 Found 2733 hits of ic50 for UniProtKB: Q08499
Found 2733 hits of ic50 for UniProtKB: Q08499 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50017295
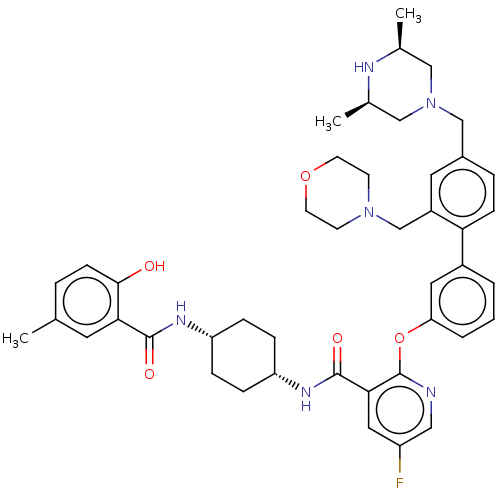
(CHEMBL3288030)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3cc(C)ccc3O)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,53.59,1.0,(46.59,-30.6,;45.26,-29.83,;43.94,-30.6,;42.61,-29.83,;41.28,-30.6,;39.95,-29.84,;38.61,-30.62,;37.28,-29.86,;37.27,-28.31,;38.6,-27.53,;38.58,-25.99,;39.91,-25.21,;41.23,-25.98,;42.56,-25.21,;42.55,-23.67,;41.21,-22.91,;39.88,-23.68,;39.93,-28.29,;35.93,-27.55,;34.59,-28.33,;33.26,-27.56,;33.25,-26.02,;34.59,-25.25,;34.59,-23.7,;33.25,-22.93,;31.91,-23.71,;30.57,-22.93,;30.57,-21.38,;29.23,-20.62,;31.91,-20.61,;33.25,-21.38,;34.58,-20.6,;34.57,-19.06,;35.92,-21.37,;37.25,-20.59,;38.59,-21.37,;39.92,-20.6,;39.93,-19.05,;38.59,-18.28,;37.25,-19.06,;41.27,-18.28,;42.61,-19.05,;42.61,-20.6,;43.94,-18.29,;43.93,-16.76,;45.26,-15.99,;45.25,-14.45,;46.6,-16.76,;46.59,-18.29,;45.26,-19.06,;45.26,-20.6,;35.92,-26.01,;42.59,-28.3,;43.93,-27.53,;43.92,-25.99,;45.26,-28.29,)| Show InChI InChI=1S/C44H53FN6O5/c1-28-7-14-41(52)39(19-28)42(53)48-35-9-11-36(12-10-35)49-43(54)40-22-34(45)23-46-44(40)56-37-6-4-5-32(21-37)38-13-8-31(26-51-24-29(2)47-30(3)25-51)20-33(38)27-50-15-17-55-18-16-50/h4-8,13-14,19-23,29-30,35-36,47,52H,9-12,15-18,24-27H2,1-3H3,(H,48,53)(H,49,54)/t29-,30+,35-,36+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50017294
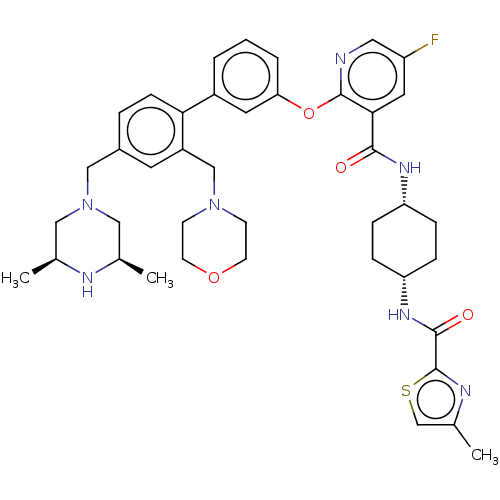
(CHEMBL3288029)Show SMILES C[C@H]1CN(Cc2ccc(c(CN3CCOCC3)c2)-c2cccc(Oc3ncc(F)cc3C(=O)N[C@@H]3CC[C@@H](CC3)NC(=O)c3nc(C)cs3)c2)C[C@@H](C)N1 |r,wU:37.43,34.36,1.0,51.57,(20.55,-53.47,;19.22,-52.7,;17.9,-53.47,;16.57,-52.7,;15.24,-53.48,;13.91,-52.72,;12.58,-53.49,;11.24,-52.73,;11.24,-51.19,;12.56,-50.4,;12.55,-48.86,;13.88,-48.09,;15.21,-48.85,;16.54,-48.08,;16.53,-46.54,;15.2,-45.78,;13.86,-46.55,;13.9,-51.17,;9.9,-50.42,;8.56,-51.21,;7.23,-50.43,;7.22,-48.89,;8.56,-48.13,;8.56,-46.58,;7.22,-45.81,;5.88,-46.59,;4.54,-45.81,;4.54,-44.27,;3.21,-43.5,;5.88,-43.5,;7.22,-44.26,;8.55,-43.49,;8.54,-41.94,;9.88,-44.25,;11.22,-43.48,;12.56,-44.25,;13.89,-43.48,;13.89,-41.94,;12.55,-41.16,;11.21,-41.94,;15.23,-41.16,;16.57,-41.94,;16.57,-43.48,;17.78,-40.99,;19.26,-41.44,;20.12,-40.16,;21.66,-40.11,;19.18,-38.94,;17.73,-39.47,;9.89,-48.88,;16.55,-51.17,;17.88,-50.4,;17.88,-48.87,;19.22,-51.17,)| Show