

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sortilin (Homo sapiens (Human)) | BDBM50445041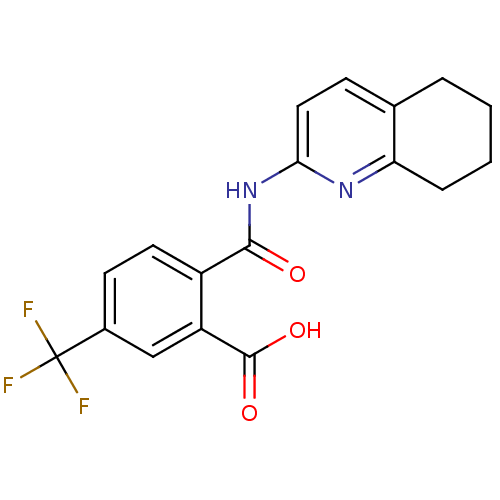 (CHEMBL3098768 | US10195186, Example 48 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 84 | n/a | 88 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159226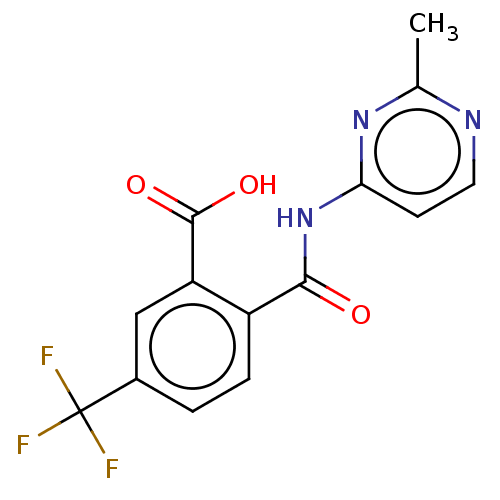 (US10195186, Example 46 | US9682967, 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445041 (CHEMBL3098768 | US10195186, Example 48 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445042 (CHEMBL3098767 | US10195186, Example 7 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 162 | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445042 (CHEMBL3098767 | US10195186, Example 7 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445042 (CHEMBL3098767 | US10195186, Example 7 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159232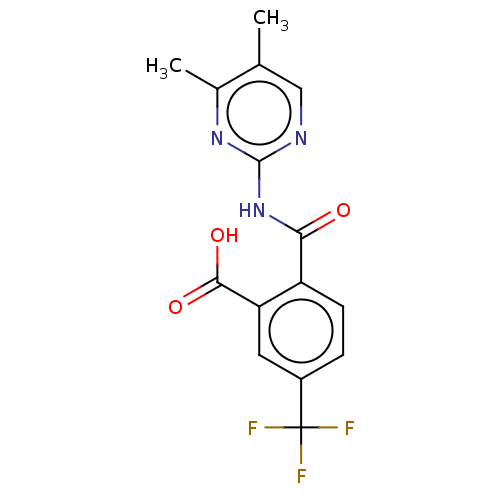 (US10195186, Example 47 | US9682967, 45) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 190 | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445055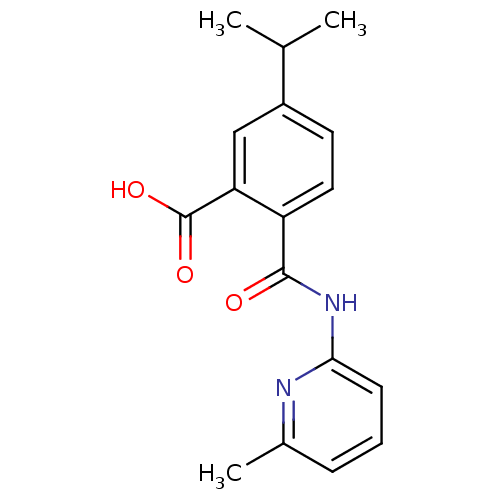 (CHEMBL3098744 | US10195186, Example 45 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50248034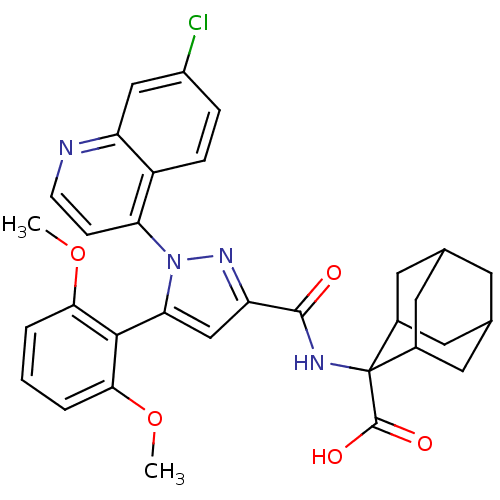 (2-{[1-(7-Chloro-quinolin-4-yl)-5-(2,6-dimethoxy-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Durham University Curated by ChEMBL | Assay Description Binding affinity to NTR3 (unknown