50259613

(CHEMBL467720 | sodium 6-(5-chloro-2-(2,4-dichlorob...)Show SMILES [O-]C(=O)c1cccc(Cc2cc(Cl)ccc2OCc2ccc(Cl)cc2Cl)n1 Show InChI InChI=1S/C20H14Cl3NO3/c21-14-6-7-19(27-11-12-4-5-15(22)10-17(12)23)13(8-14)9-16-2-1-3-18(24-16)20(25)26/h1-8,10H,9,11H2,(H,25,26)/p-1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as inhibition of PGE2-mediated intracellular calcium mobilizati... |
Bioorg Med Chem Lett 19: 2599-603 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.112
BindingDB Entry DOI: 10.7270/Q2HT2P68 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Mus musculus (Mouse)) | BDBM50101863
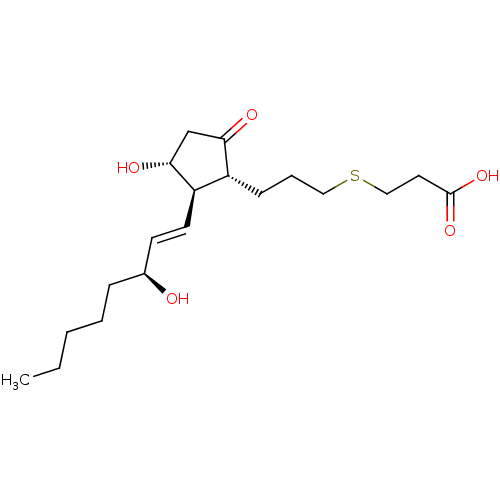
(3-{(S)-3-[(R)-3-Hydroxy-2-((1R,2R)-3-hydroxy-oct-1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1CCCSCCC(O)=O Show InChI InChI=1S/C19H32O5S/c1-2-3-4-6-14(20)8-9-16-15(17(21)13-18(16)22)7-5-11-25-12-10-19(23)24/h8-9,14-16,18,20,22H,2-7,10-13H2,1H3,(H,23,24)/b9-8+/t14-,15+,16+,18+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute
Curated by ChEMBL
| Assay Description
Affinity for mouse Prostanoid EP1 receptor expressed in CHO cells |
Bioorg Med Chem Lett 11: 2033-5 (2001)
BindingDB Entry DOI: 10.7270/Q24B30K2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50183182
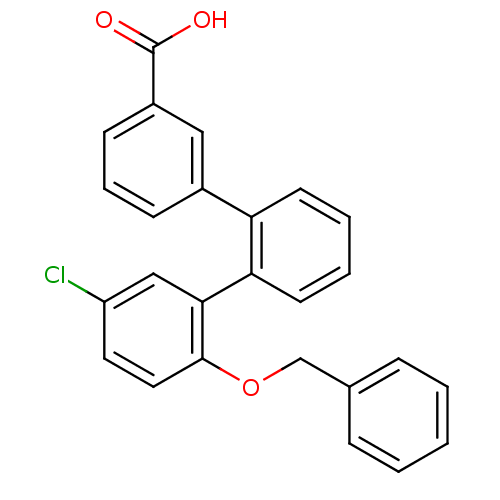
(2''-benzyloxy-5''-chloro-[1,1';2',1'']terphenyl-3-...)Show SMILES OC(=O)c1cccc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1 Show InChI InChI=1S/C26H19ClO3/c27-21-13-14-25(30-17-18-7-2-1-3-8-18)24(16-21)23-12-5-4-11-22(23)19-9-6-10-20(15-19)26(28)29/h1-16H,17H2,(H,28,29) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor assessed as calcium mobilization by FLIPR assay |
Bioorg Med Chem Lett 19: 497-501 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.032
BindingDB Entry DOI: 10.7270/Q2N879N5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data