InChI InChI=1S/C41H50FN7O4S/c1-26-21-49(22-27(2)44-26)23-29-7-12-36(31(17-29)24-48-13-15-52-16-14-48)30-5-4-6-35(18-30)53-40-37(19-32(42)20-43-40)38(50)46-33-8-10-34(11-9-33)47-39(51)41-45-28(3)25-54-41/h4-7,12,17-20,25-27,33-34,44H,8-11,13-16,21-24H2,1-3H3,(H,46,50)(H,47,51)/t26-,27+,33-,34+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D using [3H]cAMP by packard topcount scintillation counting analysis |
J Med Chem 57: 4661-76 (2014)
Article DOI: 10.1021/jm5001216
BindingDB Entry DOI: 10.7270/Q2708302 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human PDE4D by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02170
BindingDB Entry DOI: 10.7270/Q2W95DVV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PDE4D catalytic domain expressed in Escherichia coli BL21-CodonPlus(DE3) cells using [3H]cAMP or [3H]cGMP as substrate... |
J Med Chem 59: 7029-65 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01813
BindingDB Entry DOI: 10.7270/Q2RX9GJ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PDE4D catalytic domain expressed in Escherichia coli BL21-CodonPlus(DE3) cells using [3H]cAMP or [3H]cGMP as substrate... |
J Med Chem 59: 7029-65 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01813
BindingDB Entry DOI: 10.7270/Q2RX9GJ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14775

(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem 18: 2204-18 (2010)
Article DOI: 10.1016/j.bmc.2010.01.070
BindingDB Entry DOI: 10.7270/Q27945MJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183805
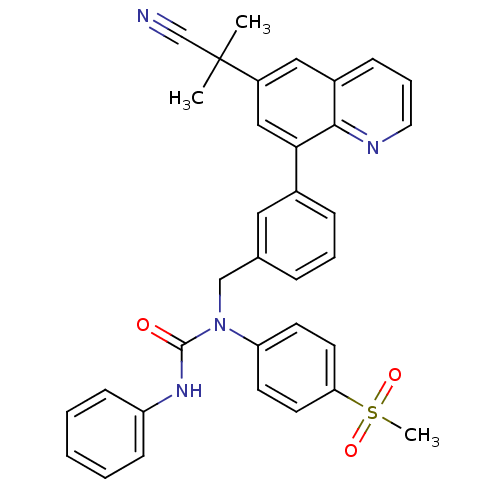
(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50353703
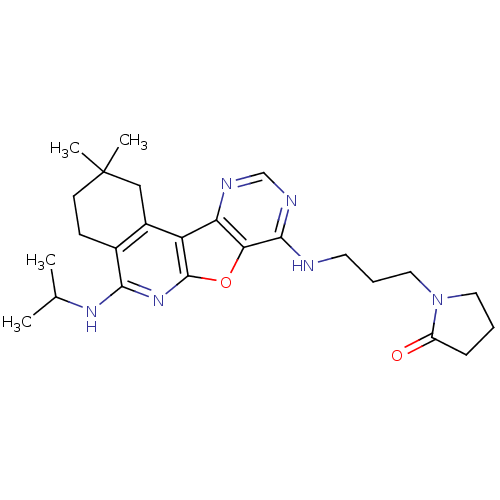
(CHEMBL1830646)Show SMILES CC(C)Nc1nc2oc3c(NCCCN4CCCC4=O)ncnc3c2c2CC(C)(C)CCc12 Show InChI InChI=1S/C25H34N6O2/c1-15(2)29-22-16-8-9-25(3,4)13-17(16)19-20-21(33-24(19)30-22)23(28-14-27-20)26-10-6-12-31-11-5-7-18(31)32/h14-15H,5-13H2,1-4H3,(H,29,30)(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183803

(CHEMBL206968 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES Cc1cc(no1)C(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H28N4O4S/c1-21-15-29(35-40-21)31(37)36(26-10-12-27(13-11-26)41(4,38)39)19-22-7-5-8-23(16-22)28-18-25(32(2,3)20-33)17-24-9-6-14-34-30(24)28/h5-18H,19H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183794
