

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM22109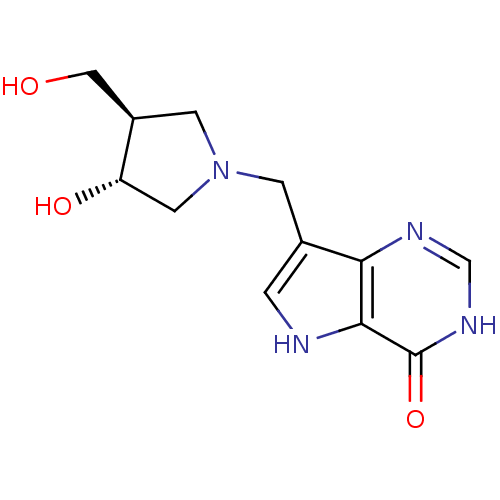 (7-{[(3R,4R)-3-hydroxy-4-(hydroxymethyl)pyrrolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 0.00850 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by ChEMBL | Assay Description Binding affinity to human PNP | Nat Chem Biol 5: 551-8 (2009) Article DOI: 10.1038/nchembio.202 BindingDB Entry DOI: 10.7270/Q2GX4BRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293091 (7-({[(1R,2S)-2,3-DIHYDROXY-1-(HYDROXYMETHYL)PROPYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB US Patent | n/a | n/a | n/a | 0.00860 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of human PNP | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50195587 (1,4-DIDEOXY-4-AZA-1-(S)-(9-DEAZAHYPOXANTHIN-9-YL)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine Curated by ChEMBL | Assay Description Binding affinity to human PNP | Nat Chem Biol 5: 551-8 (2009) Article DOI: 10.1038/nchembio.202 BindingDB Entry DOI: 10.7270/Q2GX4BRS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM92929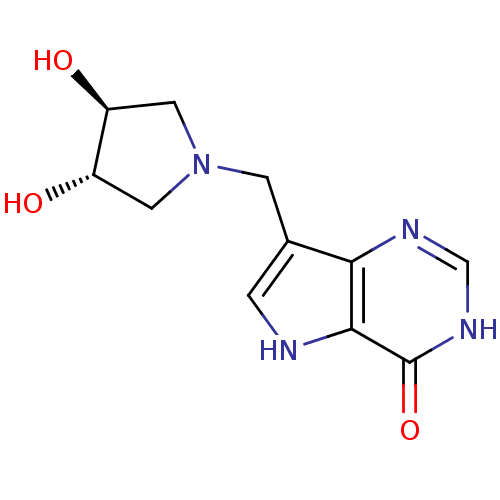 (Immucillins, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.156 