

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50322535 (3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human HCK using KVEKIGEGTYGVVYK as substrate by radiometric hotspot kinase assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.8b00081 BindingDB Entry DOI: 10.7270/Q2WM1J37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50268924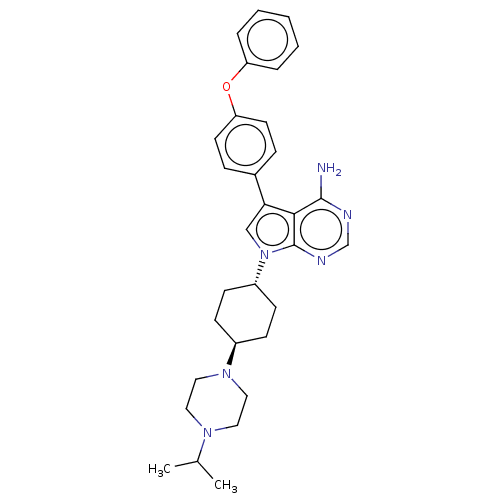 (CHEMBL4075002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.257 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50268924 (CHEMBL4075002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM8793 (7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay | Bioorg Med Chem Lett 27: 4994-4998 (2017) Article DOI: 10.1016/j.bmcl.2017.10.012 BindingDB Entry DOI: 10.7270/Q2X92DV1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM8793 (7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.427 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of HCK (75 to 526 residues) (unknown origin) expressed in Sf9 insect cells after 120 mins | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM8793 (7-[4-(4-methylpiperazin-1-yl)cyclohexyl]-5-(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of human HCK (81 to 526 residues) expressed in Sf9 insect cells after 120 mins | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473766 (US10844077, Compound I-9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473746 (US10844077, Compound I-3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473750 (US10844077, Compound I-7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473751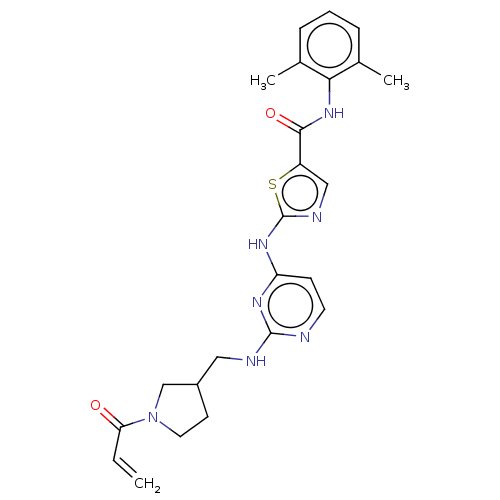 (US10844077, Compound I-8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.495 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM8794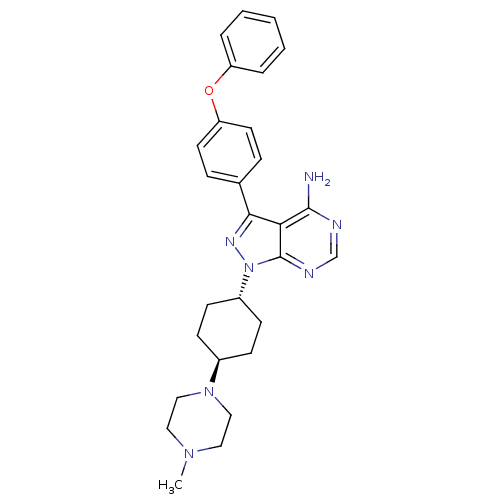 (1-[4-(4-methylpiperazin-1-yl)cyclohexyl]-3-(4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114232 BindingDB Entry DOI: 10.7270/Q27W6H6X | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM93207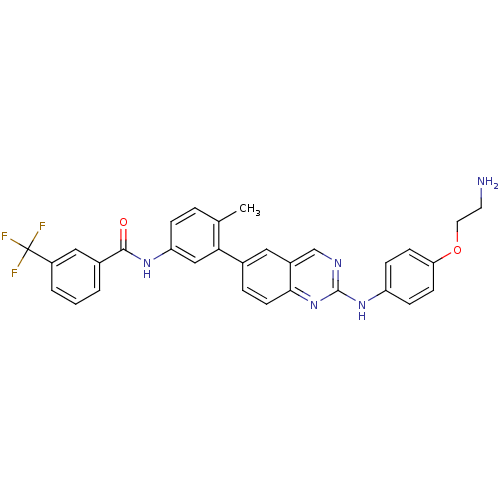 (Kinase inhibitor, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