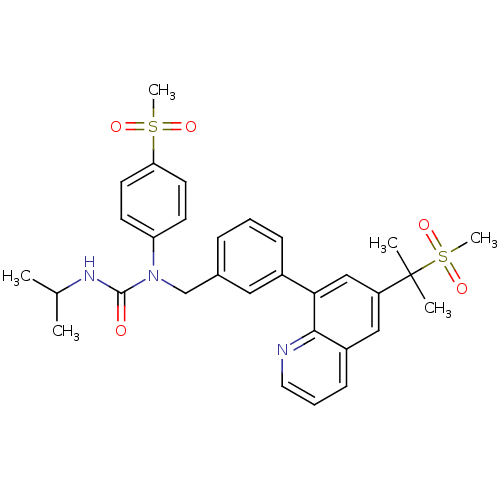
(3-isopropyl-1-(4-(methylsulfonyl)phenyl)-1-((3-(6-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H35N3O5S2/c1-21(2)33-30(35)34(26-12-14-27(15-13-26)40(5,36)37)20-22-9-7-10-23(17-22)28-19-25(31(3,4)41(6,38)39)18-24-11-8-16-32-29(24)28/h7-19,21H,20H2,1-6H3,(H,33,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183792

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N4O3S/c1-21(2)34-30(36)35(26-11-13-27(14-12-26)39(5,37)38)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3,(H,34,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PDE4D using [3H]cAMP as substrate preincubated with enzyme for 10 mins followed by substrate addition and measured af... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112492
BindingDB Entry DOI: 10.7270/Q2R21511 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174025
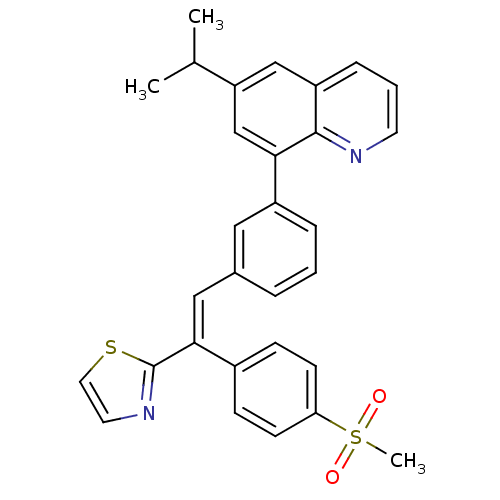
(6-isopropyl-8-(3-(2-(4-(methylsulfonyl)phenyl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nccs3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H26N2O2S2/c1-20(2)25-18-24-8-5-13-31-29(24)27(19-25)23-7-4-6-21(16-23)17-28(30-32-14-15-35-30)22-9-11-26(12-10-22)36(3,33)34/h4-20H,1-3H3/b28-17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174030
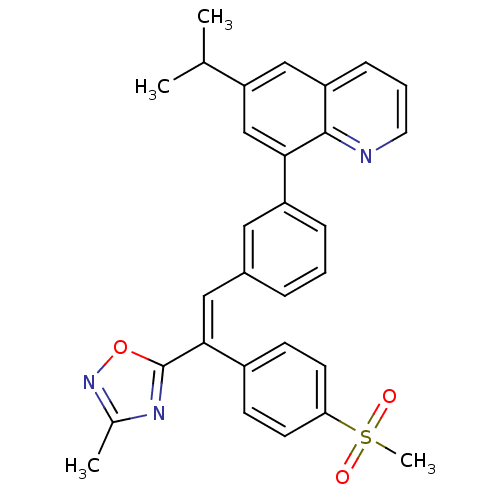
((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H27N3O3S/c1-19(2)25-17-24-9-6-14-31-29(24)27(18-25)23-8-5-7-21(15-23)16-28(30-32-20(3)33-36-30)22-10-12-26(13-11-22)37(4,34)35/h5-19H,1-4H3/b28-16+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50325788
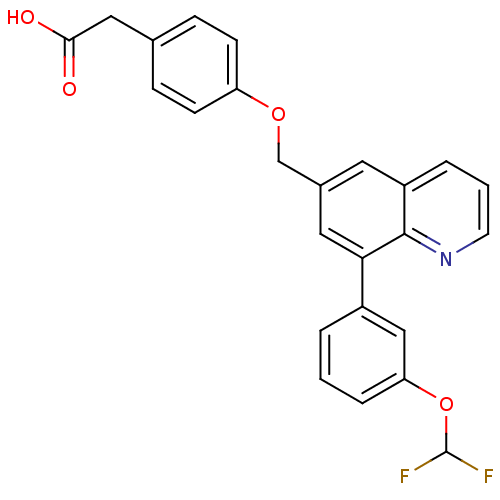
(2-(4-((8-(3-(difluoromethoxy)phenyl)quinolin-6-yl)...)Show SMILES OC(=O)Cc1ccc(OCc2cc(-c3cccc(OC(F)F)c3)c3ncccc3c2)cc1 Show InChI InChI=1S/C25H19F2NO4/c26-25(27)32-21-5-1-3-18(14-21)22-12-17(11-19-4-2-10-28-24(19)22)15-31-20-8-6-16(7-9-20)13-23(29)30/h1-12,14,25H,13,15H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 5502-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.076
BindingDB Entry DOI: 10.7270/Q2K35TVZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hyderabad Campus
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D (unknown origin) |
Eur J Med Chem 174: 198-215 (2019)
Article DOI: 10.1016/j.ejmech.2019.04.020
BindingDB Entry DOI: 10.7270/Q2G44TQ6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174028