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine | Assay Description Inhibition assay using human PNP. | Biochemistry 48: 5226-38 (2009) Article DOI: 10.1021/bi9005896 BindingDB Entry DOI: 10.7270/Q2SB44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293060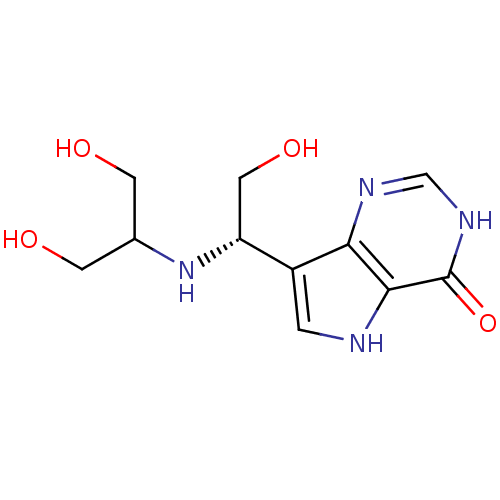 (7-{(1S)-1-[(1,3-Dihydroxypropan-2-yl)amino]-2-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM92927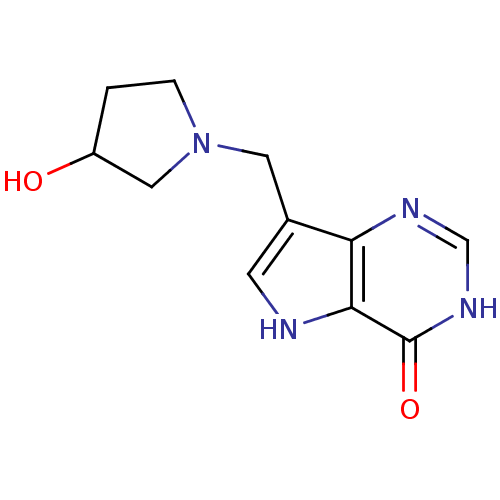 (Immucillins, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 0.383 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine | Assay Description Inhibition assay using human PNP. | Biochemistry 48: 5226-38 (2009) Article DOI: 10.1021/bi9005896 BindingDB Entry DOI: 10.7270/Q2SB44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293063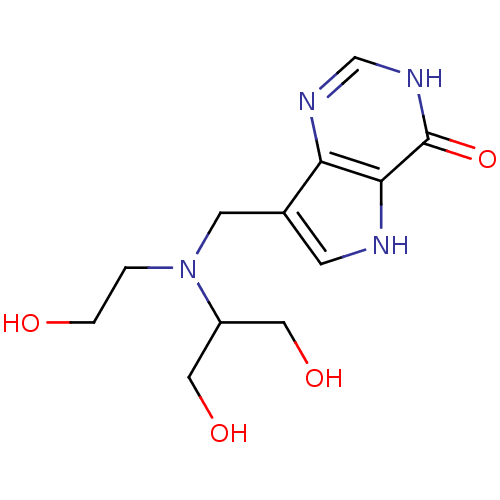 (7-{[(1,3-Dihydroxypropan-2-yl)(2-hydroxyethyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 0.469 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293064 (7-({[1,3-Dihydroxy-2-(hydroxymethyl)propan-2-yl]am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293085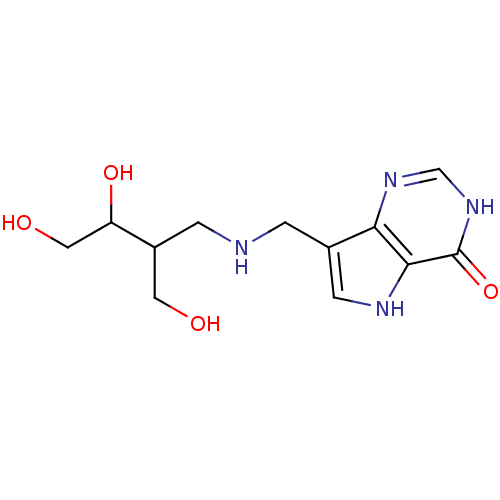 (7-({[(2R/S,3S/R)-3,4-Dihydroxy-2-(hydroxymethyl)bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293067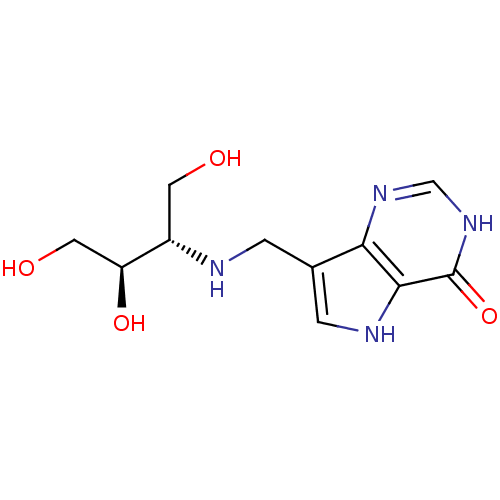 (7-({[(2S,3R)-1,3,4-Trihydroxybutan-2-yl]amino}meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293068 (7-{[(2-Hydroxyethyl)amino]methyl}-3,5-dihydro-4H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136302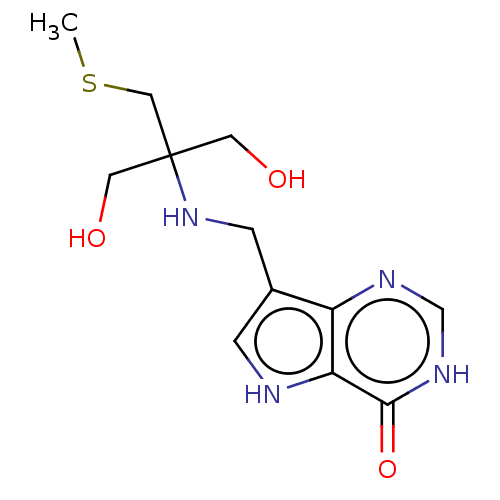 (US8853224, 12 | US9290501, MT-TrisMe-ImmH) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293070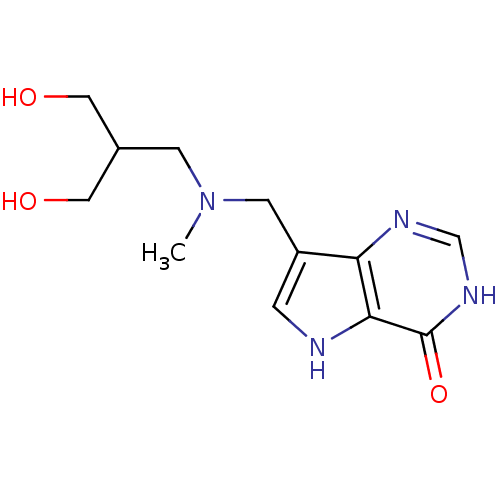 (7-({[3-Hydroxy-2-(hydroxymethyl)propyl](methyl)ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293071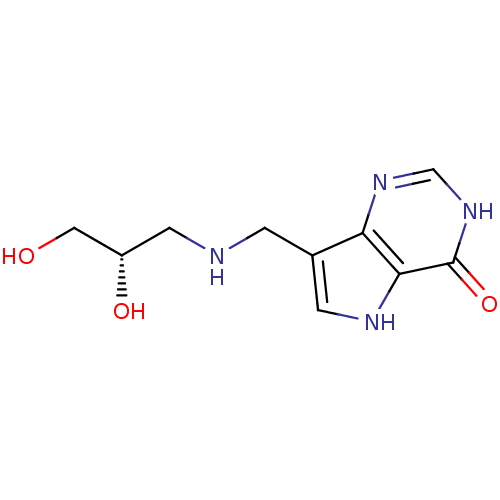 (7-({[(2S)-2,3-Dihydroxypropyl]amino}methyl)-3,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293072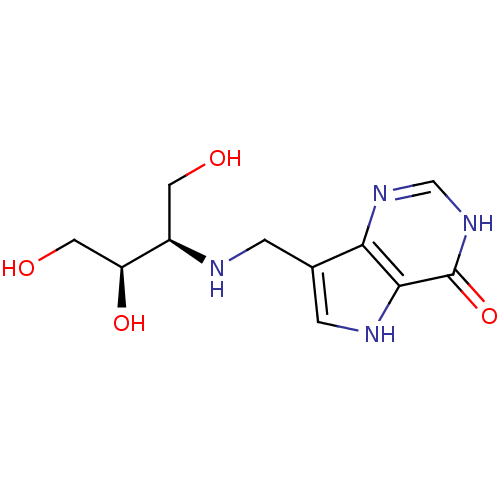 (7-({[(2R,3R)-1,3,4-Trihydroxybutan-2-yl]amino}meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293073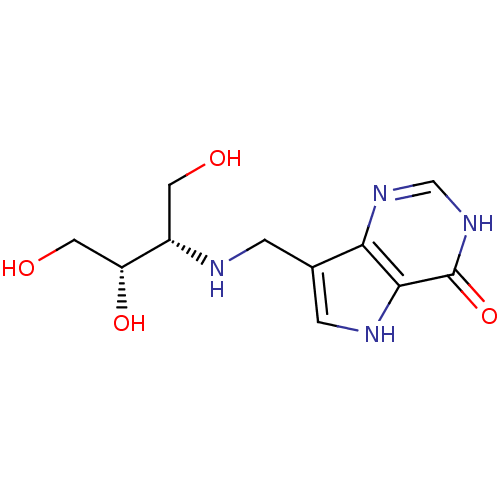 (7-({[(2S,3S)-1,3,4-Trihydroxybutan-2-yl]amino}meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50330392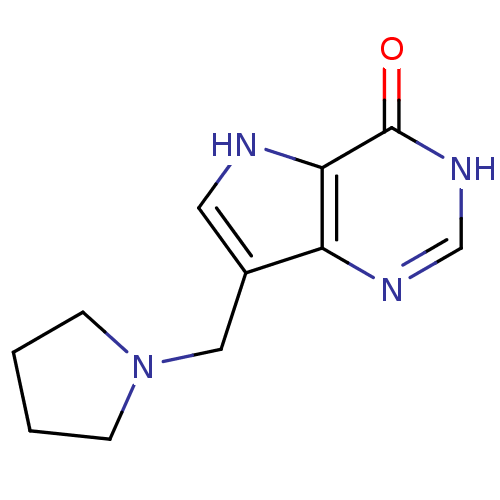 (7-(pyrrolidin-1-ylmethyl)-3H-pyrrolo[3,2-d]pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a |
