

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490888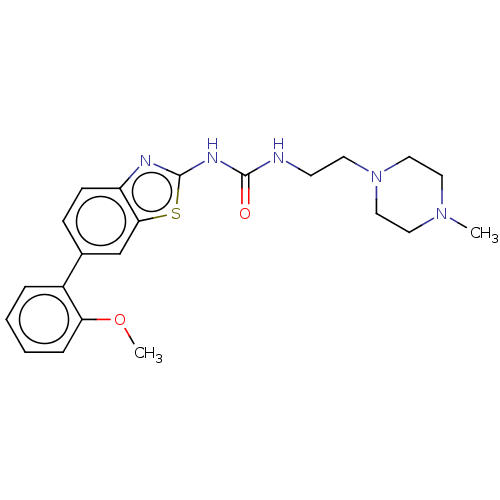 (CHEMBL2347725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490888 (CHEMBL2347725) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490916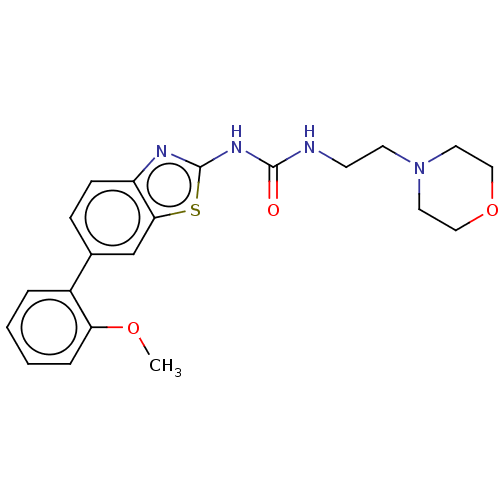 (CHEMBL2347722) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490895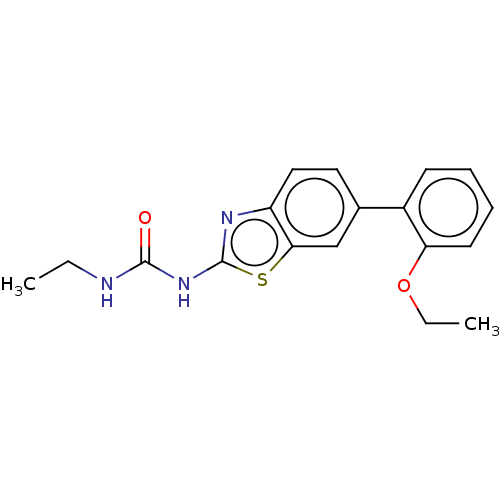 (CHEMBL2347711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490918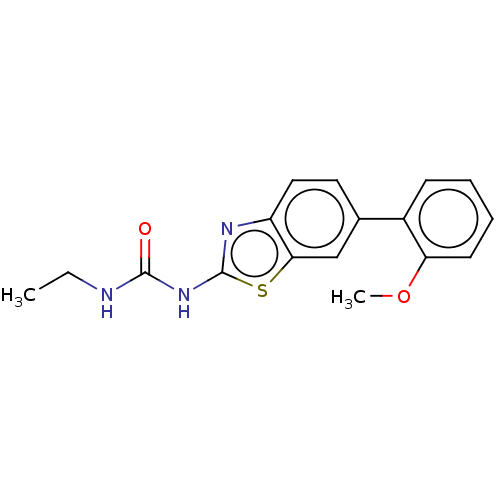 (CHEMBL2347710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490895 (CHEMBL2347711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490889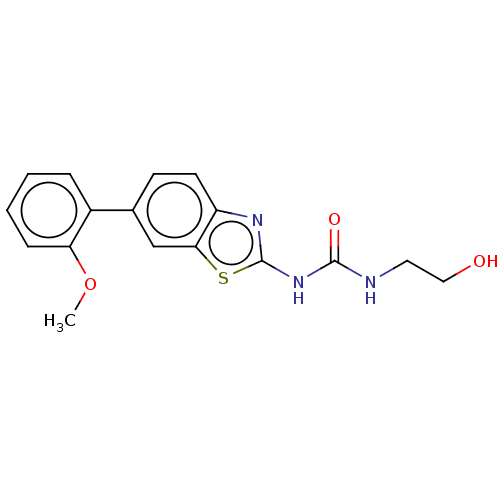 (CHEMBL2347723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490889 (CHEMBL2347723) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490895 (CHEMBL2347711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230809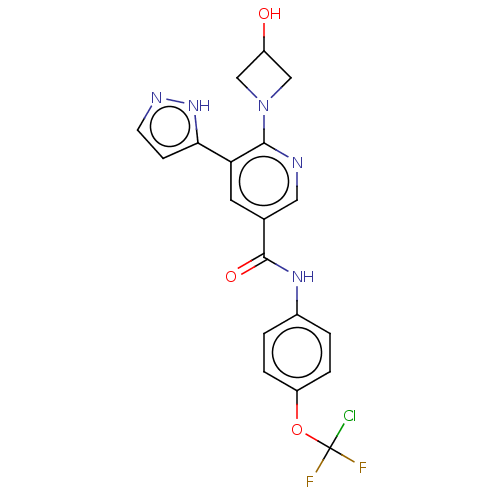 (US9340537, 63 | US9896444, Example 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Radio ABL1 (64-515) Assay: For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213682 (US9278981, 274) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 uL of the compound pre-... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230777 (US9340537, 31 | US9896444, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Radio ABL1 (64-515) Assay: For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230795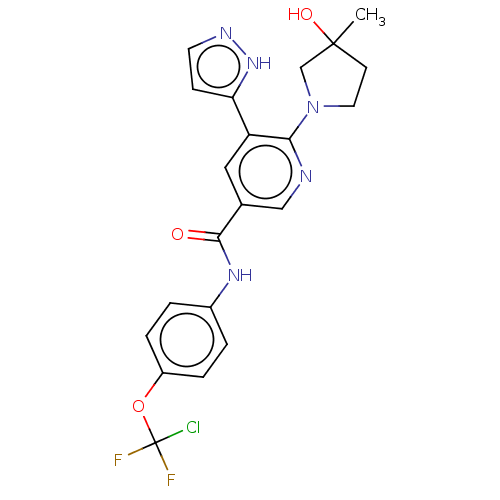 (US9340537, 49 | US9896444, Example 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Radio ABL1 (64-515) Assay: For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230799 (US9340537, 53 | US9896444, Example 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Radio ABL1 (64-515) Assay: For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230775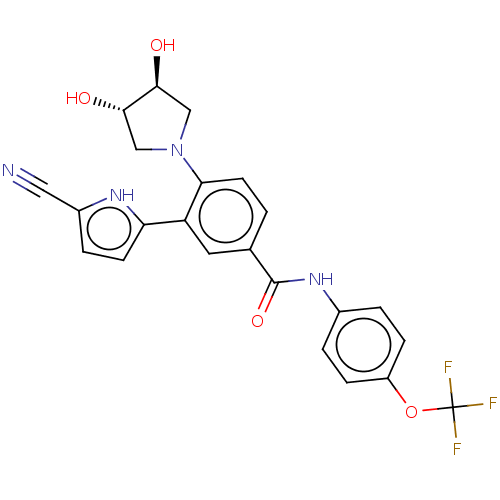 (US9340537, 29 | US9896444, Example 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Radio ABL1 (64-515) Assay: For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230775 (US9340537, 29 | US9896444, Example 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVATIS AG US Patent | Assay Description The assay was performed by mixing 10 μL of the compound pre-diluted with 10 μL of ATP (20 μM ATP with 0.1 μCi [γ-33P]-ATP) with the... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230777 (US9340537, 31 | US9896444, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVATIS AG US Patent | Assay Description The assay was performed by mixing 10 μL of the compound pre-diluted with 10 μL of ATP (20 μM ATP with 0.1 μCi [γ-33P]-ATP) with the... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230795 (US9340537, 49 | US9896444, Example 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVATIS AG US Patent | Assay Description The assay was performed by mixing 10 μL of the compound pre-diluted with 10 μL of ATP (20 μM ATP with 0.1 μCi [γ-33P]-ATP) with the... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230799 (US9340537, 53 | US9896444, Example 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVATIS AG US Patent | Assay Description The assay was performed by mixing 10 μL of the compound pre-diluted with 10 μL of ATP (20 μM ATP with 0.1 μCi [γ-33P]-ATP) with the... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230809 (US9340537, 63 | US9896444, Example 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
NOVATIS AG US Patent | Assay Description The assay was performed by mixing 10 μL of the compound pre-diluted with 10 μL of ATP (20 μM ATP with 0.1 μCi [γ-33P]-ATP) with the... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213659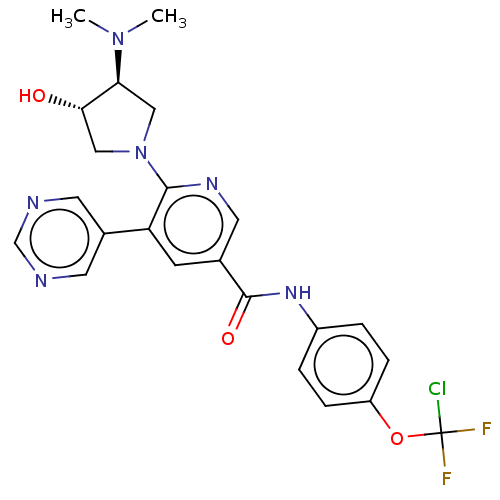 (US9278981, 251) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 uL of the compound pre-... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213662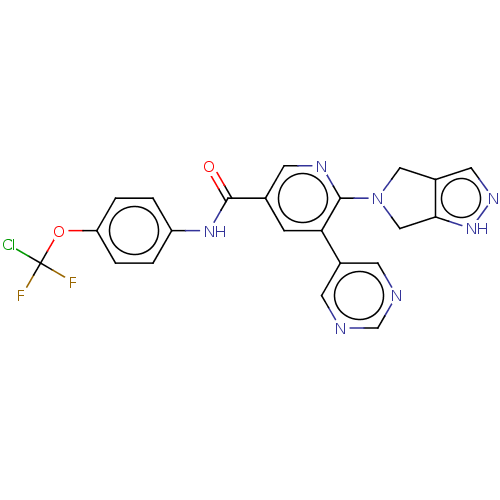 (US9278981, 254) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 uL of the compound pre-... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213677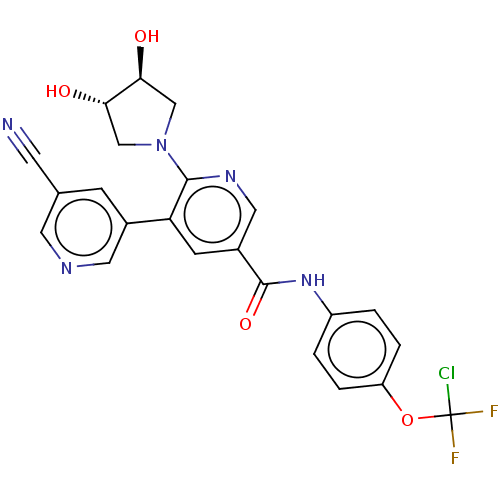 (US9278981, 269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Novartis AG US Patent | Assay Description For determination of ABL kinase activity, the radiometric filter-binding assay was used. The assay was performed by mixing 10 uL of the compound pre-... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM223091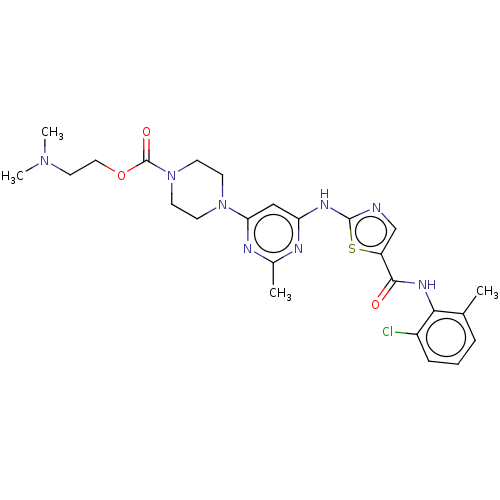 (2-(Dimethylamino)ethyl 4-(6-((5-((2-chloro-6-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.105 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences | Assay Description ABL activity assays were performed in 384-well plate using the FRET-based Z'-Lyteassay system and Tyr-2 peptide substrate according to the manufa... | Chem Biol Drug Des 89: 420-427 (2017) Article DOI: 10.1111/cbdd.12863 BindingDB Entry DOI: 10.7270/Q29C6W98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490918 (CHEMBL2347710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490918 (CHEMBL2347710) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490890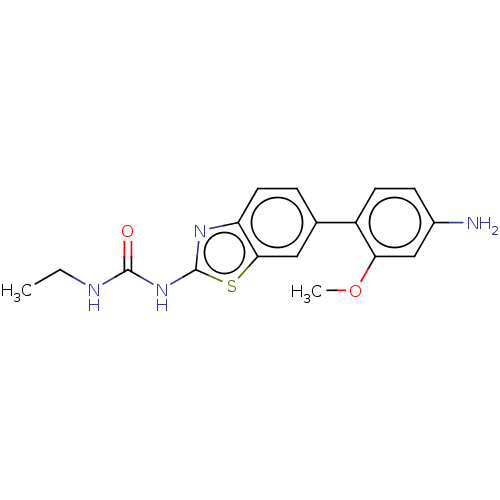 (CHEMBL2347719) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490932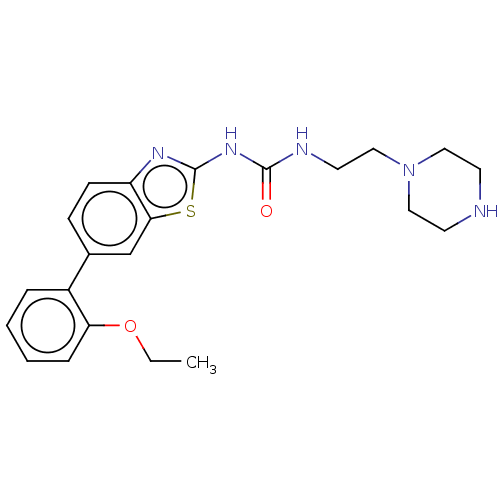 (CHEMBL2347729) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230814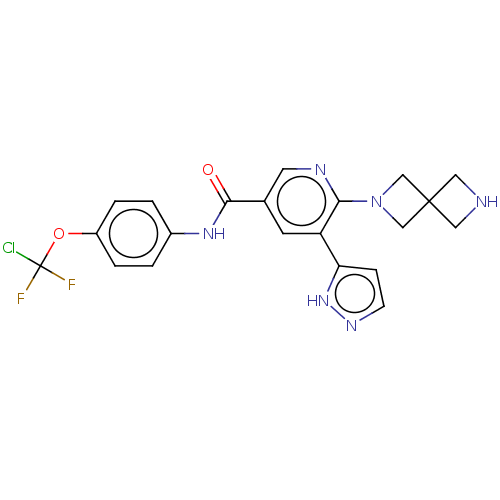 (US9340537, 68 | US9896444, Example 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Caliper ABL1 (64-515) Assay: The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213581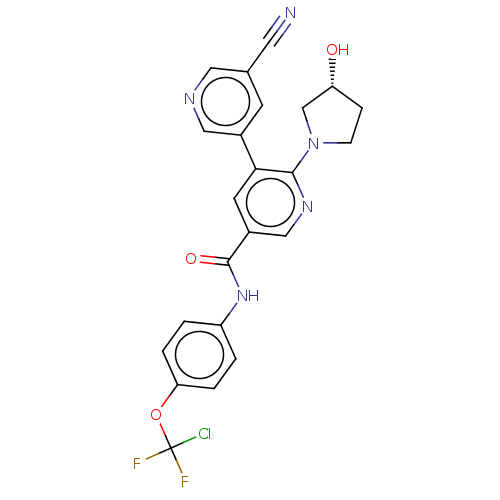 (US9278981, 173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 (Homo sapiens (Human)) | BDBM50490886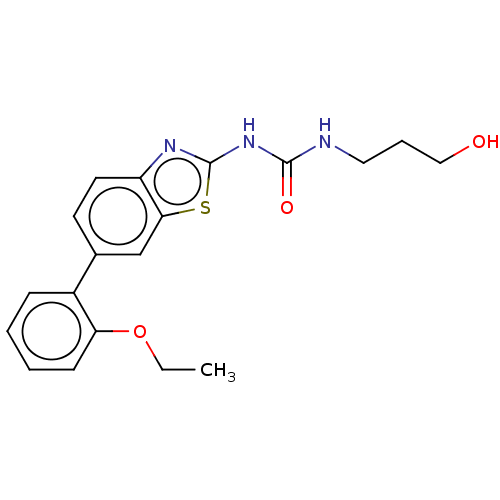 (CHEMBL2347728) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KAIST) Curated by ChEMBL | Assay Description Inhibition of breakpoint cluster region-Abelson tyrosine kinase T315I mutant (unknown origin) using [gamma-33P]ATP as substrate by radiometric kinase... | J Med Chem 56: 3531-45 (2013) Article DOI: 10.1021/jm301891t BindingDB Entry DOI: 