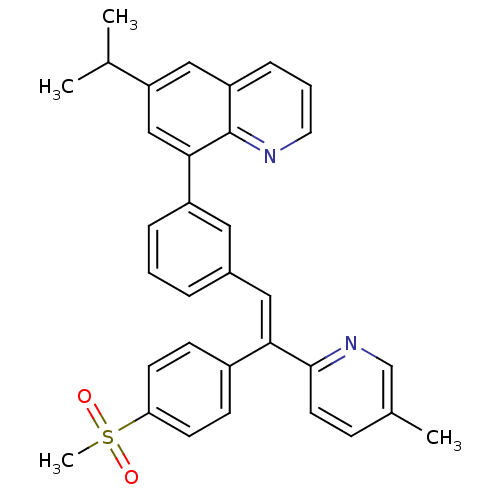
((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183808

(CHEMBL383225 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)c3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H29N3O3S/c1-34(2,23-35)28-20-27-13-8-18-36-32(27)31(21-28)26-12-7-9-24(19-26)22-37(33(38)25-10-5-4-6-11-25)29-14-16-30(17-15-29)41(3,39)40/h4-21H,22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183791
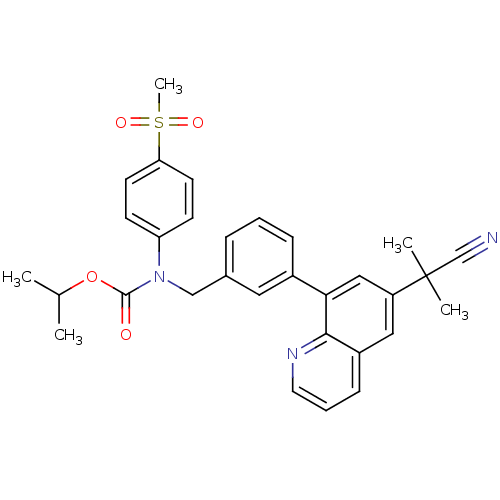
(CHEMBL209295 | isopropyl (3-(6-(2-cyanopropan-2-yl...)Show SMILES CC(C)OC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H31N3O4S/c1-21(2)38-30(35)34(26-11-13-27(14-12-26)39(5,36)37)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50304417
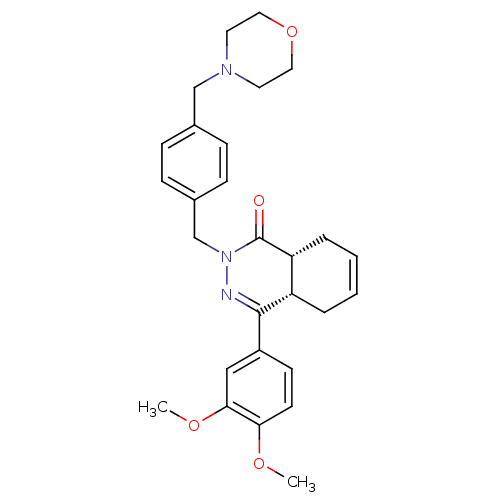
(CHEMBL593656 | CHEMBL593877 | cis-(+/-)-4-(3,4-Dim...)Show SMILES COc1ccc(cc1OC)C1=NN(Cc2ccc(CN3CCOCC3)cc2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:34,t:11| Show InChI InChI=1S/C28H33N3O4/c1-33-25-12-11-22(17-26(25)34-2)27-23-5-3-4-6-24(23)28(32)31(29-27)19-21-9-7-20(8-10-21)18-30-13-15-35-16-14-30/h3-4,7-12,17,23-24H,5-6,13-16,18-19H2,1-2H3/t23-,24+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis |
Bioorg Med Chem 17: 6959-70 (2009)
Article DOI: 10.1016/j.bmc.2009.08.014
BindingDB Entry DOI: 10.7270/Q2WH2Q26 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347344
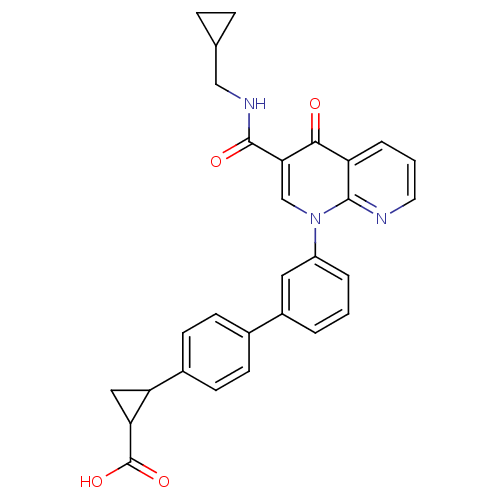
(CHEMBL1801156)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-5-2-12-30-27(22)32(16-25(26)28(34)31-15-17-6-7-17)21-4-1-3-20(13-21)18-8-10-19(11-9-18)23-14-24(23)29(35)36/h1-5,8-13,16-17,23-24H,6-7,14-15H2,(H,31,34)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50017340
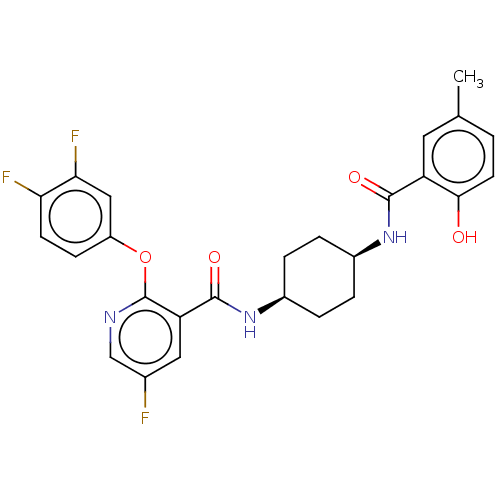
(CHEMBL3113734)Show SMILES Cc1ccc(O)c(c1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1ccc(F)c(F)c1 |r,wU:14.18,11.11,(24.27,-11.72,;24.28,-13.26,;25.62,-14.03,;25.62,-15.57,;24.29,-16.34,;24.28,-17.88,;22.96,-15.57,;22.95,-14.04,;21.63,-16.34,;21.63,-17.88,;20.29,-15.57,;18.96,-16.34,;18.96,-17.88,;17.63,-18.65,;16.29,-17.87,;16.28,-16.34,;17.62,-15.57,;14.96,-18.65,;13.62,-17.88,;13.62,-16.34,;12.29,-18.66,;10.95,-17.9,;9.63,-18.67,;8.29,-17.9,;9.62,-20.21,;10.96,-20.98,;12.3,-20.21,;13.63,-20.98,;13.63,-22.52,;12.3,-23.29,;12.3,-24.83,;13.64,-25.6,;13.64,-27.14,;14.97,-24.81,;16.31,-25.58,;14.96,-23.28,)| Show InChI InChI=1S/C26H24F3N3O4/c1-14-2-9-23(33)19(10-14)24(34)31-16-3-5-17(6-4-16)32-25(35)20-11-15(27)13-30-26(20)36-18-7-8-21(28)22(29)12-18/h2,7-13,16-17,33H,3-6H2,1H3,(H,31,34)(H,32,35)/t16-,17+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4D (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02170
