 Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50039393
Found 62 hits Enz. Inhib. hit(s) with all data for entry = 50039393 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338363
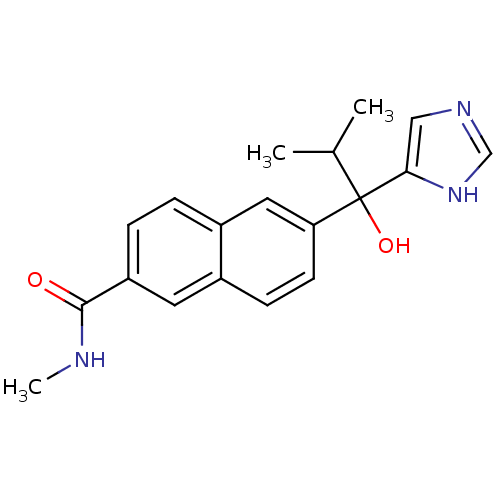
(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338355
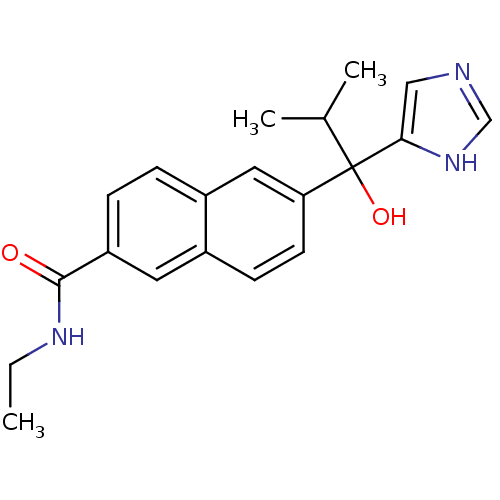
(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338349
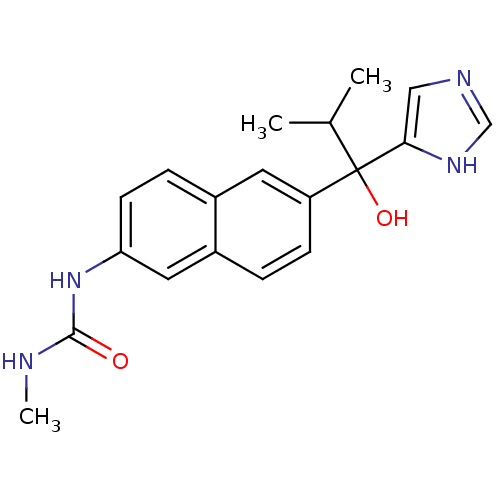
(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338353
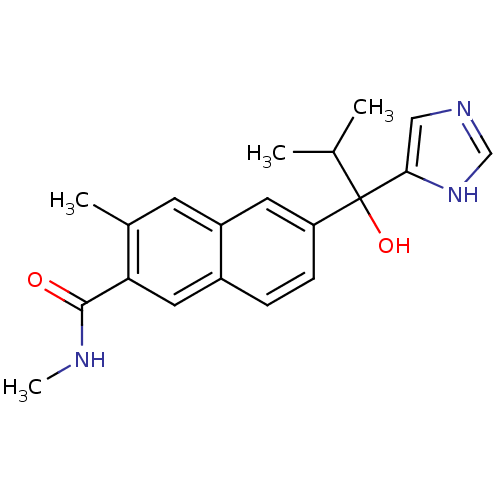
(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338358
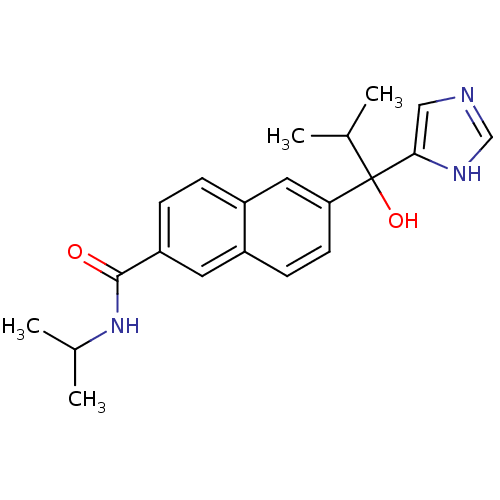
(CHEMBL1682896 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)NC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-13(2)21(26,19-11-22-12-23-19)18-8-7-15-9-17(6-5-16(15)10-18)20(25)24-14(3)4/h5-14,26H,1-4H3,(H,22,23)(H,24,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338351
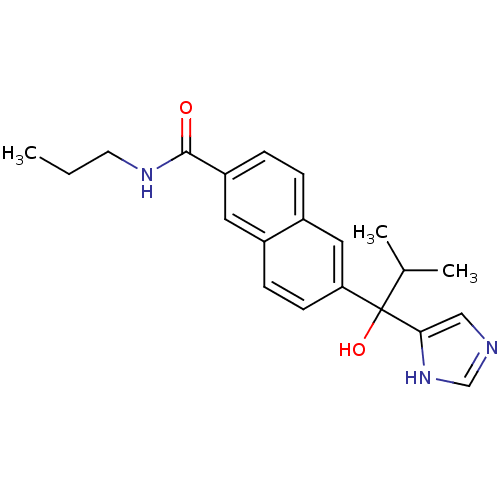
(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338362
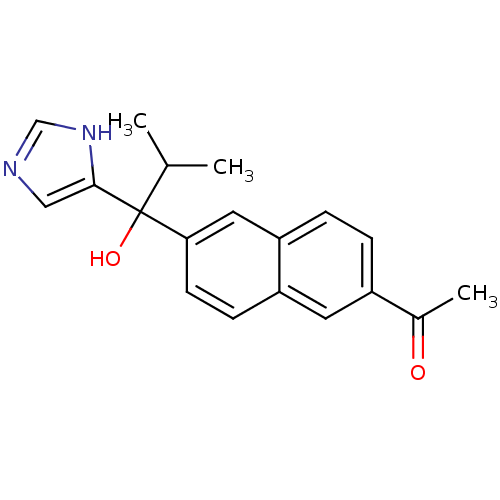
(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338365
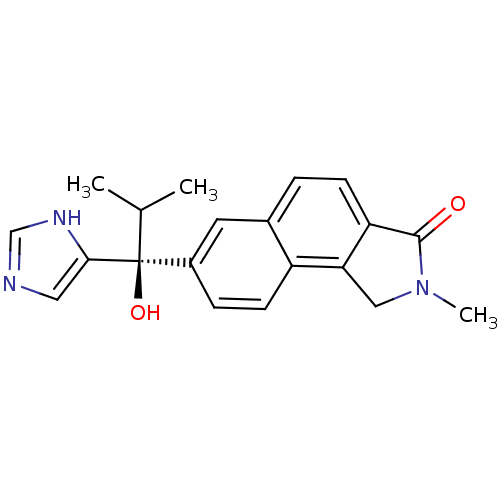
((-)-(S)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338357

