

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231167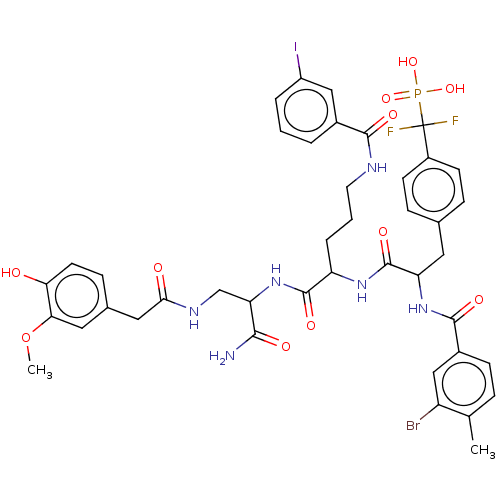 (US9340574, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 34 | n/a | 75 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231166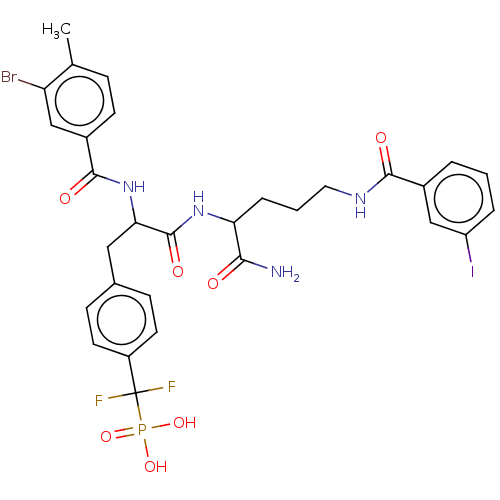 (US9340574, 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50531902 (CHEMBL4569405) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged MEG2 catalytic domain (285 to 593 residues) expressed in Escherichia coli using p-nitrophenol a... | Bioorg Med Chem Lett 29: 797-801 (2019) Article DOI: 10.1016/j.bmcl.2019.01.026 BindingDB Entry DOI: 10.7270/Q2NC64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50436358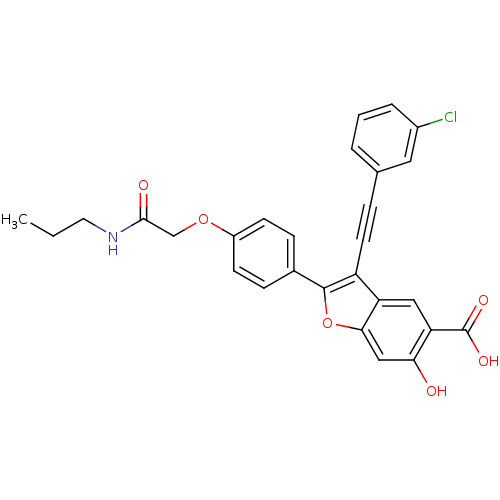 (CHEMBL2396718) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTP-MEG2 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 [277-582] (Homo sapiens (Human)) | BDBM231165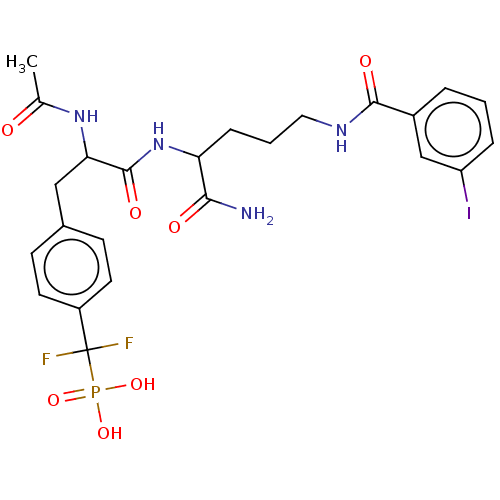 (US9340574, 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Indiana University Research and Technology Corporation US Patent | Assay Description PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in 3,3-dimethylglutarate buffer (50 mM 3,3-dimethylglutarate, pH 7.0, 1 ... | US Patent US9340574 (2016) BindingDB Entry DOI: 10.7270/Q2NV9H4J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425815 (CHEMBL2316908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425808 (CHEMBL2316906) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50531903 (CHEMBL4530344) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged MEG2 catalytic domain (285 to 593 residues) expressed in Escherichia coli using p-nitrophenol a... | Bioorg Med Chem Lett 29: 797-801 (2019) Article DOI: 10.1016/j.bmcl.2019.01.026 BindingDB Entry DOI: 10.7270/Q2NC64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50436357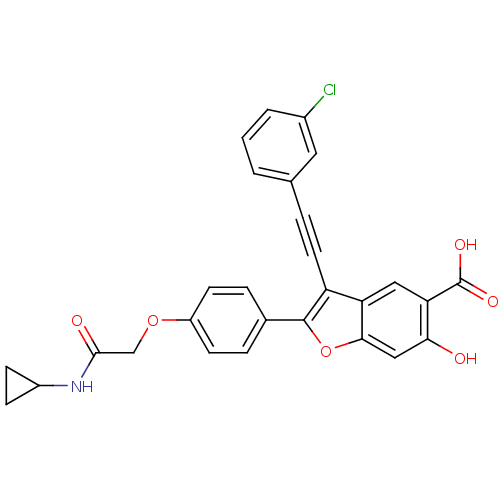 (CHEMBL2396719) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of recombinant PTP-MEG2 (unknown origin) using pNPP as substrate by spectrophotometric analysis | J Med Chem 56: 4990-5008 (2013) Article DOI: 10.1021/jm400248c BindingDB Entry DOI: 10.7270/Q2N017XW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425806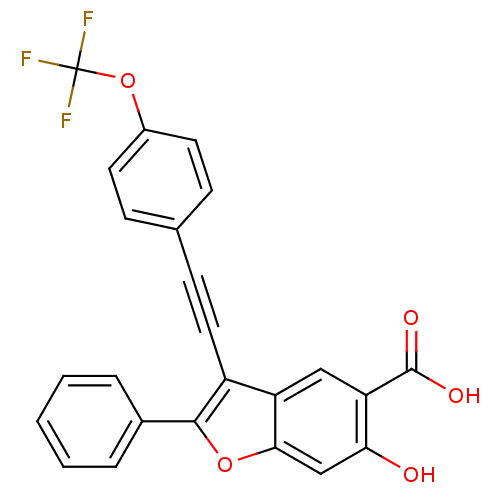 (CHEMBL2316907) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425818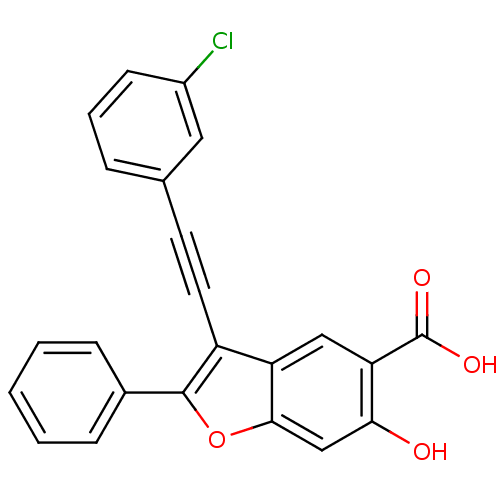 (CHEMBL2316905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425807 (CHEMBL2316902) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425816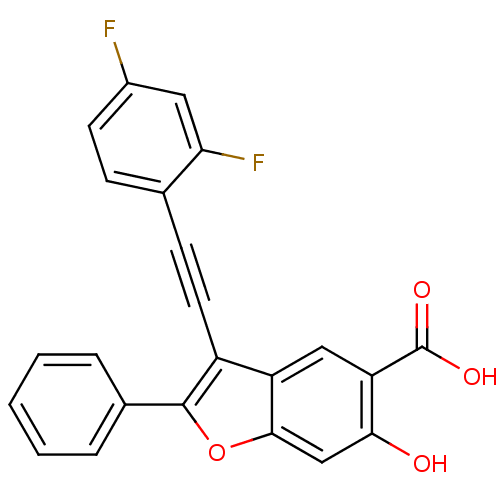 (CHEMBL2316910) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50531901 (CHEMBL4540813) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
V.P. Kukhar Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged MEG2 catalytic domain (285 to 593 residues) expressed in Escherichia coli using p-nitrophenol a... | Bioorg Med Chem Lett 29: 797-801 (2019) Article DOI: 10.1016/j.bmcl.2019.01.026 BindingDB Entry DOI: 