BindingDB Entry DOI: 10.7270/Q2W95DVV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D (unknown origin) using cAMP as substrate |
J Med Chem 62: 5579-5593 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00518
BindingDB Entry DOI: 10.7270/Q2S75KRZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Guangzhou University of Chinese Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D2 (unknown origin) |
J Med Chem 63: 3370-3380 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00060
BindingDB Entry DOI: 10.7270/Q20Z76QT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02058
BindingDB Entry DOI: 10.7270/Q2DF6W9W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14359

((+)-1 | (S)-(+)-3-{2-[(3-Cyclopropyloxy-4-diflurom...)Show SMILES OC(c1cnc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)19-32-10-18(38-19)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of North Carolina at Chapel Hill
| Assay Description
PDE4 catalytic activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using PDE-SPA kit (Amersham International). [3H]-AMP was c... |
J Med Chem 49: 1867-73 (2006)
Article DOI: 10.1021/jm051273d
BindingDB Entry DOI: 10.7270/Q2MK6B48 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50304410

(CHEMBL594108 | cis-2-[(E)-4-(1H-Imidazol-1-yl)but-...)Show SMILES COc1ccc(cc1OC)C1=NN(C\C=C\Cn2ccnc2)C(=O)[C@@H]2CC=CC[C@H]12 |r,c:28,t:11| Show InChI InChI=1S/C23H26N4O3/c1-29-20-10-9-17(15-21(20)30-2)22-18-7-3-4-8-19(18)23(28)27(25-22)13-6-5-12-26-14-11-24-16-26/h3-6,9-11,14-16,18-19H,7-8,12-13H2,1-2H3/b6-5+/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis |
Bioorg Med Chem 17: 6959-70 (2009)
Article DOI: 10.1016/j.bmc.2009.08.014
BindingDB Entry DOI: 10.7270/Q2WH2Q26 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50325790
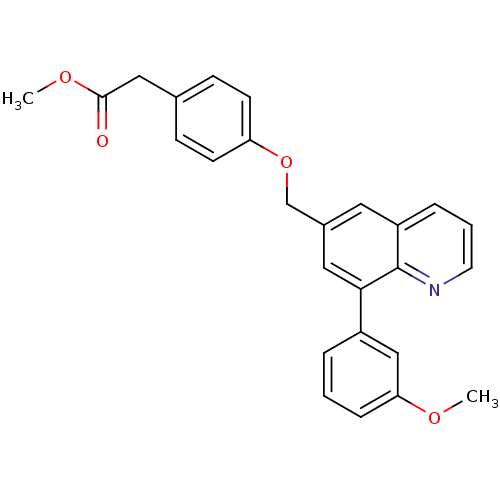
(CHEMBL1224717 | methyl 2-(4-((8-(3-methoxyphenyl)q...)Show SMILES COC(=O)Cc1ccc(OCc2cc(-c3cccc(OC)c3)c3ncccc3c2)cc1 Show InChI InChI=1S/C26H23NO4/c1-29-23-7-3-5-20(16-23)24-14-19(13-21-6-4-12-27-26(21)24)17-31-22-10-8-18(9-11-22)15-25(28)30-2/h3-14,16H,15,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