10.7270/Q2BG2RW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230768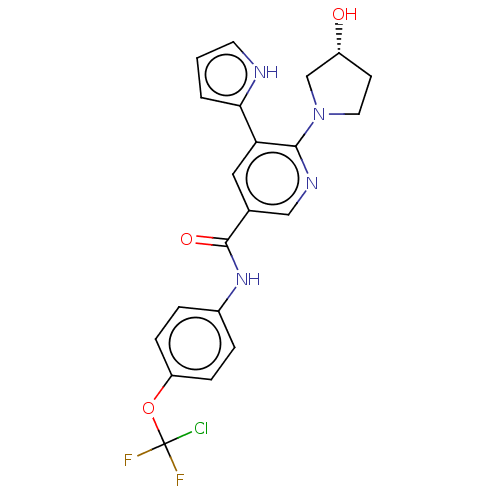 (US9340537, 22 | US9896444, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Caliper ABL1 (64-515) Assay: The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230777 (US9340537, 31 | US9896444, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Caliper ABL1 (64-515) Assay: The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230768 (US9340537, 22 | US9896444, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVATIS AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230777 (US9340537, 31 | US9896444, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVATIS AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230814 (US9340537, 68 | US9896444, Example 68) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVATIS AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230826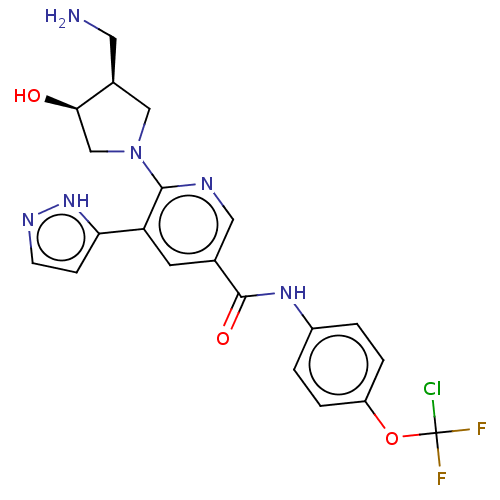 (US9340537, 80 | US9896444, Example 80) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVATIS AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9340537 (2016) BindingDB Entry DOI: 10.7270/Q2FB51T5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213603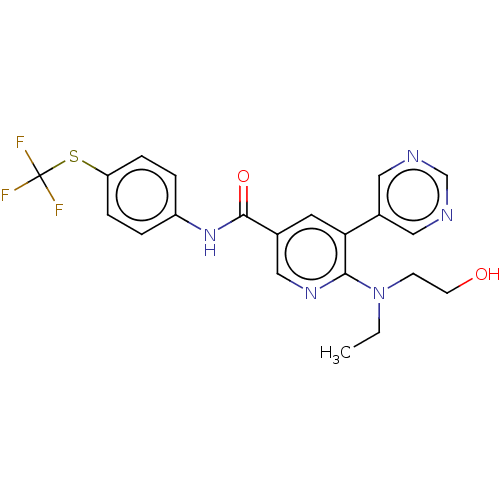 (US9278981, 195) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213613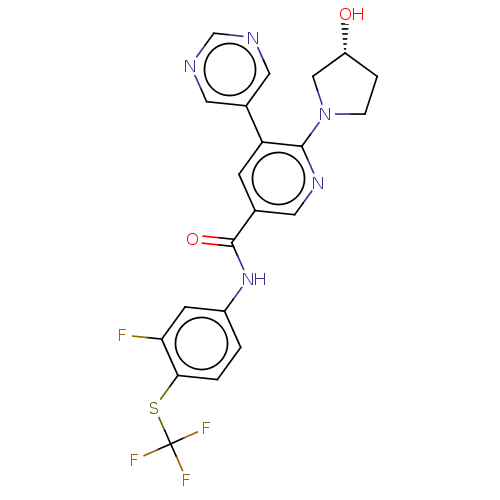 (US9278981, 205) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213655 (US9278981, 247) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213656 (US9278981, 