(CHEMBL1682903 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)N(C)Cc3cc2c1 Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)16-5-4-13-8-17-15(6-14(13)7-16)10-23(3)19(17)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338366
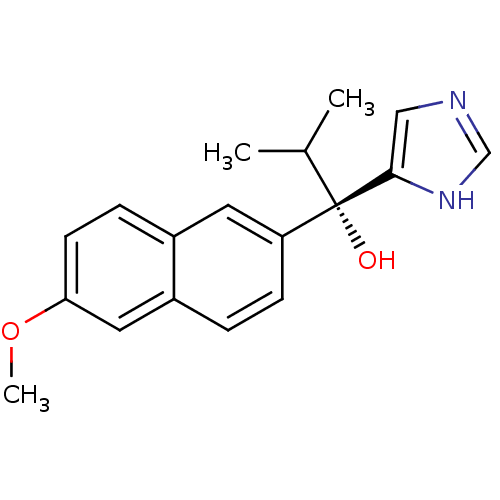
((S)-1-(1H-imidazol-4-yl)-1-(6-methoxynaphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C18H20N2O2/c1-12(2)18(21,17-10-19-11-20-17)15-6-4-14-9-16(22-3)7-5-13(14)8-15/h4-12,21H,1-3H3,(H,19,20)/t18-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338354
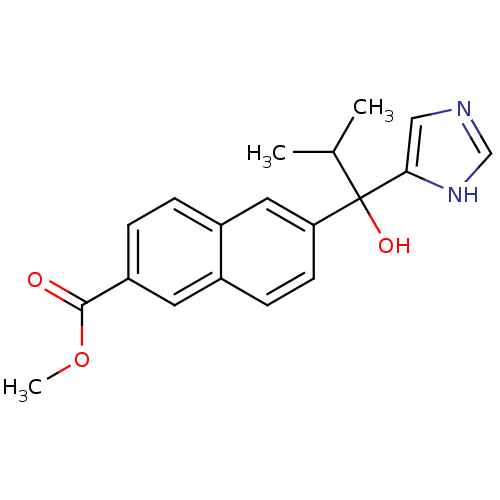
(CHEMBL1682891 | rac-Methyl 6-[1-hydroxy-1-(1H-imid...)Show SMILES COC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20N2O3/c1-12(2)19(23,17-10-20-11-21-17)16-7-6-13-8-15(18(22)24-3)5-4-14(13)9-16/h4-12,23H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338366