Bioorg Med Chem Lett 20: 5502-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.076
BindingDB Entry DOI: 10.7270/Q2K35TVZ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50353700

(CHEMBL1830643)Show SMILES CC(C)Nc1nc2oc3c(NCCN4CCOCC4)ncnc3c2c2CC(C)(C)CCc12 Show InChI InChI=1S/C24H34N6O2/c1-15(2)28-21-16-5-6-24(3,4)13-17(16)18-19-20(32-23(18)29-21)22(27-14-26-19)25-7-8-30-9-11-31-12-10-30/h14-15H,5-13H2,1-4H3,(H,28,29)(H,25,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174020
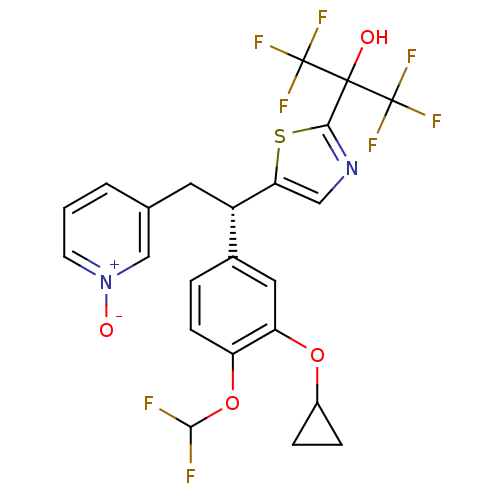
((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347349
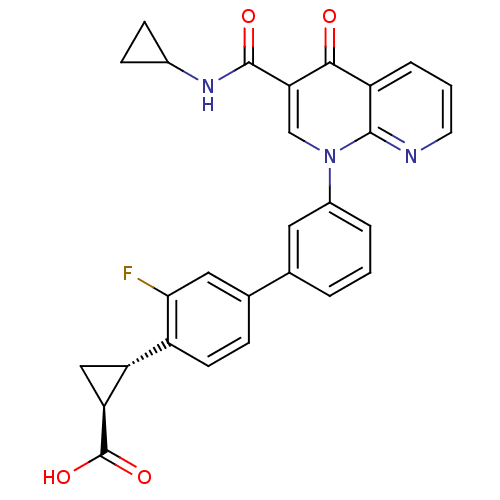
(CHEMBL1801161)Show SMILES OC(=O)[C@H]1C[C@@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50532272
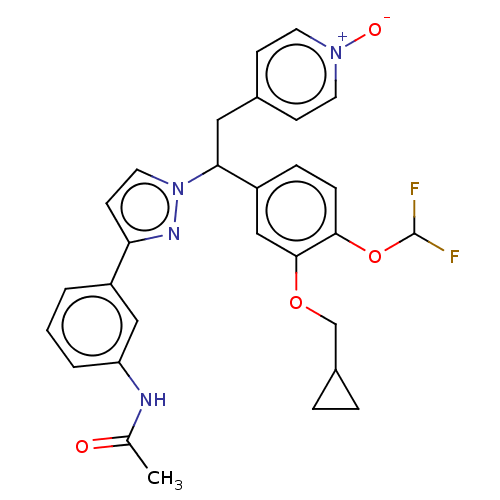
(CHEMBL4534321)Show SMILES CC(=O)Nc1cccc(c1)-c1ccn(n1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OCC2CC2)c1 Show InChI InChI=1S/C29H28F2N4O4/c1-19(36)32-24-4-2-3-22(16-24)25-11-14-35(33-25)26(15-20-9-12-34(37)13-10-20)23-7-8-27(39-29(30)31)28(17-23)38-18-21-5-6-21/h2-4,7-14,16-17,21,26,29H,5-6,15,18H2,1H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D (unknown origin) |
J Med Chem 59: 7029-65 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01813
BindingDB Entry DOI: 10.7270/Q2RX9GJ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174021
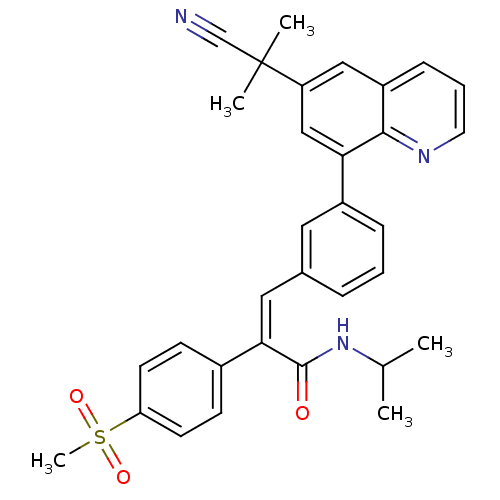
((Z)-3-(3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phen...)Show SMILES CC(C)NC(=O)C(=C/c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H31N3O3S/c1-21(2)35-31(36)29(23-11-13-27(14-12-23)39(5,37)38)17-22-8-6-9-24(16-22)28-19-26(32(3,4)20-33)18-25-10-7-15-34-30(25)28/h6-19,21H,1-5H3,(H,35,36)/b29-17- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174031
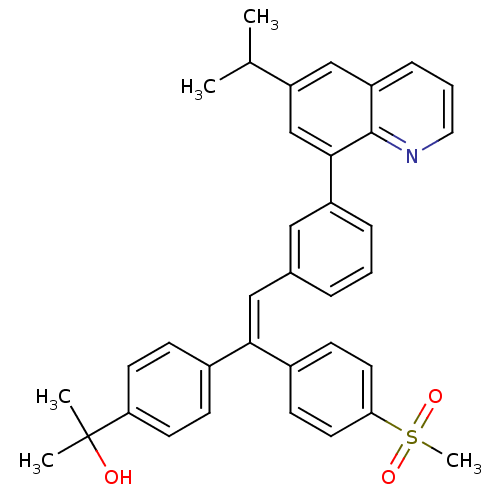
((Z)-2-(4-(2-(3-(6-isopropylquinolin-8-yl)phenyl)-1...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3ccc(cc3)C(C)(C)O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C36H35NO3S/c1-24(2)30-22-29-10-7-19-37-35(29)34(23-30)28-9-6-8-25(20-28)21-33(26-11-15-31(16-12-26)36(3,4)38)27-13-17-32(18-14-27)41(5,39)40/h6-24,38H,1-5H3/b33-21- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50174013
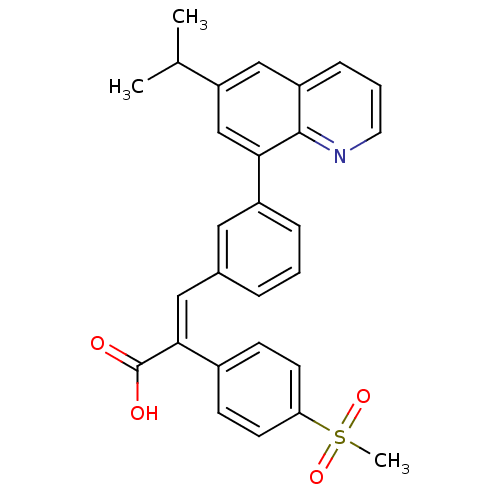