Instituto de Parasitología y Biomedicina López-Neyra Curated by ChEMBL | Assay Description Inhibition of human PNP | Eur J Med Chem 45: 5140-9 (2010) Article DOI: 10.1016/j.ejmech.2010.08.026 BindingDB Entry DOI: 10.7270/Q24J0FB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136310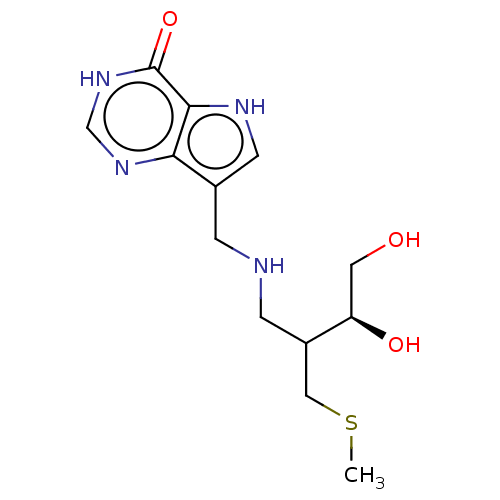 (US8853224, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293075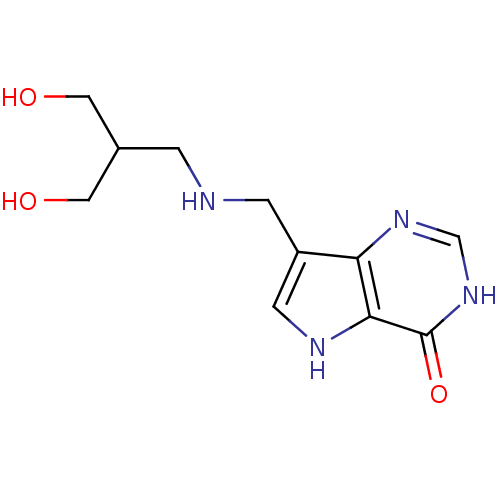 (7-({[3-Hydroxy-2-(hydroxymethyl)propyl]amino}methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 14.1 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293076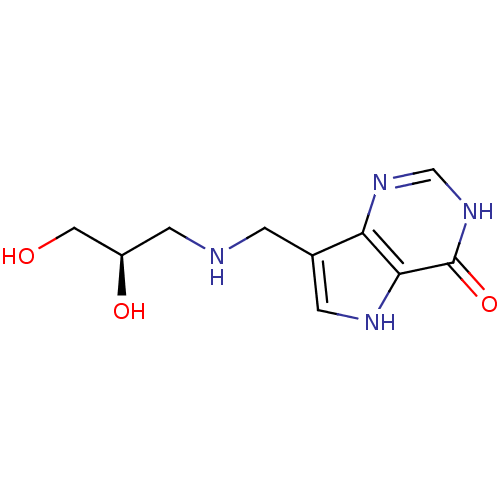 (7-({[(2R)-2,3-Dihydroxypropyl]amino}methyl)-3,5-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 14.9 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136306 (US8853224, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136312 (US8853224, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293077 (7-{[(4-Hydroxybutyl)amino]methyl}-3,5-dihydro-4H-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293078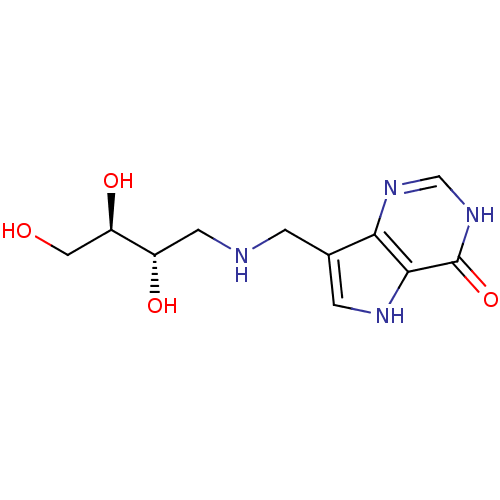 (7-({[(2S,3R)-2,3,4-Trihydroxybutyl]amino}methyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136313 (US8853224, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM92930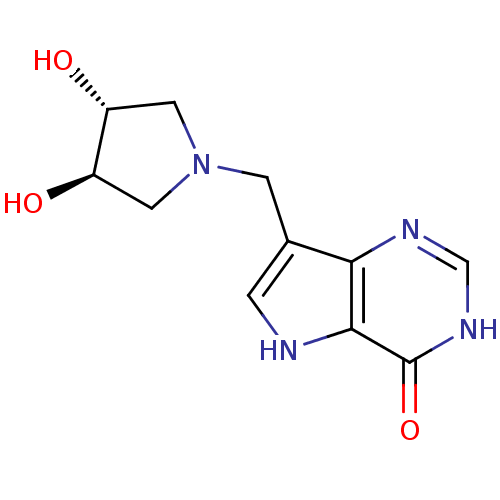 (Immucillins, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a |