University of Washington | Assay Description Fluorescence assay used for determination of catch and release efficiency. | ACS Chem Biol 8: 691-9 (2013) Article DOI: 10.1021/cb300623a BindingDB Entry DOI: 10.7270/Q20R9N1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473701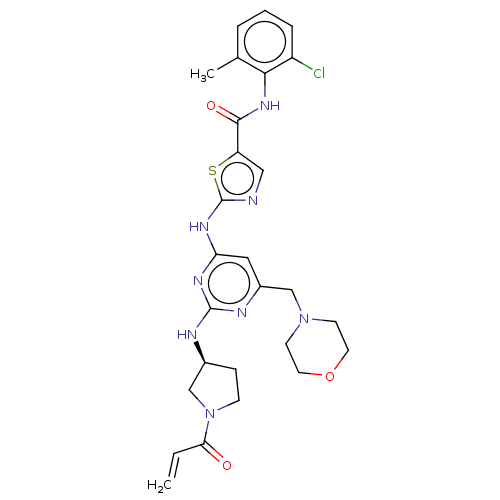 (US10844077, Compound I-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473749 (US10844077, Compound I-4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473748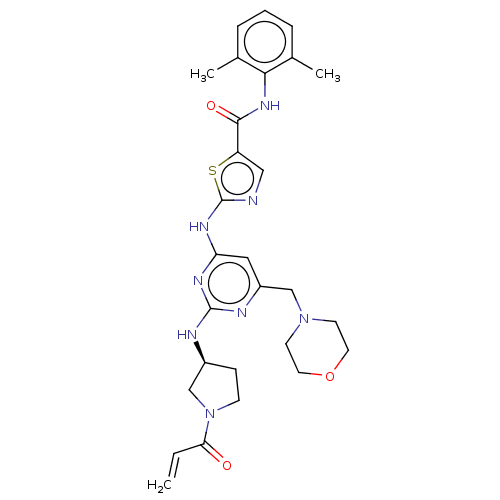 (US10844077, Compound I-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM97672 (US8476284, 40 | US9133201, 10 | US9181263, 9 | US9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473763 (US10844077, Compound II-1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.64 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50451482 (CHEMBL4208645) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay | Bioorg Med Chem Lett 27: 4994-4998 (2017) Article DOI: 10.1016/j.bmcl.2017.10.012 BindingDB Entry DOI: 10.7270/Q2X92DV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Inhibition of human HCK using KVEKIGEGTYGVVYK as substrate by [gamma-33P]-ATP assay | Eur J Med Chem 161: 456-467 (2019) Article DOI: 10.1016/j.ejmech.2018.10.052 BindingDB Entry DOI: 10.7270/Q2W380MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473764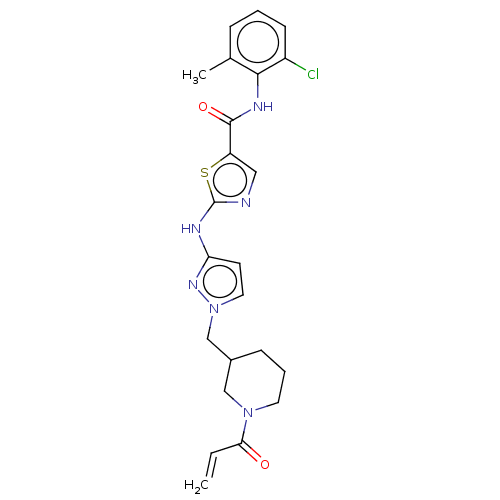 (US10844077, Compound II-2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.94 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142600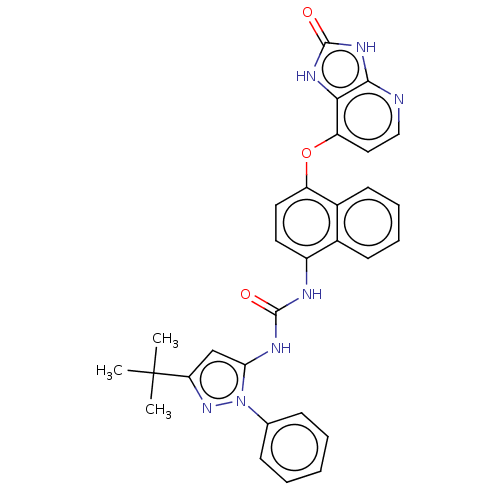 (US8933228, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142607 (US8933228, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142618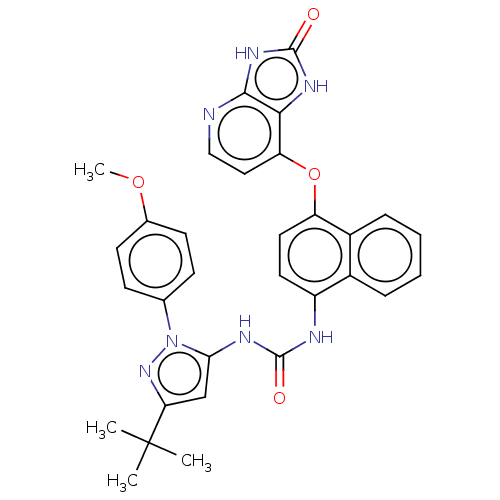 (US8933228, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142599 (US8933228, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM13268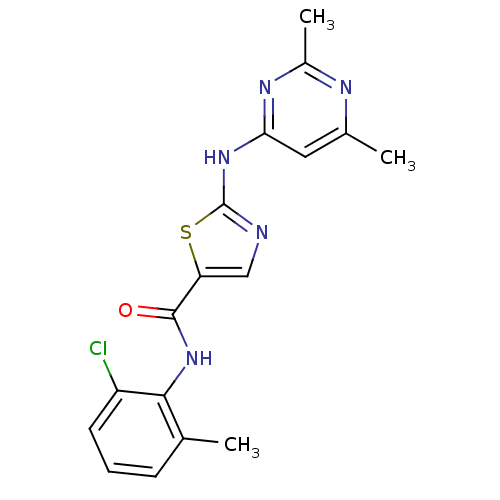 (BMS-354825 2-Heteroarylamino-thiazole Analog 12m |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description inhibitory activity against Hck kinase | Bioorg Med Chem Lett 14: 6061-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.093 BindingDB Entry DOI: 10.7270/Q2FQ9W3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142601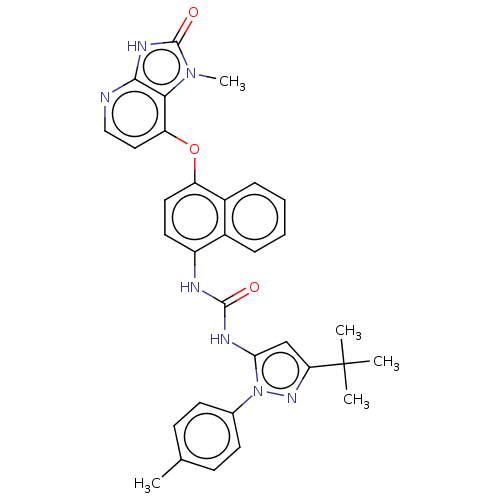 (US8933228, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM473752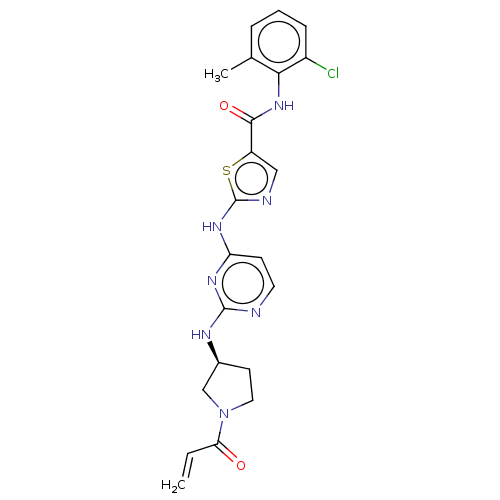 (US10844077, Compound I-10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.42 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The inhibitory activities of exemplary compounds described herein against select protein kinases. | US Patent US10844077 (2020) BindingDB Entry DOI: 10.7270/Q2862KJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM163700 (N-(5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.43 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana-Farber Cancer Institute, Inc. US Patent | Assay Description The in vitro activity of the compounds described herein in inhibiting TAK1, HCK, and other kinases were obtained using an Invitrogen Select Screening... | US Patent US10597387 (2020) BindingDB Entry DOI: 10.7270/Q2ZC85X9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50536679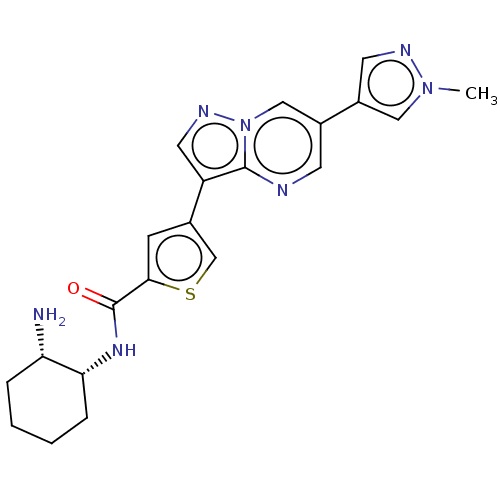 (CHEMBL4568087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck and Co. Curated by ChEMBL | Assay Description Inhibition of full-length human N-terminal GST-tagged HCK expressed in baculovirus expression system by Z'-LYTE assay | Bioorg Med Chem Lett 26: 4362-6 (2016) Article DOI: 10.1016/j.bmcl.2016.02.003 BindingDB Entry DOI: 10.7270/Q2NP27X5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142610 (US8933228, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142628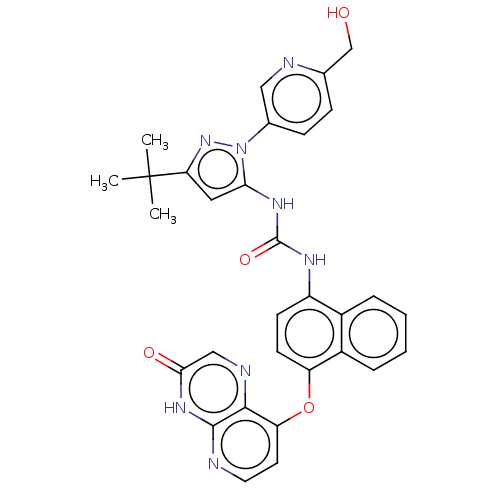 (US8933228, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HCK (230 to 497 residues) using GGMEDIYFEFMGGKKK as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2M90DC6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50514534 (CHEMBL4515441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) | J Med Chem 63: 1624-1641 (2020) Article DOI: 10.1021/acs.jmedchem.9b01502 BindingDB Entry DOI: 10.7270/Q2H998J8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human HCK using KVEKIGEGTYGVVYK as substrate by [gamma-33P]-ATP assay | Citation and Details Article DOI: 10.1016/j.bmc.2018.02.022 BindingDB Entry DOI: 10.7270/Q2DJ5KB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142629 (US8933228, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142619 (US8933228, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142605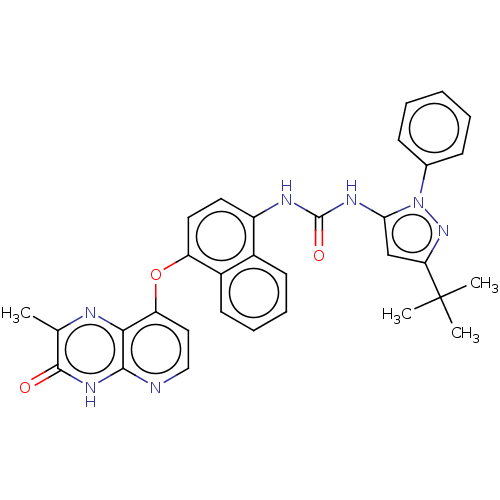 (US8933228, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM378885 (US10266537, Compound 93) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kalypsys, Inc. | Assay Description The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isot... | Bioorg Med Chem Lett 17: 3562-9 (2007) BindingDB Entry DOI: 10.7270/Q21C2065 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142615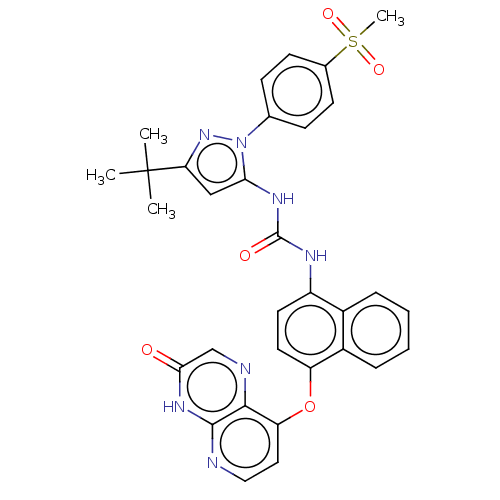 (US8933228, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142598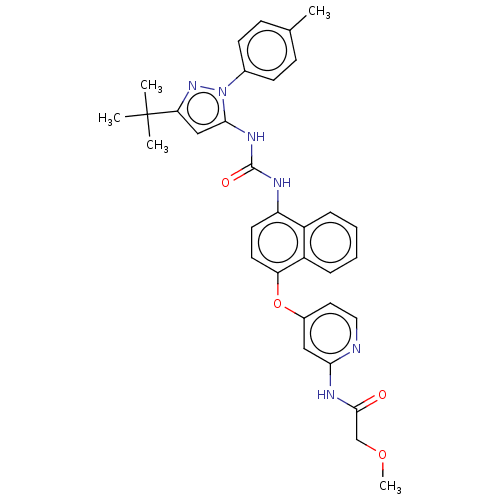 (US10238658, Example Reference | US10813932, Refere...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142603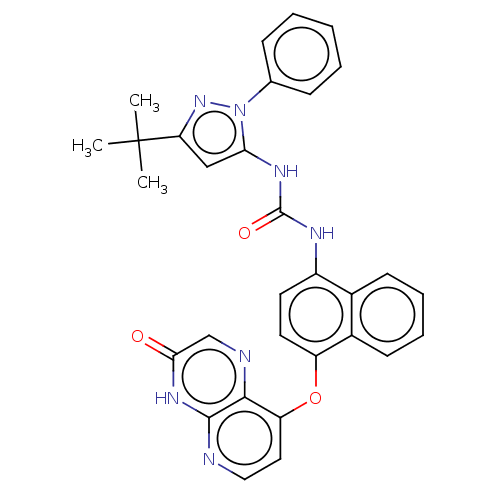 (US8933228, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM142604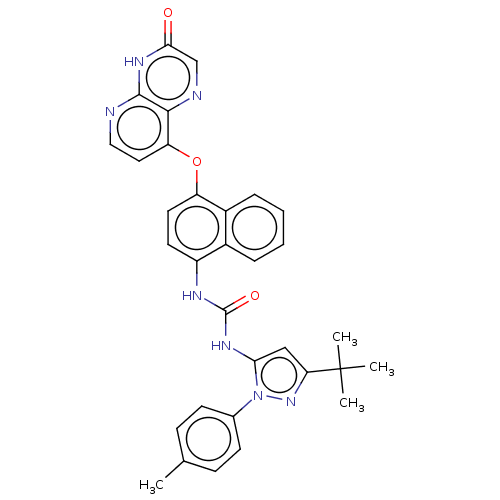 (US8933228, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Respivert, Ltd. US Patent | Assay Description The enzyme inhibitory activity of test compounds was determined by fluorescence resonance energy transfer (FRET) using synthetic peptides labelled wi... | US Patent US8933228 (2015) BindingDB Entry DOI: 10.7270/Q2RV0MDW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM4552 (4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Center for Molecular Medicine of the Austrian Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of HCK | Leukemia 23: 477-85 (2009) Article DOI: 10.1038/leu.2008.334 BindingDB Entry DOI: 10.7270/Q22Z15R6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50268923 (CHEMBL4095434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50268923 (CHEMBL4095434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of HCK (unknown origin) | Bioorg Med Chem 25: 4259-4264 (2017) Article DOI: 10.1016/j.bmc.2017.05.053 BindingDB Entry DOI: 10.7270/Q2SB4875 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50268922 (CHEMBL4066401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
RIKEN Center for Life Science Technologies Curated by ChEMBL | Assay Description Inhibition of recombinant human HCK SH3-SH2-KD (75 to 526 residues) after 20 mins by mobility shift assay | Bioorg Med Chem Lett 27: 4994-4998 (2017) Article DOI: 10.1016/j.bmcl.2017.10.012 BindingDB Entry DOI: 10.7270/Q2X92DV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HCK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2017.12.079 BindingDB Entry DOI: 10.7270/Q2P272TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PHARMACYCLICS LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 μM ATP.). For enzyme in... | US Patent US9181263 (2015) BindingDB Entry DOI: 10.7270/Q2765D5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacyclics LLC US Patent | Assay Description IC50s were determined using the in vitro HotSpot kinase assay (purified enzymes, 33P-ATP, an appropriate substrate and 1 uM ATP.). Reaction condition... | US Patent US9278100 (2016) BindingDB Entry DOI: 10.7270/Q20C4TMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 439 total ) | Next | Last >> |