((S)-1-(1H-imidazol-4-yl)-1-(6-methoxynaphthalen-2-...)Show SMILES COc1ccc2cc(ccc2c1)[C@@](O)(C(C)C)c1cnc[nH]1 |r| Show InChI InChI=1S/C18H20N2O2/c1-12(2)18(21,17-10-19-11-20-17)15-6-4-14-9-16(22-3)7-5-13(14)8-15/h4-12,21H,1-3H3,(H,19,20)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338362

(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338352

(CHEMBL1682889 | rac-N-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(NC(C)=O)ccc2c1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,18-10-20-11-21-18)16-6-4-15-9-17(22-13(3)23)7-5-14(15)8-16/h4-12,24H,1-3H3,(H,20,21)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338364
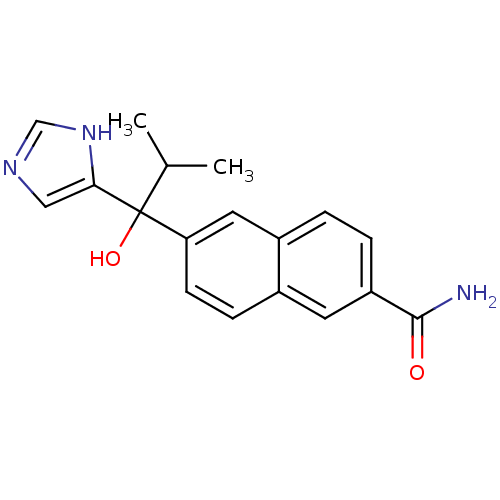
(CHEMBL1682892 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(N)=O Show InChI InChI=1S/C18H19N3O2/c1-11(2)18(23,16-9-20-10-21-16)15-6-5-12-7-14(17(19)22)4-3-13(12)8-15/h3-11,23H,1-2H3,(H2,19,22)(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338349