Albert Einstein College of Medicine | Assay Description Inhibition assay using human PNP. | Biochemistry 48: 5226-38 (2009) Article DOI: 10.1021/bi9005896 BindingDB Entry DOI: 10.7270/Q2SB44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136308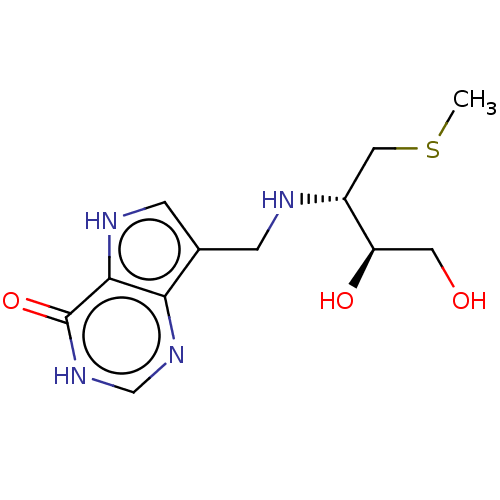 (US8853224, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136307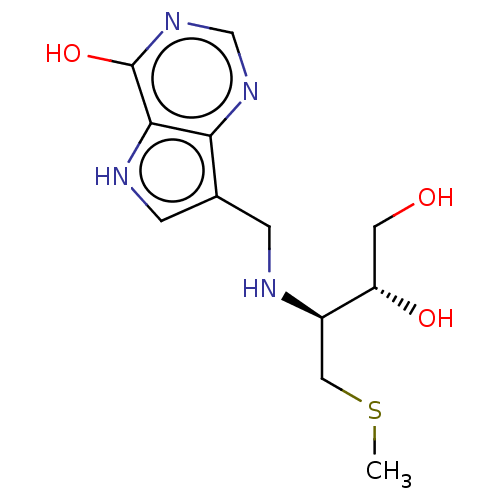 (US8853224, 25) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136304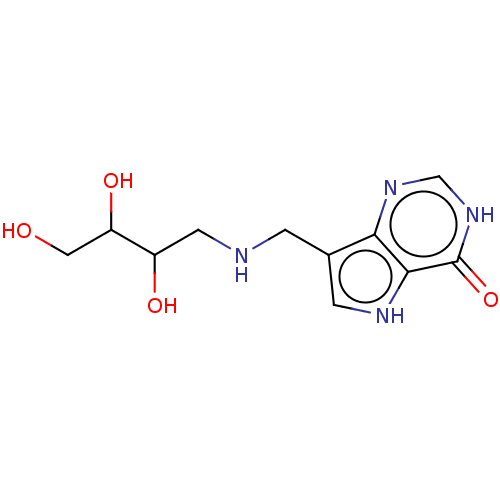 (US8853224, 20.5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293081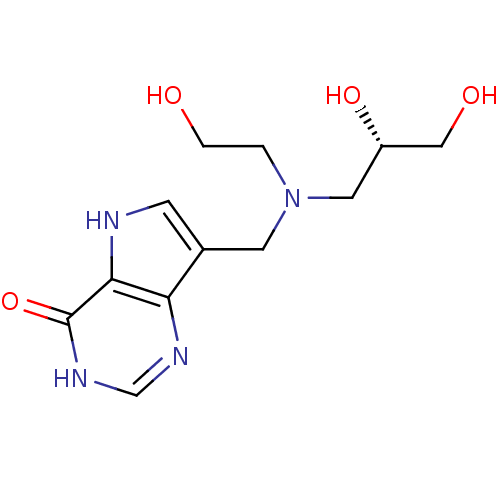 (7-({[(2R)-2,3-Dihydroxypropyl](2-hydroxyethyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 96 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136309 (US8853224, 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 142 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136311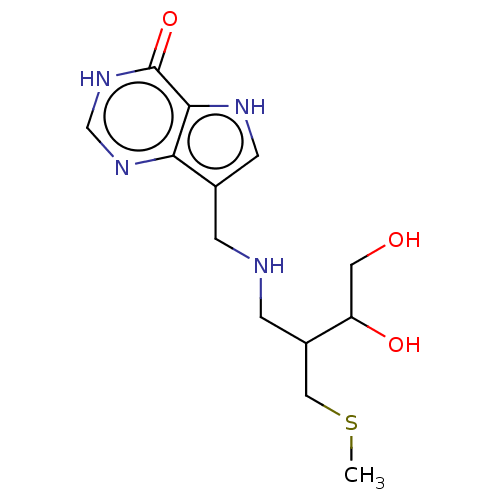 (US8853224, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 159 