((E)-3-(3-(6-isopropylquinolin-8-yl)phenyl)-2-(4-(m...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\C(O)=O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C28H25NO4S/c1-18(2)23-16-22-8-5-13-29-27(22)25(17-23)21-7-4-6-19(14-21)15-26(28(30)31)20-9-11-24(12-10-20)34(3,32)33/h4-18H,1-3H3,(H,30,31)/b26-15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4D |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50353706
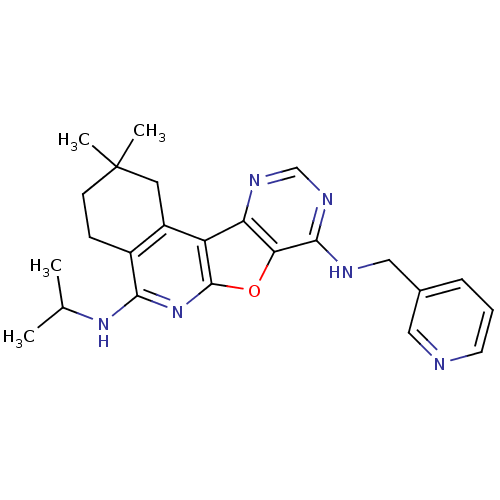
(CHEMBL1828652)Show SMILES CC(C)Nc1nc2oc3c(NCc4cccnc4)ncnc3c2c2CC(C)(C)CCc12 Show InChI InChI=1S/C24H28N6O/c1-14(2)29-21-16-7-8-24(3,4)10-17(16)18-19-20(31-23(18)30-21)22(28-13-27-19)26-12-15-6-5-9-25-11-15/h5-6,9,11,13-14H,7-8,10,12H2,1-4H3,(H,29,30)(H,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50532272

(CHEMBL4534321)Show SMILES CC(=O)Nc1cccc(c1)-c1ccn(n1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OCC2CC2)c1 Show InChI InChI=1S/C29H28F2N4O4/c1-19(36)32-24-4-2-3-22(16-24)25-11-14-35(33-25)26(15-20-9-12-34(37)13-10-20)23-7-8-27(39-29(30)31)28(17-23)38-18-21-5-6-21/h2-4,7-14,16-17,21,26,29H,5-6,15,18H2,1H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D (unknown origin) |
J Med Chem 59: 7029-65 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01813
BindingDB Entry DOI: 10.7270/Q2RX9GJ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50601869
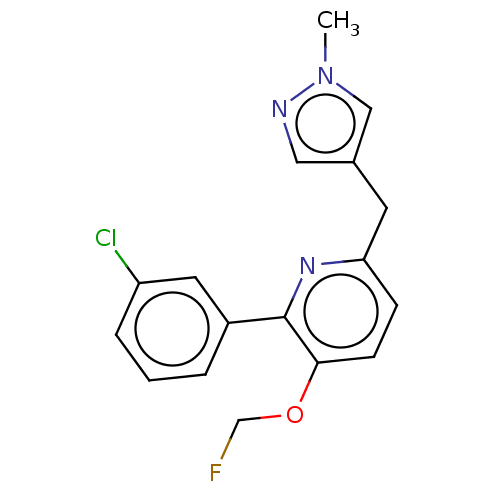
(CHEMBL5184286)Show SMILES [11CH3]n1cc(Cc2ccc(OCF)c(n2)-c2cccc(Cl)c2)cn1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01571
BindingDB Entry DOI: 10.7270/Q25H7MBX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183795
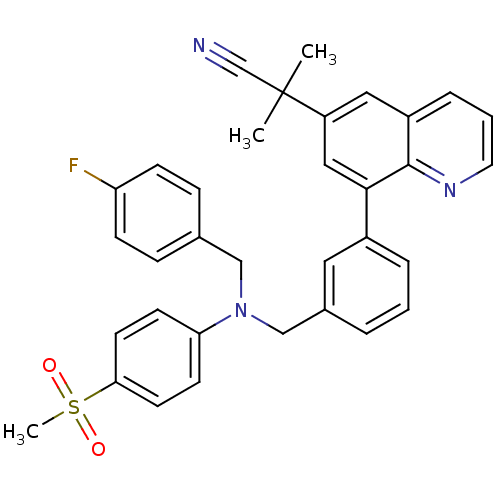
(2-(8-(3-(((4-fluorobenzyl)(4-(methylsulfonyl)pheny...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(Cc3ccc(F)cc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30FN3O2S/c1-34(2,23-36)28-19-27-8-5-17-37-33(27)32(20-28)26-7-4-6-25(18-26)22-38(21-24-9-11-29(35)12-10-24)30-13-15-31(16-14-30)41(3,39)40/h4-20H,21-22H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50183804
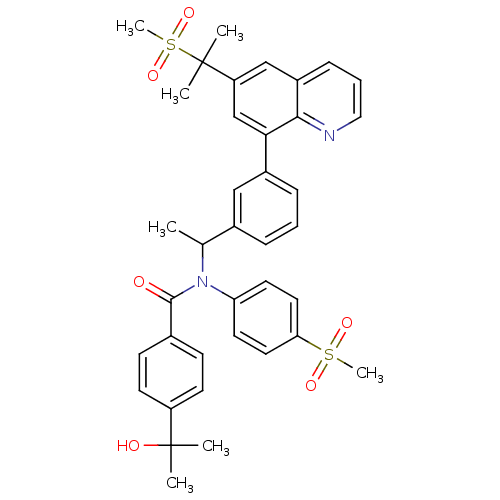
(4-(2-hydroxypropan-2-yl)-N-(4-(methylsulfonyl)phen...)Show SMILES CC(N(C(=O)c1ccc(cc1)C(C)(C)O)c1ccc(cc1)S(C)(=O)=O)c1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C38H40N2O6S2/c1-25(40(32-17-19-33(20-18-32)47(6,43)44)36(41)26-13-15-30(16-14-26)37(2,3)42)27-10-8-11-28(22-27)34-24-31(38(4,5)48(7,45)46)23-29-12-9-21-39-35(29)34/h8-25,42H,1-7H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4D |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347343