248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213667 (US9278981, 259) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213671 (US9278981, 263) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213673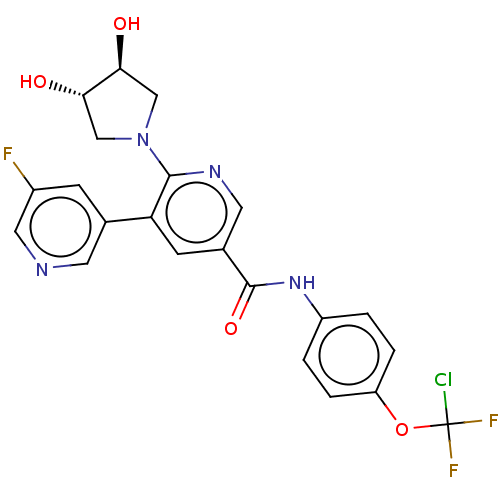 (US9278981, 265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213677 (US9278981, 269) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM230826 (US9340537, 80 | US9896444, Example 80) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description Caliper ABL1 (64-515) Assay: The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were... | J Med Chem 50: 4953-75 (2007) BindingDB Entry DOI: 10.7270/Q2B27XKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213496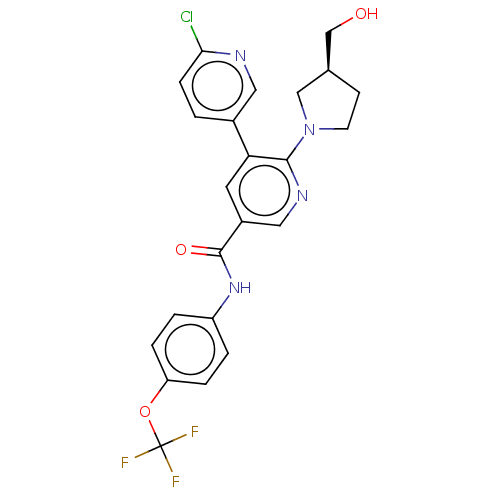 (US9278981, 88) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213559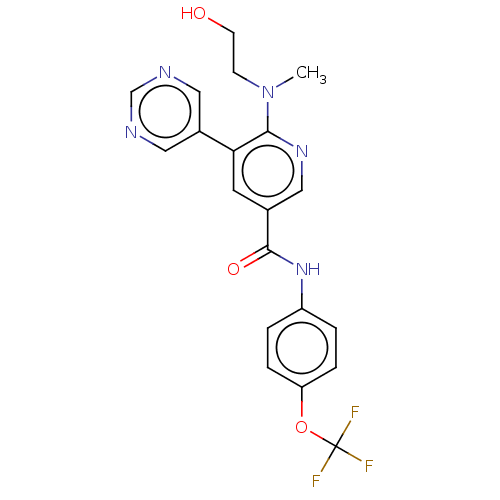 (US9278981, 151) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213578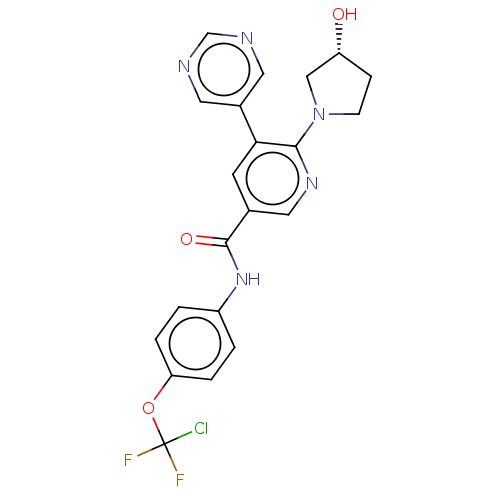 (US9278981, 170) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase ABL1 [64-515] (Homo sapiens (Human)) | BDBM213579 (US9278981, 171) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.130 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The assay plates were prepared by addition of 50 nL per well of compound solution in 90% DMSO. The kinase reactions were started by stepwise addition... | US Patent US9278981 (2016) BindingDB Entry DOI: 10.7270/Q2DV1HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4966 total ) | Next | Last >> |