origin) | Bioorg Med Chem 21: 4378-87 (2013) Article DOI: 10.1016/j.bmc.2013.04.075 BindingDB Entry DOI: 10.7270/Q27M09B6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159140 (US10195186, Example 18 | US9682967, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 229 | n/a | 240 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159140 (US10195186, Example 18 | US9682967, 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445054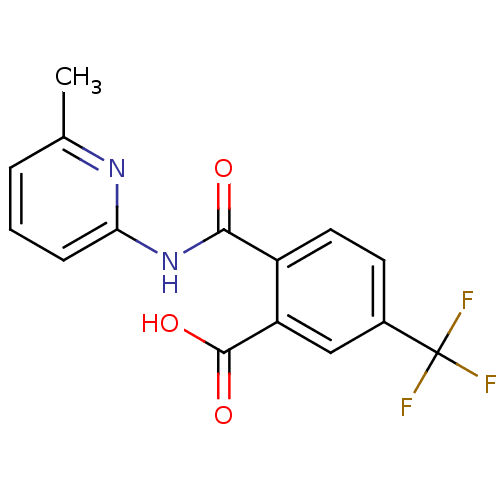 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The pro Sortilin assay was performed in total volume of 20 μl in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159226 (US10195186, Example 46 | US9682967, 44) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 286 | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159213 (US10195186, Example 44 | US9682967, 42) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445054 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Aarhus University Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from human His6-tagged sortilin pretreated for 30 mins followed by [3H]-neurotensin addition after 6 hrs measured fo... | Bioorg Med Chem Lett 27: 2629-2633 (2017) Article DOI: 10.1016/j.bmcl.2017.02.028 BindingDB Entry DOI: 10.7270/Q2TX3HS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445054 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159139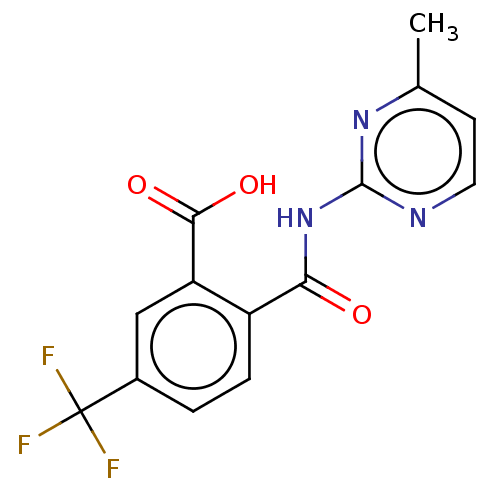 (US10195186, Example 17 | US9682967, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 343 | n/a | 360 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159138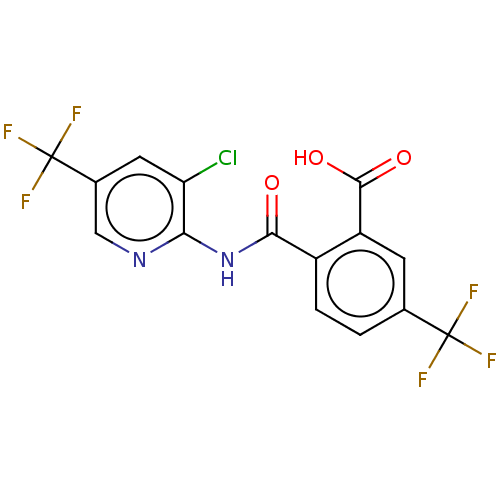 (US10195186, Example 16 | US9682967, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 343 | n/a | 360 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159138 (US10195186, Example 16 | US9682967, 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159139 (US10195186, Example 17 | US9682967, 17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from human sortilin incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintillation proxi... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50545414 (CHEMBL1852155) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nevada Curated by ChEMBL | Assay Description Inhibition of GFP-tagged Sortilin (unknown origin) expressed in HEK293T cells after 42 hrs by FACS analysis | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127115 BindingDB Entry DOI: 10.7270/Q2SB49BX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133194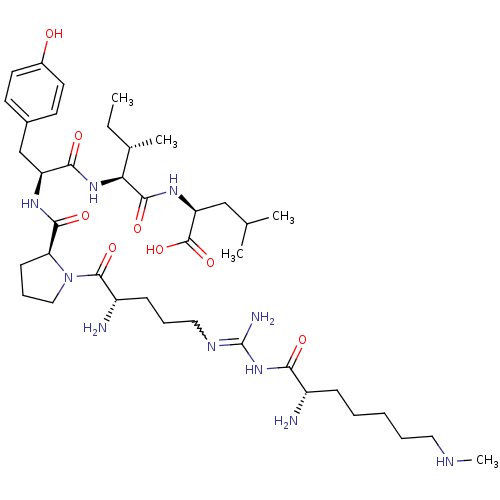 (CHEMBL133849 | Compound KK2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133192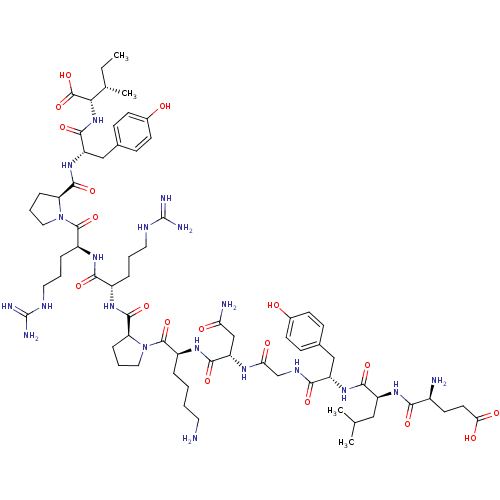 (CHEMBL439494 | Compound NT) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445054 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | 429 | n/a | 450 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445054 (CHEMBL3098745 | US10195186, Example 