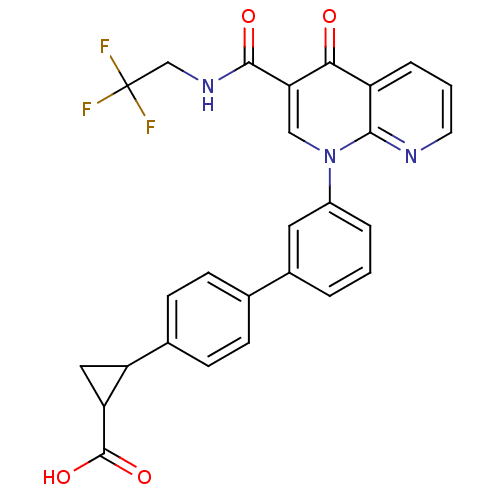
(CHEMBL1801155)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC(F)(F)F)c(=O)c2cccnc12 Show InChI InChI=1S/C27H20F3N3O4/c28-27(29,30)14-32-25(35)22-13-33(24-19(23(22)34)5-2-10-31-24)18-4-1-3-17(11-18)15-6-8-16(9-7-15)20-12-21(20)26(36)37/h1-11,13,20-21H,12,14H2,(H,32,35)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347345
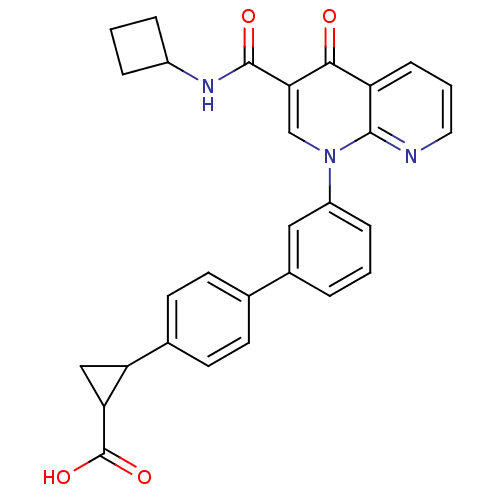
(CHEMBL1801157)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NC2CCC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-8-3-13-30-27(22)32(16-25(26)28(34)31-20-5-2-6-20)21-7-1-4-19(14-21)17-9-11-18(12-10-17)23-15-24(23)29(35)36/h1,3-4,7-14,16,20,23-24H,2,5-6,15H2,(H,31,34)(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347348
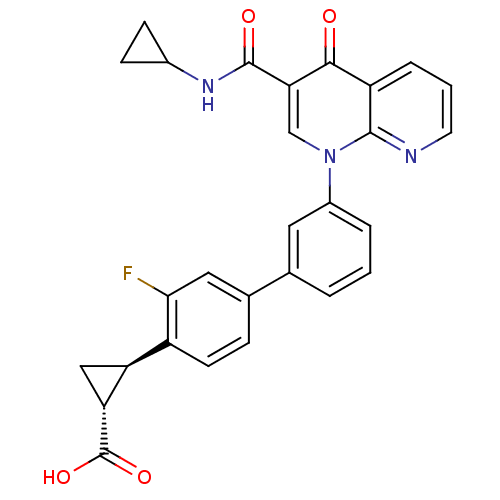
(CHEMBL1801160)Show SMILES OC(=O)[C@@H]1C[C@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50232731
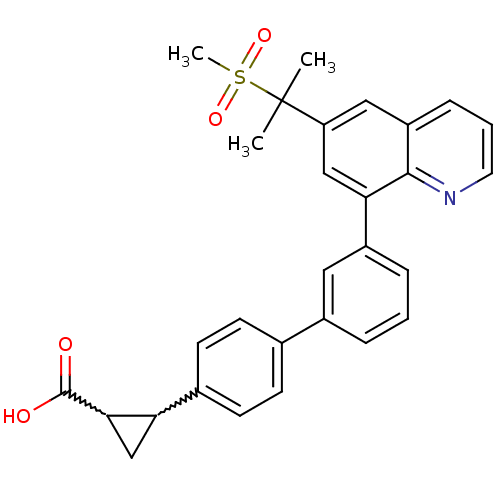
(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347335
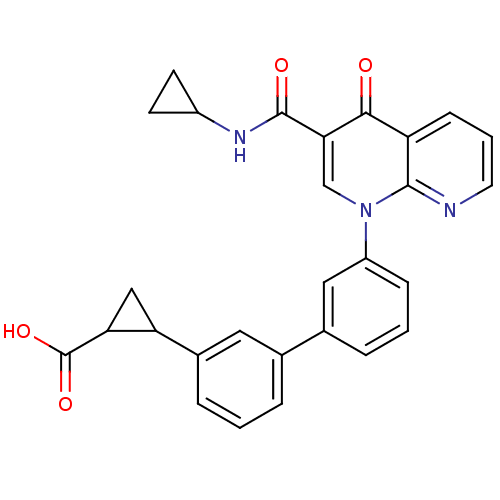
(CHEMBL1801068)Show SMILES OC(=O)C1CC1c1cccc(c1)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C28H23N3O4/c32-25-21-8-3-11-29-26(21)31(15-24(25)27(33)30-19-9-10-19)20-7-2-5-17(13-20)16-4-1-6-18(12-16)22-14-23(22)28(34)35/h1-8,11-13,15,19,22-23H,9-10,14H2,(H,30,33)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50232731

(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50347339
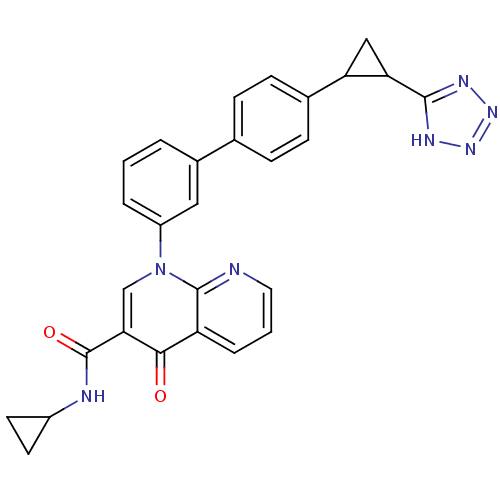
(CHEMBL1801151)Show SMILES O=C(NC1CC1)c1cn(-c2cccc(c2)-c2ccc(cc2)C2CC2c2nnn[nH]2)c2ncccc2c1=O Show InChI InChI=1S/C28H23N7O2/c36-25-21-5-2-12-29-27(21)35(15-24(25)28(37)30-19-10-11-19)20-4-1-3-18(13-20)16-6-8-17(9-7-16)22-14-23(22)26-31-33-34-32-26/h1-9,12-13,15,19,22-23H,10-11,14H2,(H,30,37)(H,31,32,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4D |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Cairo University
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D by ELISA |
Eur J Med Chem 148: 477-486 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.038
BindingDB Entry DOI: 10.7270/Q22Z185T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data