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293079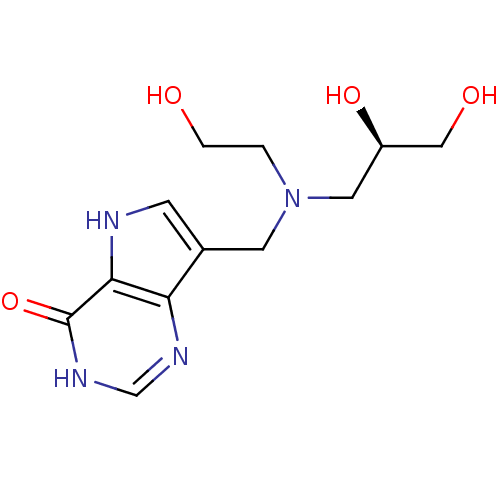 (7-({[(2S)-2,3-Dihydroxypropyl](2-hydroxyethyl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 165 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM50293083 (7-({[(2R/S)-2,4-Dihydroxybutyl](methyl)amino}methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136303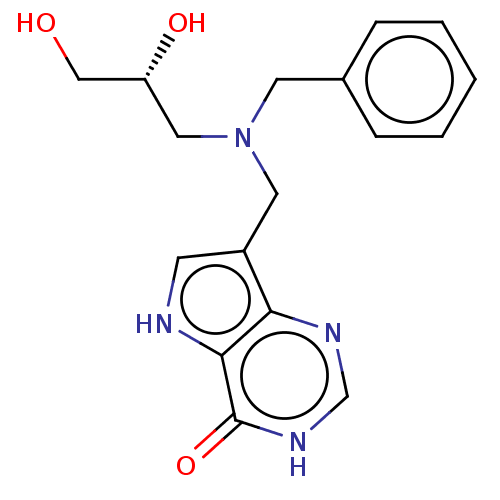 (US8853224, 3.4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136314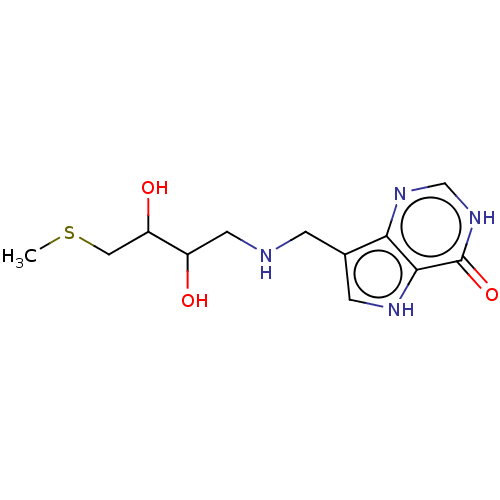 (US8853224, 20.8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 789 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Purine nucleoside phosphorylase (Homo sapiens (Human)) | BDBM136305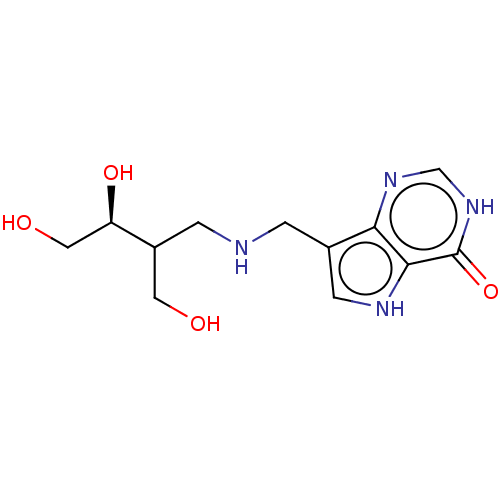 (US8853224, 22) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 25 |
Industrial Research Limited; Albert Einstein College of Medicine of Yeshiva University US Patent | Assay Description The inhibitor dissociation constants reported in Table 1 below are for phosphorolysis of inosine by PNP and were based on reaction rates measurements... | US Patent US8853224 (2014) BindingDB Entry DOI: 10.7270/Q27H1H84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||