1 | US9682967,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159123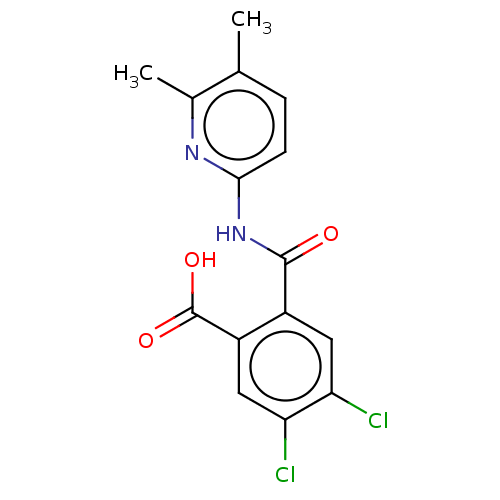 (US10195186, Example 11 | US9682967, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 457 | n/a | 480 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159129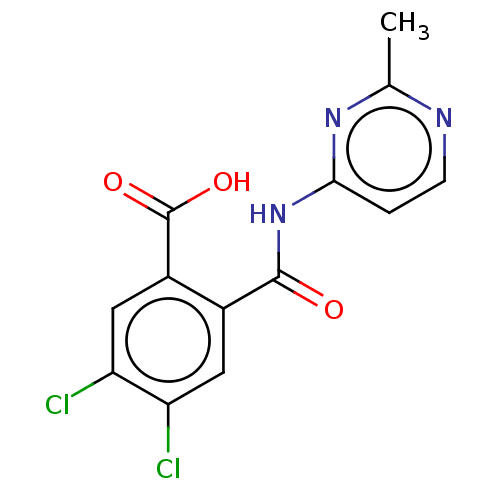 (US10195186, Example 13 | US9682967, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 457 | n/a | 480 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133207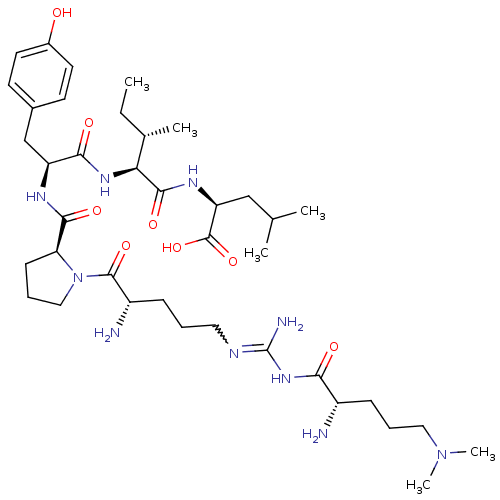 (CHEMBL133378 | Compound KK11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133188 (CHEMBL336836 | Compound KK17) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159129 (US10195186, Example 13 | US9682967, 13) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159123 (US10195186, Example 11 | US9682967, 11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445044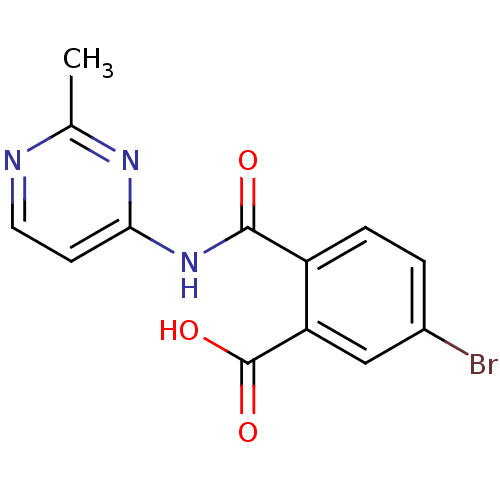 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | 467 | n/a | 490 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159178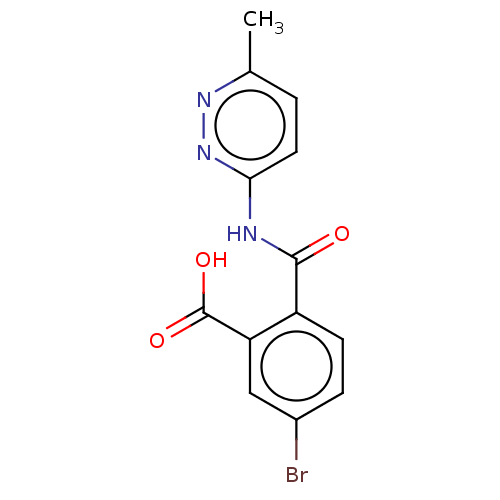 (US10195186, Example 34 | US9682967, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50445044 (CHEMBL3098765 | US10195186, Example 36 | US9682967...