(CHEMBL1682890 | rac-N'-{6-[1-Hydroxy-1-(1H-imidazo...)Show SMILES CNC(=O)Nc1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H22N4O2/c1-12(2)19(25,17-10-21-11-22-17)15-6-4-14-9-16(23-18(24)20-3)7-5-13(14)8-15/h4-12,25H,1-3H3,(H,21,22)(H2,20,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338350
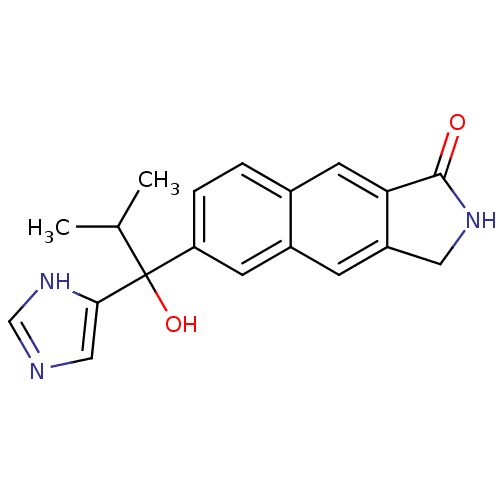
(CHEMBL1682902 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)NCc3cc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)15-4-3-12-7-16-14(5-13(12)6-15)8-21-18(16)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338351

(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338352

(CHEMBL1682889 | rac-N-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(NC(C)=O)ccc2c1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,18-10-20-11-21-18)16-6-4-15-9-17(22-13(3)23)7-5-14(15)8-16/h4-12,24H,1-3H3,(H,20,21)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338353

(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338350

(CHEMBL1682902 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)NCc3cc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)15-4-3-12-7-16-14(5-13(12)6-15)8-21-18(16)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338354

(CHEMBL1682891 | rac-Methyl 6-[1-hydroxy-1-(1H-imid...)Show SMILES COC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20N2O3/c1-12(2)19(23,17-10-20-11-21-17)16-7-6-13-8-15(18(22)24-3)5-4-14(13)9-16/h4-12,23H,1-3H3,(H,20,21) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338355

(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338357

(CHEMBL1682903 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)N(C)Cc3cc2c1 Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)16-5-4-13-8-17-15(6-14(13)7-16)10-23(3)19(17)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338358

(CHEMBL1682896 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)NC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-13(2)21(26,19-11-22-12-23-19)18-8-7-15-9-17(6-5-16(15)10-18)20(25)24-14(3)4/h5-14,26H,1-4H3,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338359
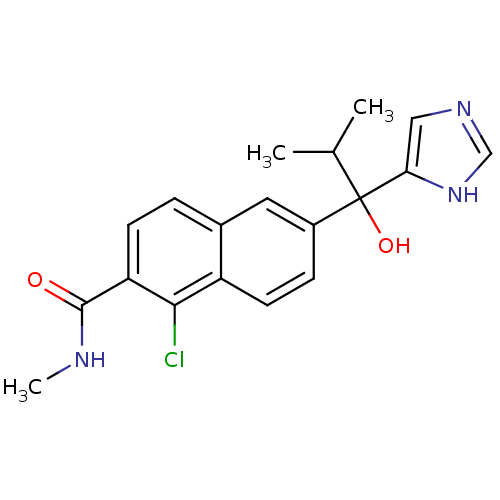
(CHEMBL1682897 | rac-1-Chloro-6-[1-hydroxy-1-(1H-im...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1Cl)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20ClN3O2/c1-11(2)19(25,16-9-22-10-23-16)13-5-7-14-12(8-13)4-6-15(17(14)20)18(24)21-3/h4-11,25H,1-3H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338361

(CHEMBL1682898 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)15-6-8-16-13(3)17(19(24)21-4)7-5-14(16)9-15/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338356

((+)-7-[1-Hydroxy-1-(1H-imidazol-4-yl)-2-methylprop...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2c3CNC(=O)c3ccc2c1 Show InChI InChI=1S/C19H19N3O2/c1-11(2)19(24,17-9-20-10-22-17)13-4-6-14-12(7-13)3-5-15-16(14)8-21-18(15)23/h3-7,9-11,24H,8H2,1-2H3,(H,20,22)(H,21,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Rattus norvegicus (Rat)) | BDBM50338361

(CHEMBL1682898 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)15-6-8-16-13(3)17(19(24)21-4)7-5-14(16)9-15/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in Sprague-Dawley rat testicular microsomes |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338359

(CHEMBL1682897 | rac-1-Chloro-6-[1-hydroxy-1-(1H-im...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1Cl)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20ClN3O2/c1-11(2)19(25,16-9-22-10-23-16)13-5-7-14-12(8-13)4-6-15(17(14)20)18(24)21-3/h4-11,25H,1-3H3,(H,21,24)(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 17-alpha-hydroxylase/17,20 lyase
(Homo sapiens (Human)) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 17,20 lyase activity in human |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338365

((-)-(S)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m0/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Steroid 11-beta-monooxygenase
(Rattus norvegicus) | BDBM50338360

((+)-(R)-7-(1-hydroxy-1-(1H-imidazol-4-yl)-2-methyl...)Show SMILES CC(C)[C@](O)(c1cnc[nH]1)c1ccc2c3CN(C)C(=O)c3ccc2c1 |r| Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)14-5-7-15-13(8-14)4-6-16-17(15)10-23(3)19(16)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22)/t20-/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of 11-hydroxylase activity in Sprague-Dawley rat adrenal gland |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338362

(CHEMBL1682888 | rac-1-{6-[1-Hydroxy-1-(1H-imidazol...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(C)=O Show InChI InChI=1S/C19H20N2O2/c1-12(2)19(23,18-10-20-11-21-18)17-7-6-15-8-14(13(3)22)4-5-16(15)9-17/h4-12,23H,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338354

(CHEMBL1682891 | rac-Methyl 6-[1-hydroxy-1-(1H-imid...)Show SMILES COC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H20N2O3/c1-12(2)19(23,17-10-20-11-21-17)16-7-6-13-8-15(18(22)24-3)5-4-14(13)9-16/h4-12,23H,1-3H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338351

(CHEMBL1682895 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CCCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C21H25N3O2/c1-4-9-23-20(25)17-6-5-16-11-18(8-7-15(16)10-17)21(26,14(2)3)19-12-22-13-24-19/h5-8,10-14,26H,4,9H2,1-3H3,(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338353

(CHEMBL1682899 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1cc2ccc(cc2cc1C)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-12(2)20(25,18-10-22-11-23-18)16-6-5-14-9-17(19(24)21-4)13(3)7-15(14)8-16/h5-12,25H,1-4H3,(H,21,24)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338363

(CHEMBL1682893 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C19H21N3O2/c1-12(2)19(24,17-10-21-11-22-17)16-7-6-13-8-15(18(23)20-3)5-4-14(13)9-16/h4-12,24H,1-3H3,(H,20,23)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338364

(CHEMBL1682892 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc(ccc2c1)C(N)=O Show InChI InChI=1S/C18H19N3O2/c1-11(2)18(23,16-9-20-10-21-16)15-6-5-12-7-14(17(19)22)4-3-13(12)8-15/h3-11,23H,1-2H3,(H2,19,22)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338357

(CHEMBL1682903 | rac-6-[1-Hydroxy-1-(1H-imidazol-4-...)Show SMILES CC(C)C(O)(c1cnc[nH]1)c1ccc2cc3C(=O)N(C)Cc3cc2c1 Show InChI InChI=1S/C20H21N3O2/c1-12(2)20(25,18-9-21-11-22-18)16-5-4-13-8-17-15(6-14(13)7-16)10-23(3)19(17)24/h4-9,11-12,25H,10H2,1-3H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50338355

(CHEMBL1682894 | rac-N-Ethyl-6-[1-Hydroxy-1-(1H-imi...)Show SMILES CCNC(=O)c1ccc2cc(ccc2c1)C(O)(C(C)C)c1cnc[nH]1 Show InChI InChI=1S/C20H23N3O2/c1-4-22-19(24)16-6-5-15-10-17(8-7-14(15)9-16)20(25,13(2)3)18-11-21-12-23-18/h5-13,25H,4H2,1-3H3,(H,21,23)(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 |
Bioorg Med Chem 19: 1751-70 (2011)
Article DOI: 10.1016/j.bmc.2011.01.017
BindingDB Entry DOI: 10.7270/Q2X92C9F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data