10.7270/Q2NC64P6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425809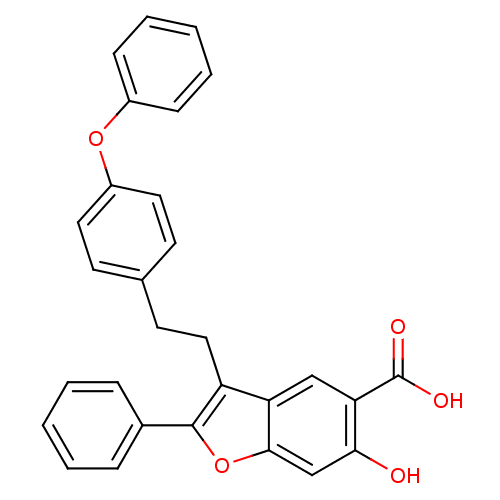 (CHEMBL2316896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425810 (CHEMBL2316895) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425811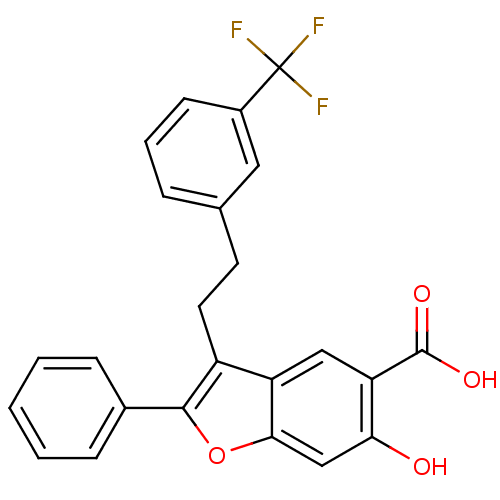 (CHEMBL2316894) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425819 (CHEMBL2316904) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425814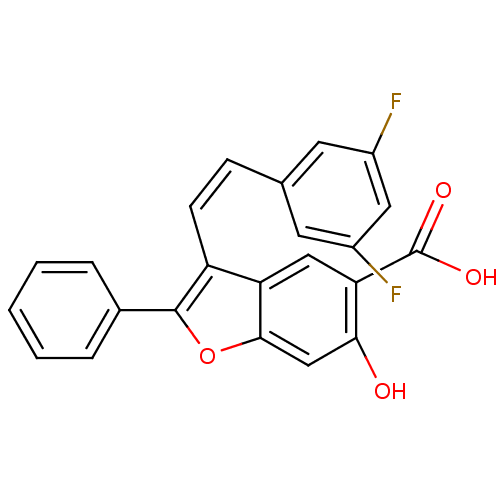 (CHEMBL2311593) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50031906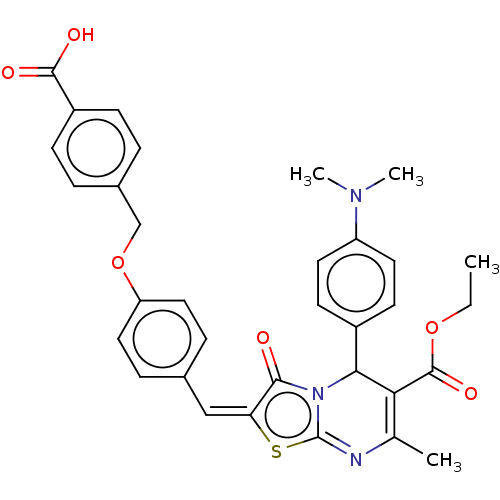 (CHEMBL3360905) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425813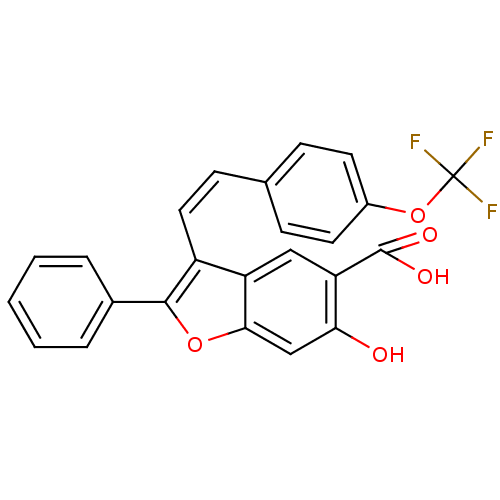 (CHEMBL2316897) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50524069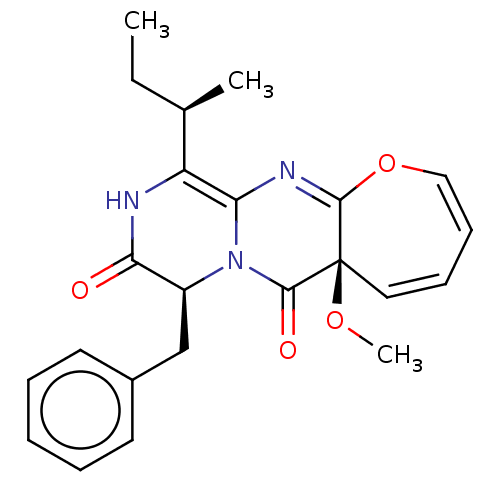 (CHEMBL4454177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human MEG2 expressed in Escherichia coli using pNPP as substrate preincubated for 15 mins followed by substrate addition an... | J Nat Prod 82: 1558-1564 (2019) Article DOI: 10.1021/acs.jnatprod.9b00055 BindingDB Entry DOI: 10.7270/Q2Z89GVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50524067 (CHEMBL4514865) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human MEG2 expressed in Escherichia coli using pNPP as substrate preincubated for 15 mins followed by substrate addition an... | J Nat Prod 82: 1558-1564 (2019) Article DOI: 10.1021/acs.jnatprod.9b00055 BindingDB Entry DOI: 