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S Curated by ChEMBL | Assay Description Displacement of [3H]-neurotensin from sortilin (unknown origin) incubated 30 mins prior to [3H]-neurotensin addition measured after 6 hrs by scintill... | Bioorg Med Chem Lett 24: 177-80 (2013) Article DOI: 10.1016/j.bmcl.2013.11.046 BindingDB Entry DOI: 10.7270/Q2RN39BB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133201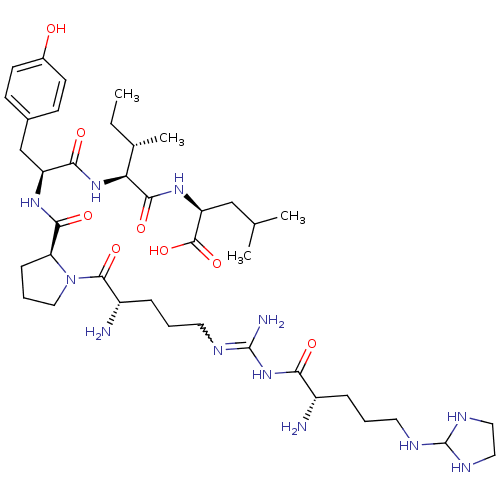 (CHEMBL132524 | Compound KK18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133190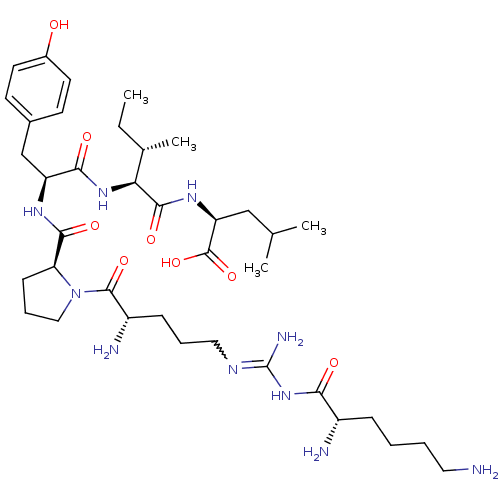 (CHEMBL430910 | Compound KK5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133202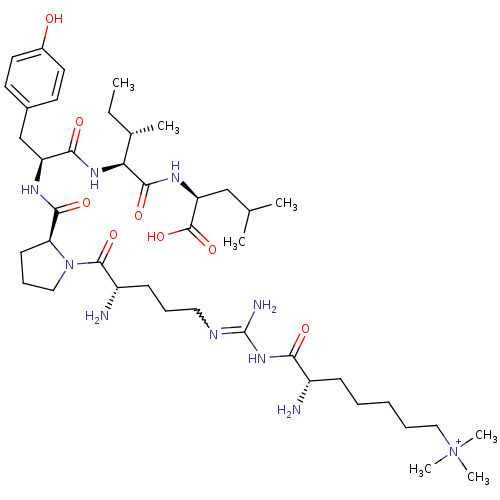 (CHEMBL132794 | Compound KK4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159154 (US10195186, Example 23 | US9682967, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 543 | n/a | 570 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159154 (US10195186, Example 23 | US9682967, 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133193 (CHEMBL337721 | Neurotensin analogue) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159285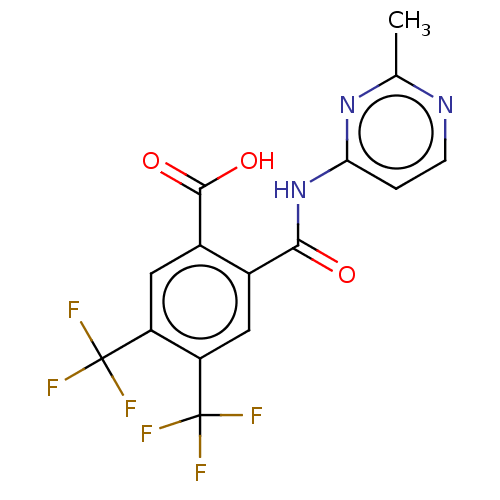 (US9682967, 50) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 599 | n/a | 620 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159272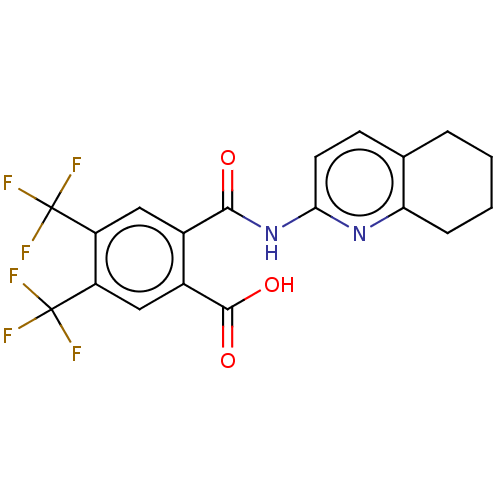 (US10195186, Example 50 | US9682967, 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159286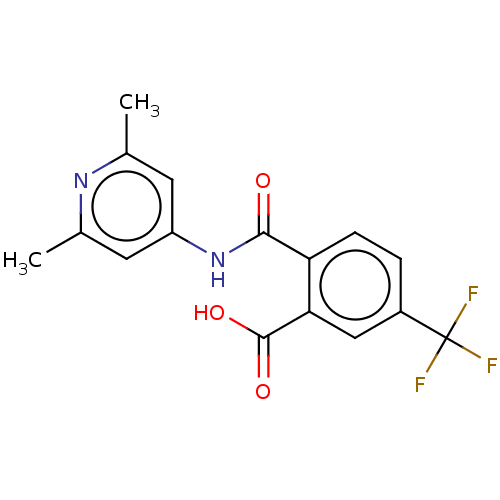 (US9682967, 51) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 614 | n/a | 640 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159273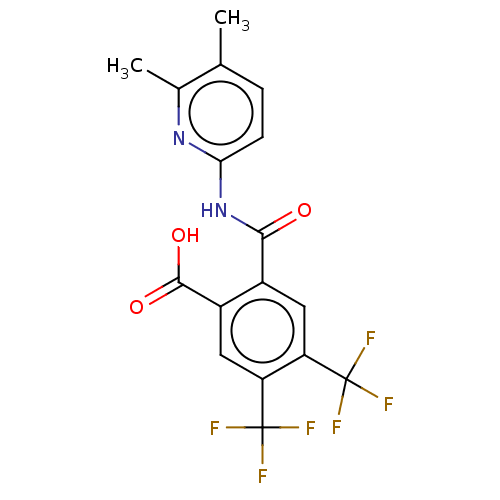 (US10195186, Example 51 | US9682967, 49) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50240845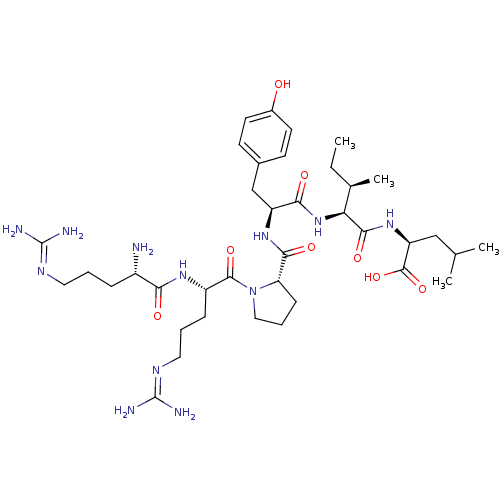 ((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133195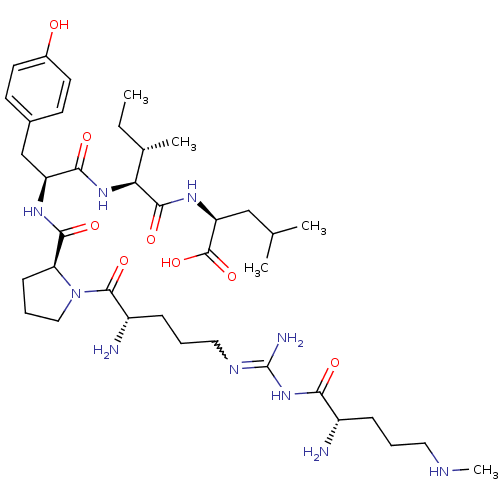 (CHEMBL133340 | Compound KK10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159121 (US10195186, Example 9 | US9682967, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 667 | n/a | 700 | n/a | n/a | n/a | n/a | 7.4 | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US9682967 (2017) BindingDB Entry DOI: 10.7270/Q22805SC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM159121 (US10195186, Example 9 | US9682967, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
H. Lundbeck A/S US Patent | Assay Description The Sortilin assay was performed in total volume of 40 ul in 50 mM HEPES pH 7.4 assay buffer containing 100 mM NaCl, 2.0 mM CaCl2, 0.1% BSA and 0.1% ... | US Patent US10195186 (2019) BindingDB Entry DOI: 10.7270/Q2474CZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sortilin (Homo sapiens (Human)) | BDBM50133187 (CHEMBL133850 | Compound KK3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description Evaluated for binding affinity by inhibiting binding of [125I]Tyr(3)-NT to human Neurotensin receptor 3 | J Med Chem 46: 4141-8 (2003) Article DOI: 10.1021/jm0300633 BindingDB Entry DOI: 10.7270/Q26M37KB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |