

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50240622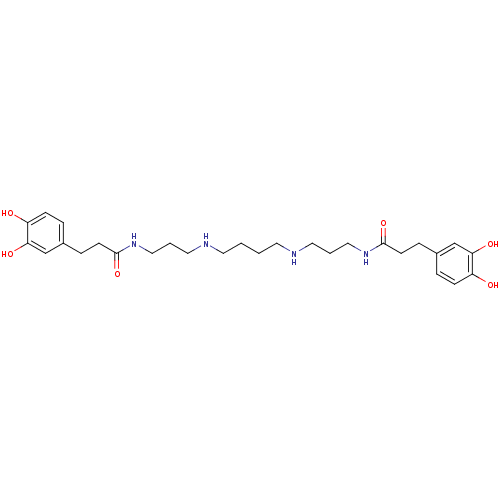 (3-(3,4-Dihydroxy-phenyl)-N-[3-(4-{3-[3-(3,4-dihydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093208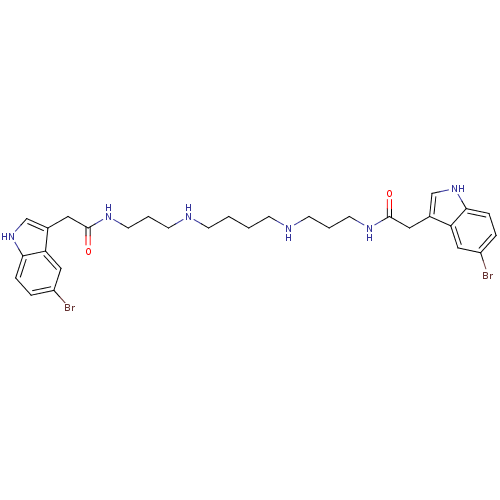 (2-(5-Bromo-1H-indol-3-yl)-N-[3-(4-{3-[2-(5-bromo-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091160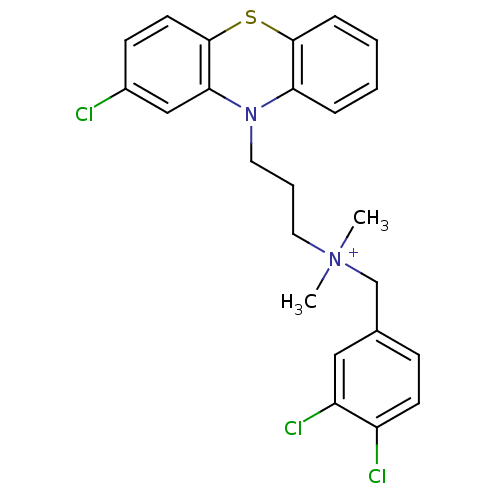 (CHEMBL106127 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178811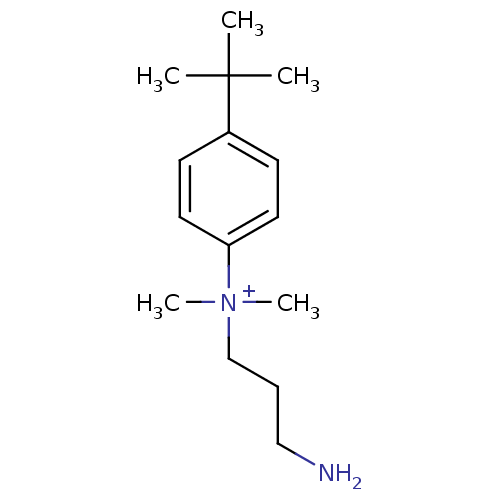 (CHEMBL199020 | N-(3-aminopropyl)-4-tert-butyl-N,N-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096495 (CHEMBL85161 | N-{3-[Bis-(3-phenyl-propyl)-amino]-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178803 (3,4-dichloro-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50612874 (CHEMBL5269349) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | UniChem | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50103373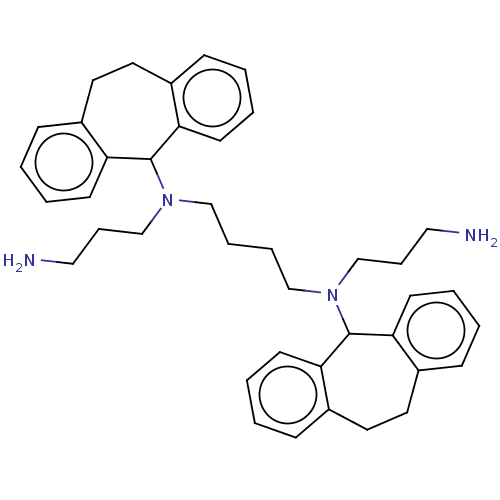 (CHEMBL3398189) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Canisius College Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction of trypanothione disulfide by spectrophotometrically | Bioorg Med Chem 23: 996-1010 (2015) Article DOI: 10.1016/j.bmc.2015.01.018 BindingDB Entry DOI: 10.7270/Q27P9166 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268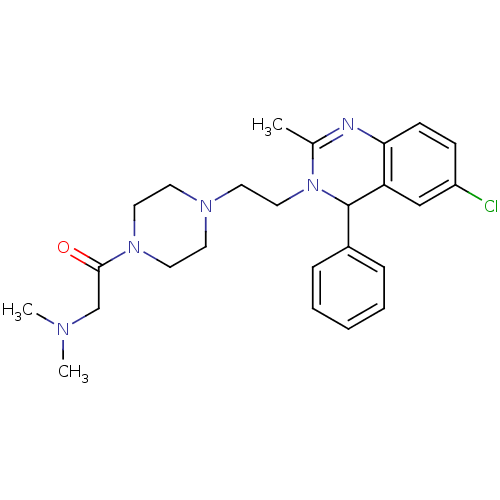 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093209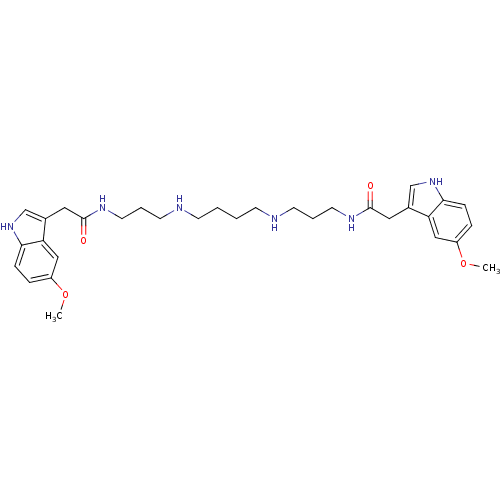 (2-(5-Methoxy-1H-indol-3-yl)-N-[3-(4-{3-[2-(5-metho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354299 (CHEMBL1836378) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093210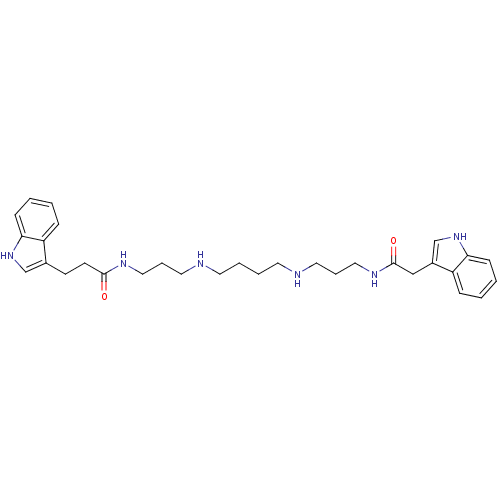 (3-(1H-Indol-3-yl)-N-(3-{4-[3-(2-1H-indol-3-yl-acet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093205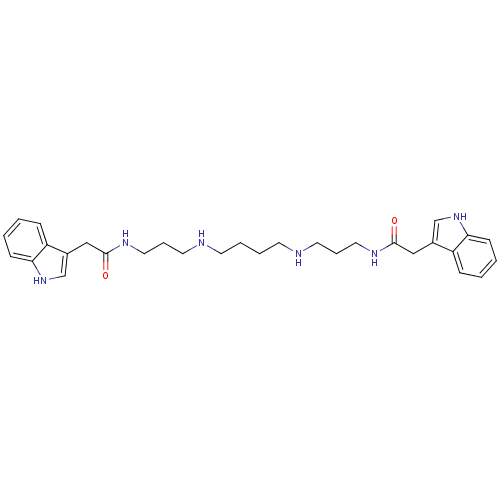 (2-(1H-Indol-3-yl)-N-(3-{4-[3-(2-1H-indol-3-yl-acet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM10469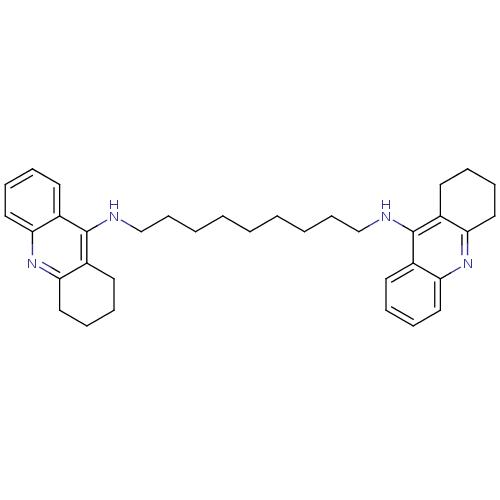 (Bis-THA inhibitor 1c | Bis-THA inhibitor 8 | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmacy and Food Chemistry, Julius-Maximilians-University of Würzburg, Am Hubland, 97074 Würzburg, Germany. Curated by ChEMBL | Assay Description Competitive inhibition of Trypanosoma cruzi trypanothione reductase using varying levels of trypanothione disulfide as substrate by Lineweaver--burk ... | Bioorg Med Chem 25: 4526-4531 (2017) Article DOI: 10.1016/j.bmc.2017.06.051 BindingDB Entry DOI: 10.7270/Q2KK9F9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257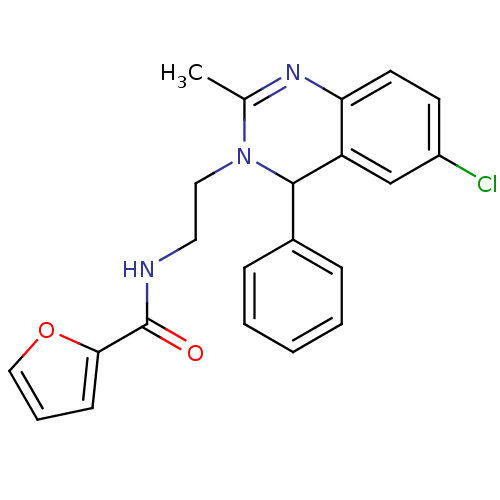 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178803 (3,4-dichloro-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Mixed inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091158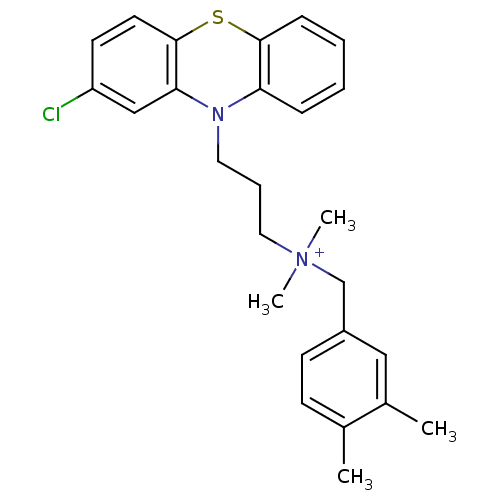 (CHEMBL322826 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091165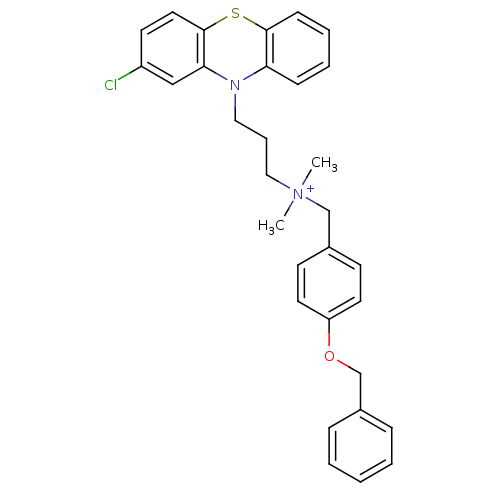 ((4-Benzyloxy-benzyl)-[3-(2-chloro-phenothiazin-10-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699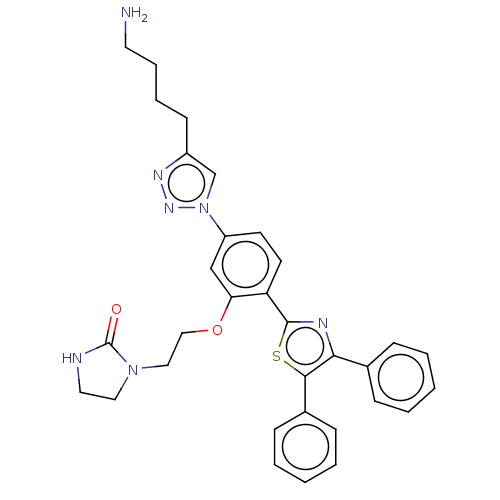 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 12.5 uM TS2 by DTNB-reagent based ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 6.2 uM TS2 by DTNB-reagent based s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091148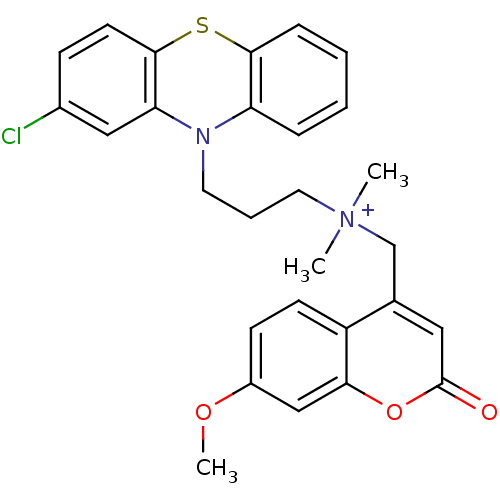 (CHEMBL106769 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178809 (4-tert-butyl-N-(3-(2-chloro-10H-phenothiazin-10-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using TSST substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50096500 (CHEMBL86281 | N,N'-Bis-(3-phenyl-propyl)-N,N'-bis-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 3.1 uM TS2 by DTNB-reagent based s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096500 (CHEMBL86281 | N,N'-Bis-(3-phenyl-propyl)-N,N'-bis-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 614 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091162 ((4-tert-Butyl-benzyl)-[3-(2-chloro-phenothiazin-10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091163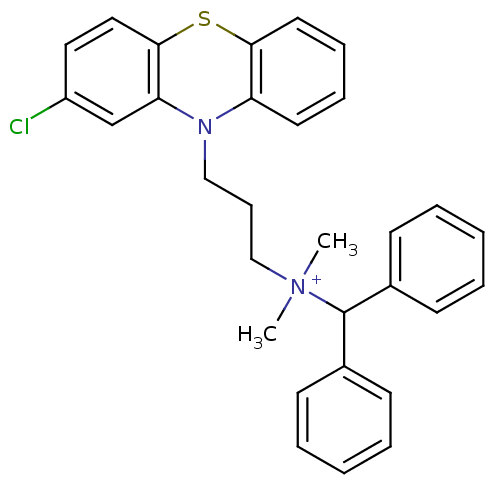 (Benzhydryl-[3-(2-chloro-phenothiazin-10-yl)-propyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093206 (CHEMBL78036 | Spermine derivative) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091154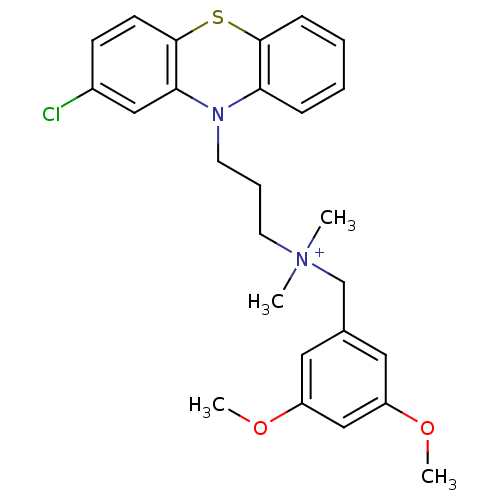 (CHEMBL106901 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 25 uM TS2 by DTNB-reagent based sp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093211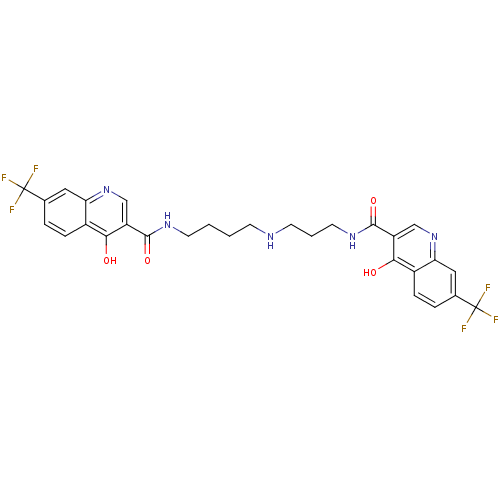 (CHEMBL308396 | Spermine derivative) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50019879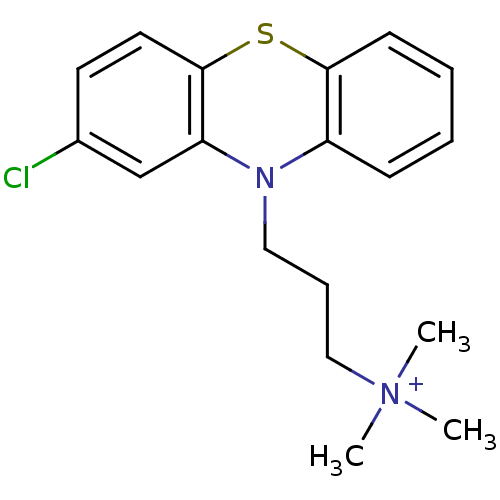 (CHEMBL279905 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50612874 (CHEMBL5269349) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | UniChem | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091157 ((2-Adamantan-1-yl-2-oxo-ethyl)-[3-(2-chloro-phenot...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093204 (4-(1H-Indol-3-yl)-N-(3-{4-[3-(4-1H-indol-3-yl-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091153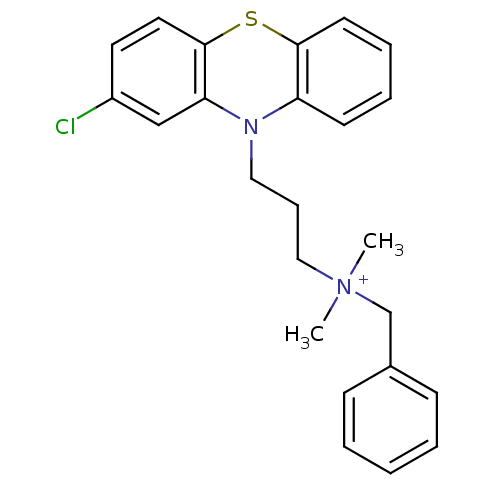 (Benzyl-[3-(2-chloro-phenothiazin-10-yl)-propyl]-di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 100 uM TS2 by DTNB-reagent based s... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50096491 (CHEMBL85189 | N-(3-Amino-propyl)-N,N'-bis-(3-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana State University Curated by ChEMBL | Assay Description Tested for binding affinity against trypanothione reductase from Trypanosoma cruzi | Bioorg Med Chem Lett 11: 251-4 (2001) BindingDB Entry DOI: 10.7270/Q2FQ9VVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50093207 ((E)-3-(3,4-Dihydroxy-phenyl)-N-[3-(4-{3-[(E)-3-(3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Southamton Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of trypanothione reductase enzyme | Bioorg Med Chem Lett 10: 2367-9 (2001) BindingDB Entry DOI: 10.7270/Q2HH6KKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Leishmania infantum) | BDBM50571699 (CHEMBL4855840) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant Leishmania infantum TryR assessed as overall apparent inhibition constant in presence of 50 uM TS2 by DTNB-reagent based sp... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00206 BindingDB Entry DOI: 10.7270/Q24T6P4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091159 (CHEMBL326458 | [2-(4-Chloro-3-methyl-phenyl)-2-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091156 ((4-Bromo-benzyl)-[3-(2-chloro-phenothiazin-10-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091155 (CHEMBL323271 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091164 (CHEMBL106236 | [3-(2-Chloro-phenothiazin-10-yl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178807 (3,4-dichloro-N-(3-(5-chloro-2-(phenylthio)phenylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Linear competitive inhibition of trypanothione reductase from Trypanosoma cruzi using (ZCG.dmapa)2 substrate | J Med Chem 48: 8087-97 (2005) Article DOI: 10.1021/jm050819t BindingDB Entry DOI: 10.7270/Q2V69J65 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50091149 ((4-Chloro-benzyl)-[3-(2-chloro-phenothiazin-10-yl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Trypanosoma cruzi trypanothione reductase | J Med Chem 43: 3148-56 (2000) BindingDB Entry DOI: 10.7270/Q2R78FXC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma cruzi) | BDBM50178807 (3,4-dichloro-N-(3-(5-chloro-2-(phenylthio)phenylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pontificia Universidad Catolica del Peru Curated by ChEMBL | Assay Description Inhibition of Trypanosoma cruzi recombinant trypanothione reductase | Bioorg Med Chem 16: 6689-95 (2008) Article DOI: 10.1016/j.bmc.2008.05.074 BindingDB Entry DOI: 10.7270/Q2F18ZHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 764 total ) | Next | Last >> |