10.7270/Q2Z89GVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50524068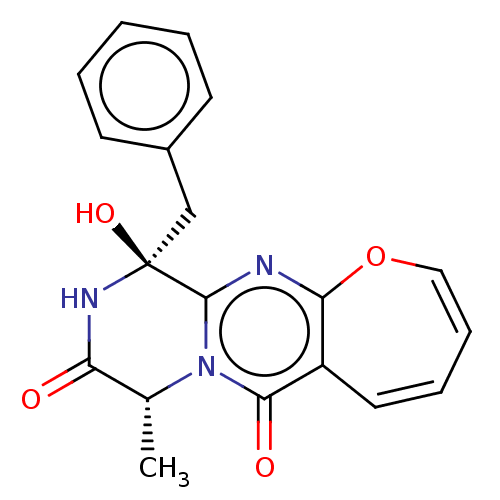 (CHEMBL4453288) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant human MEG2 expressed in Escherichia coli using pNPP as substrate preincubated for 15 mins followed by substrate addition an... | J Nat Prod 82: 1558-1564 (2019) Article DOI: 10.1021/acs.jnatprod.9b00055 BindingDB Entry DOI: 10.7270/Q2Z89GVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425820 (CHEMBL2316903) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50544440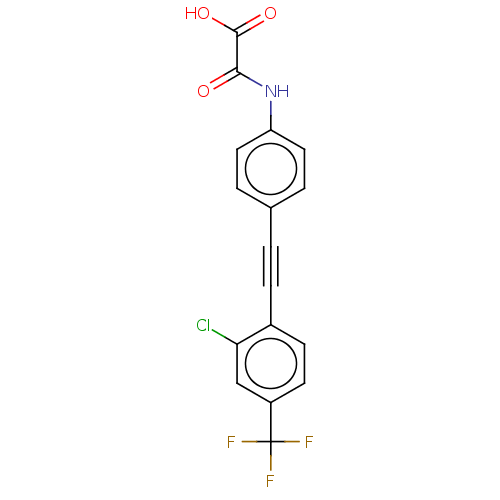 (CHEMBL4647367 | US11192850, Entry 4t) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTP-MEG2 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vi... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425817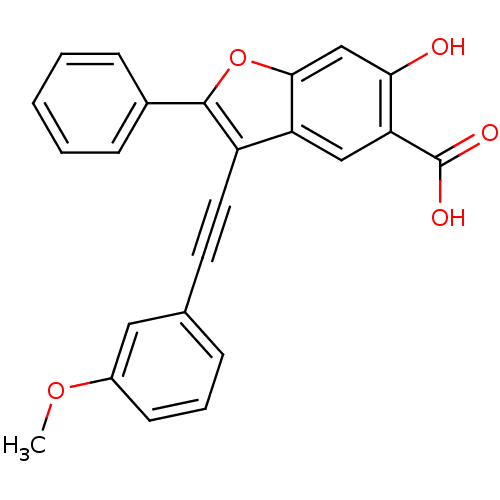 (CHEMBL2316909) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50544431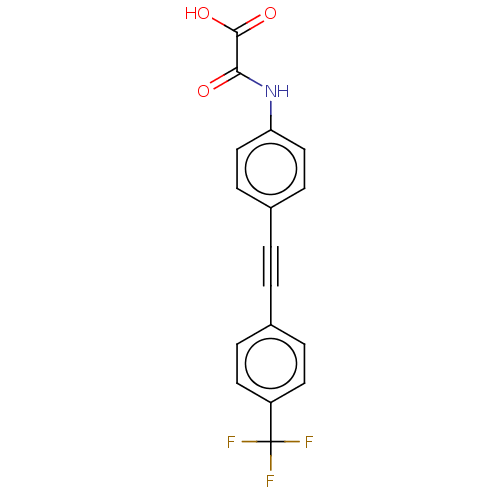 (CHEMBL4637459 | US11192850, Entry 4k) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTP-MEG2 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vi... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50335895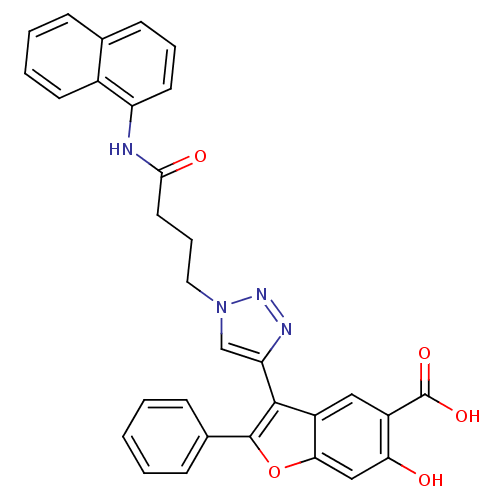 (6-hydroxy-3-(1-(4-(naphthalen-1-ylamino)-4-oxobuty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTP-Meg2 | Proc Natl Acad Sci USA 104: 19767-72 (2007) Article DOI: 10.1073/pnas.0706233104 BindingDB Entry DOI: 10.7270/Q23F4QJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50031908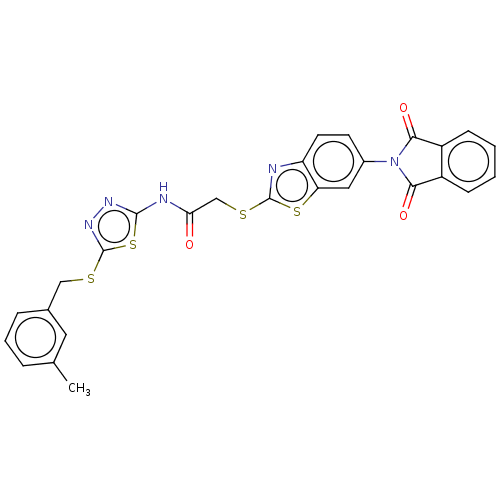 (CHEMBL3360906) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50544427 (CHEMBL4632818 | US11192850, Entry 4g) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of PTP-MEG2 (unknown origin) expressed in Escherichia coli BL21 using p-nitrophenyl phosphate as substrate measured after 30 mins by UV-vi... | J Med Chem 63: 9212-9227 (2020) Article DOI: 10.1021/acs.jmedchem.0c00302 BindingDB Entry DOI: 10.7270/Q2QV3R3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50343142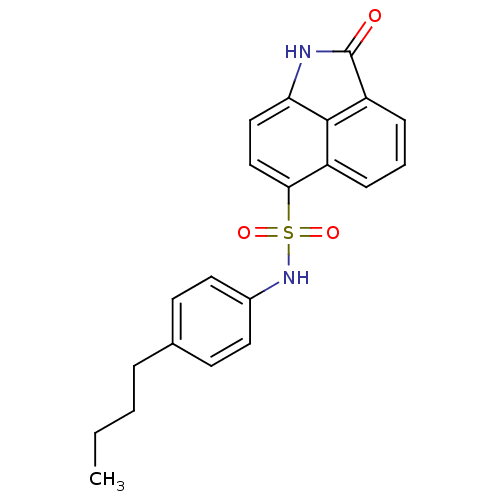 (CHEMBL1773168 | N-(4-butylphenyl)-2-oxo-1,2-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human MEG2 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50112357 (CHEMBL3609375) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human Meg2 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50112358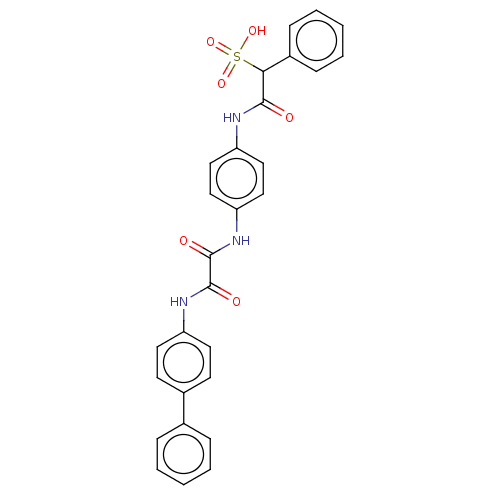 (CHEMBL3609374) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human Meg2 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50112356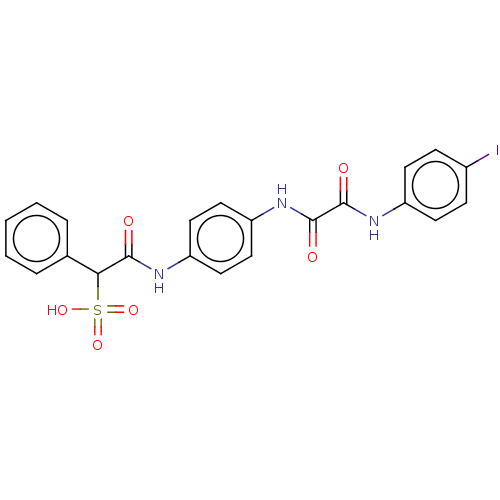 (CHEMBL3609373) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of phosphatase activity of human Meg2 using pNPP as a substrate after 10 mins by spectrophotometer analysis | ACS Med Chem Lett 6: 782-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00118 BindingDB Entry DOI: 10.7270/Q2251M0S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50343147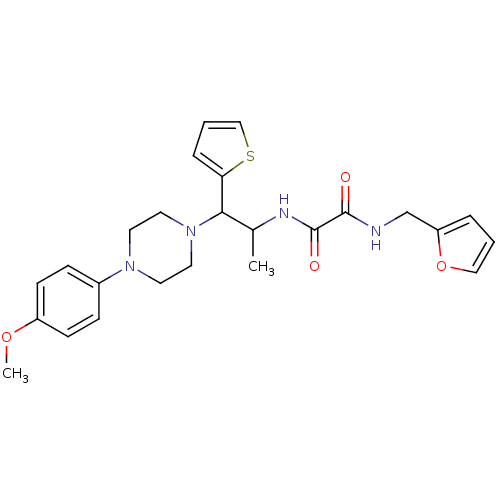 (CHEMBL1773180 | N1-(furan-2-ylmethyl)-N2-(1-(4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human MEG2 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50343148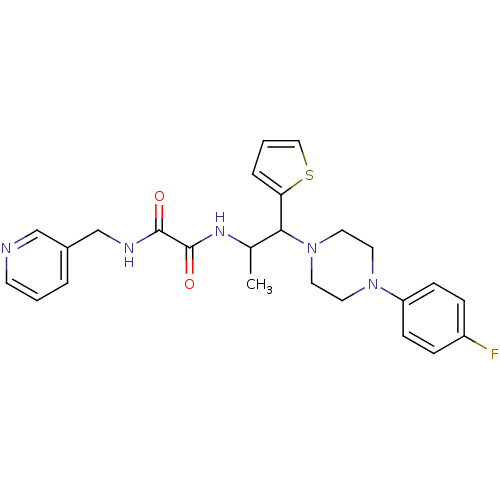 (CHEMBL1773181 | N1-(1-(4-(4-fluorophenyl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human MEG2 expressed in Escherichia coli after 5 mins by microplate spectrophotometer analysis | ACS Med Chem Lett 1: 355-359 (2010) Article DOI: 10.1021/ml1001135 BindingDB Entry DOI: 10.7270/Q26M37TG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM53072 ((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University Curated by ChEMBL | Assay Description Inhibition of GST-tagged MEG2 (unknown origin) using phospho-EGFR Asp-Ala-Asp-Glu-Tyr[PO3H2]-Leu-Ile-Pro-Gln-Gln-Gly as substrate preincubated for 30... | J Med Chem 56: 7212-21 (2013) Article DOI: 10.1021/jm400474r BindingDB Entry DOI: 10.7270/Q2CZ38M6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50343530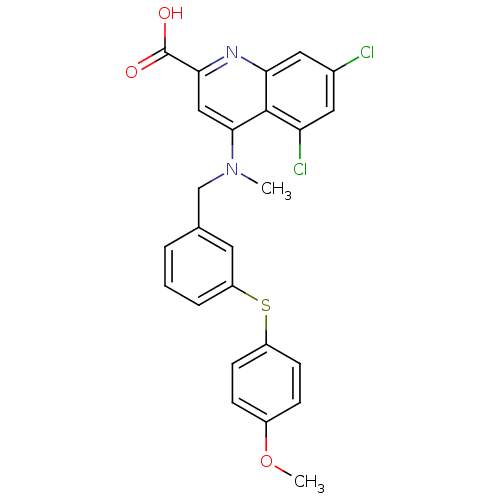 (5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50363857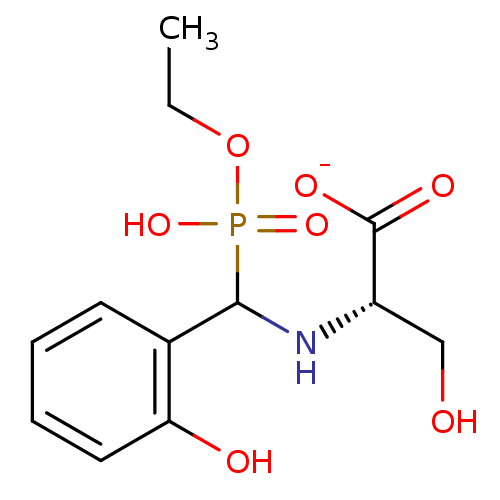 (CHEMBL1946248) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanxi University Curated by ChEMBL | Assay Description Inhibition of human PTP-MEG2 using p-nitrophenol phosphate as substrate incubated for 35 mins prior to substrate addition measured after 30 mins by s... | Eur J Med Chem 49: 354-64 (2012) Article DOI: 10.1016/j.ejmech.2012.01.038 BindingDB Entry DOI: 10.7270/Q2X92BRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50498446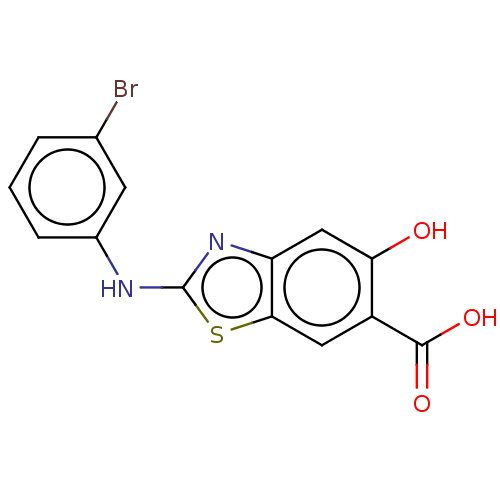 (CHEMBL3596431) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human PTP-Meg2 using pNPP substrate by spectrophotometry | Medchemcomm 5: 1496-1499 (2014) Article DOI: 10.1039/c4md00099d BindingDB Entry DOI: 10.7270/Q25D8VVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50031910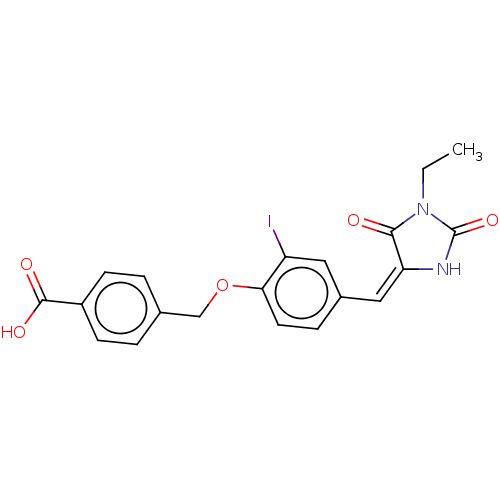 (CHEMBL3360908) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of MEG2 (unknown origin) assessed as reduction in pNPP hydrolysis | J Med Chem 57: 9309-22 (2014) Article DOI: 10.1021/jm500692u BindingDB Entry DOI: 10.7270/Q2ZG6TVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425823 (CHEMBL2316901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425821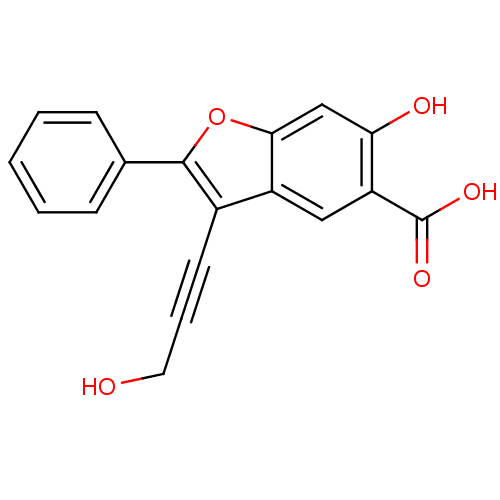 (CHEMBL2316900) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425822 (CHEMBL2316899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50558485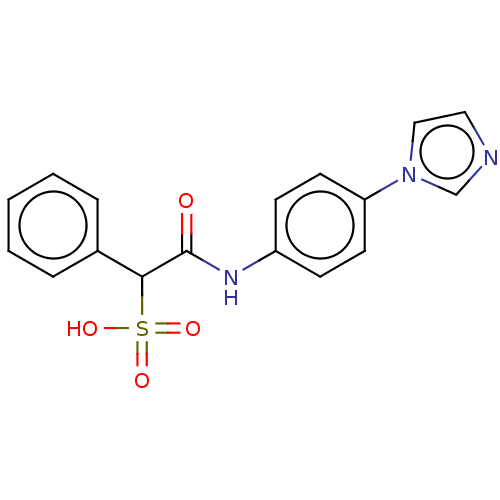 (CHEMBL4800195) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTP-MEG2 (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50558461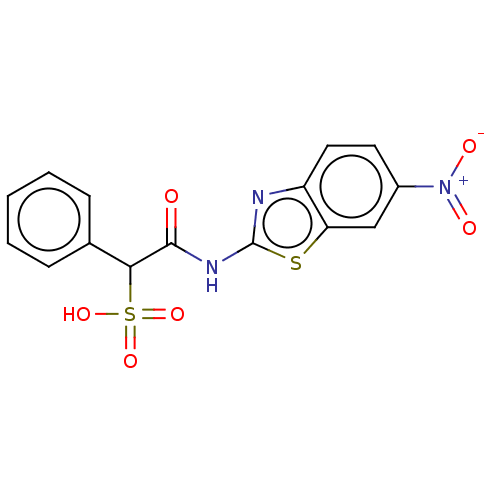 (CHEMBL4760367) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged PTP-MEG2 (unknown origin) expressed in Escherichia coli BL21 cells using para-nitrophenyl phosphate as substrate incubated f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.6b00993 BindingDB Entry DOI: 10.7270/Q2DN48Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50425812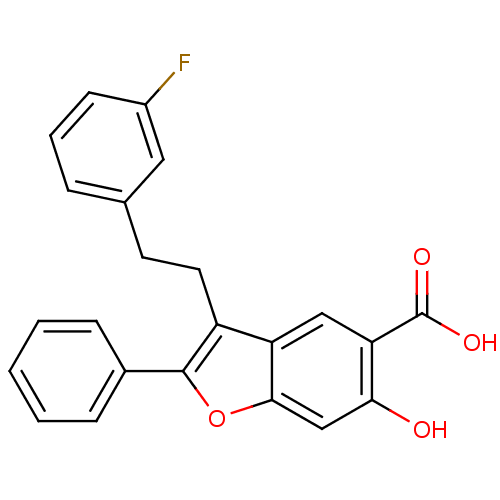 (CHEMBL2316898) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of PTPMEG2 (unknown origin) expressed in Escherichia coli using pNPP substrate after 5 mins by spectrophotometric analysis | J Med Chem 56: 832-42 (2013) Article DOI: 10.1021/jm301781p BindingDB Entry DOI: 10.7270/Q2KK9D4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50363855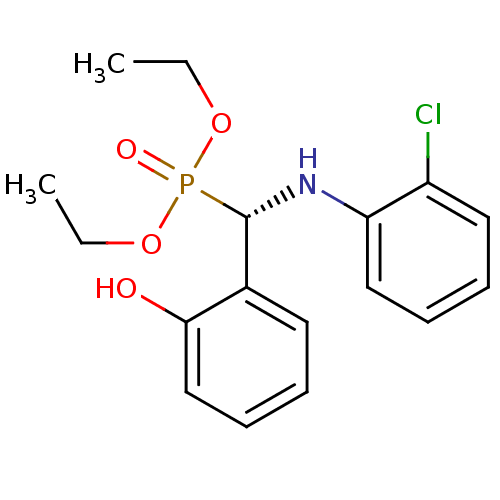 (CHEMBL1946246) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanxi University Curated by ChEMBL | Assay Description Inhibition of human PTP-MEG2 using p-nitrophenol phosphate as substrate incubated for 35 mins prior to substrate addition measured after 30 mins by s... | Eur J Med Chem 49: 354-64 (2012) Article DOI: 10.1016/j.ejmech.2012.01.038 BindingDB Entry DOI: 10.7270/Q2X92BRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 9 (Homo sapiens (Human)) | BDBM50363856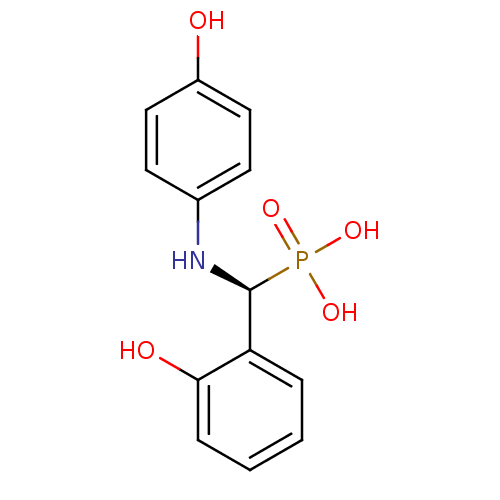 (CHEMBL1946247) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanxi University Curated by ChEMBL | Assay Description Inhibition of human PTP-MEG2 using p-nitrophenol phosphate as substrate incubated for 35 mins prior to substrate addition measured after 30 mins by s... | Eur J Med Chem 49: 354-64 (2012) Article DOI: 10.1016/j.ejmech.2012.01.038 BindingDB Entry DOI: 10.7270/Q2X92BRV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 72 total ) | Next | Last >> |