

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21653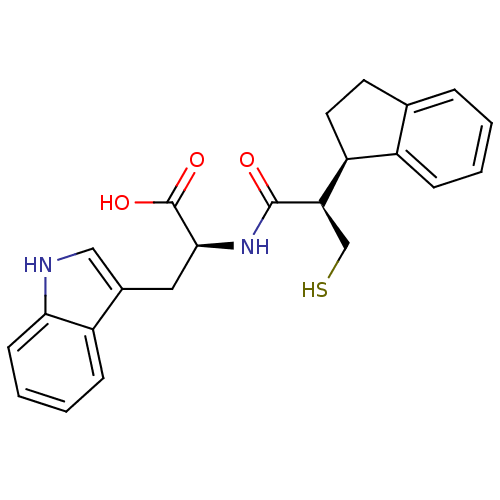 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21649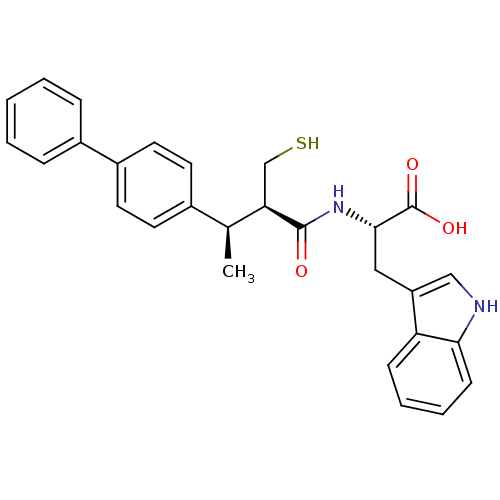 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3R)-3-(4-phenylpheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21639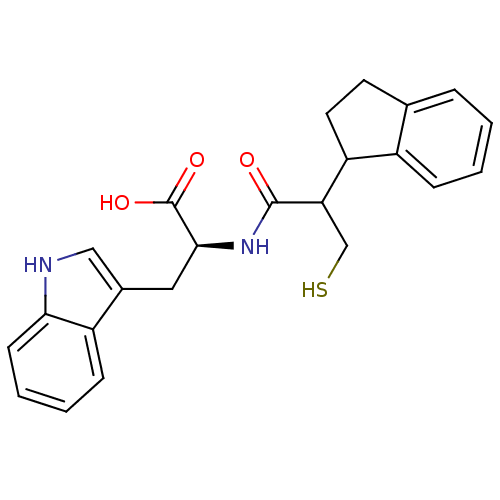 ((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21654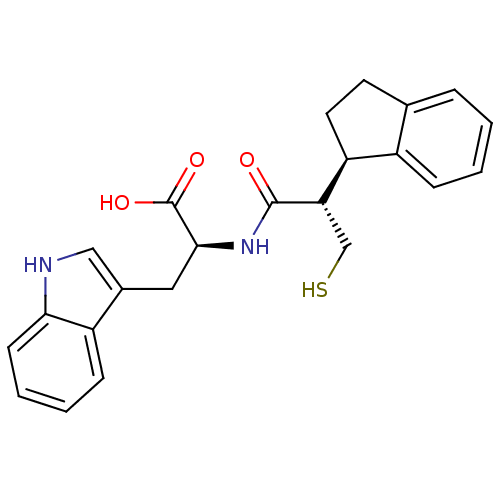 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21644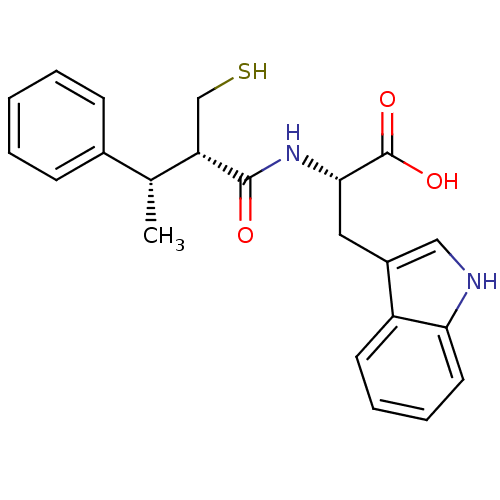 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-phenyl-2-(sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21643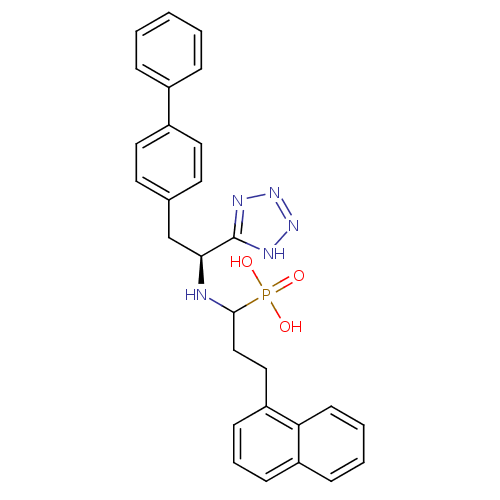 (CGS-31,447 | [3-(naphthalen-1-yl)-1-{[(1S)-2-(4-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.20 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21645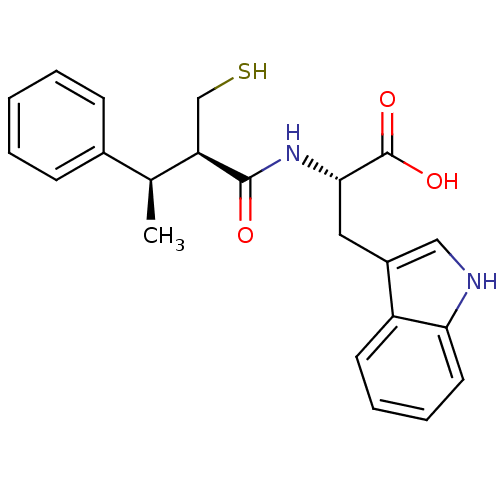 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3R)-3-phenyl-2-(sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40 | -51.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21646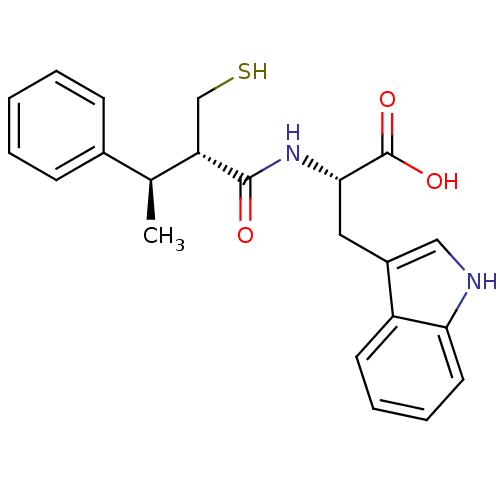 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3R)-3-phenyl-2-(sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21657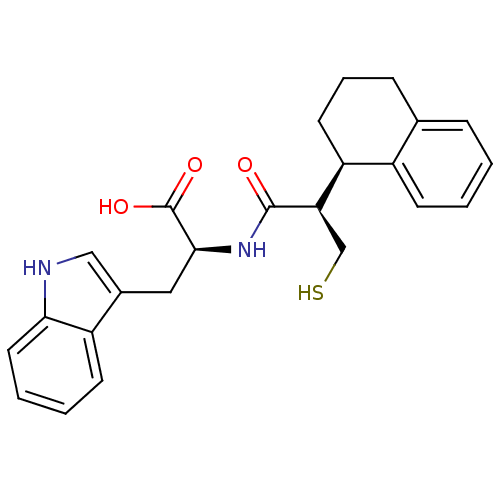 ((2S)-3-(1H-indol-3-yl)-2-[(2R)-3-sulfanyl-2-[(1R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21635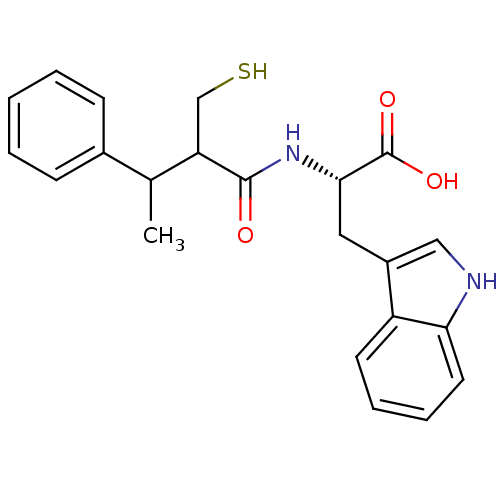 ((2S)-3-(1H-indol-3-yl)-2-[3-phenyl-2-(sulfanylmeth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21658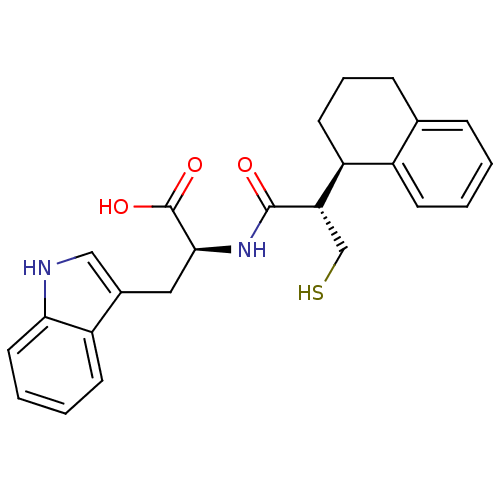 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 4 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21640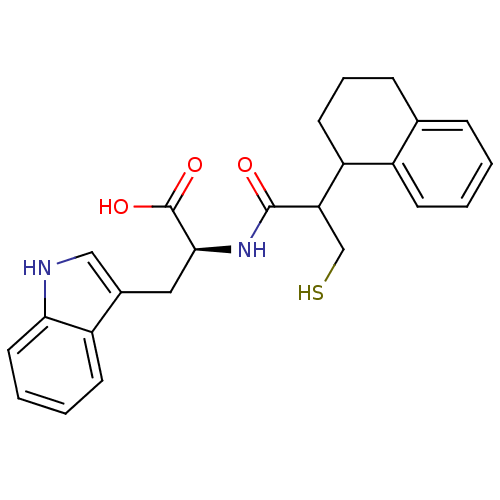 ((2S)-3-(1H-indol-3-yl)-2-[3-sulfanyl-2-(1,2,3,4-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.5 | -49.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21636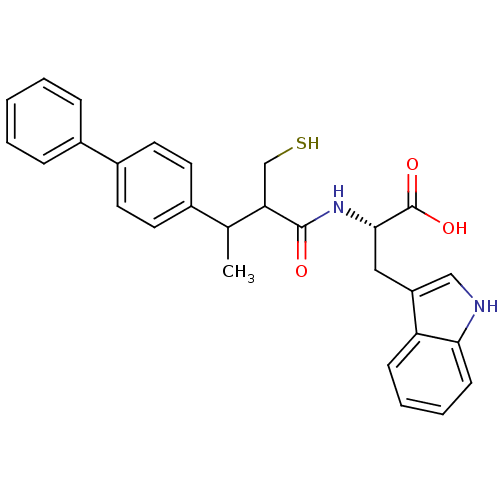 ((2S)-3-(1H-indol-3-yl)-2-[3-(4-phenylphenyl)-2-(su...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21647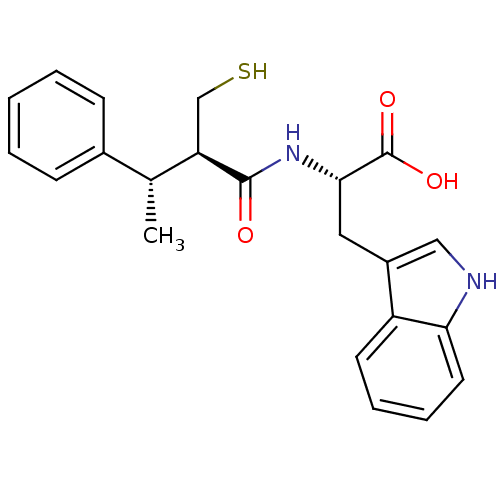 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3S)-3-phenyl-2-(sulf...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21651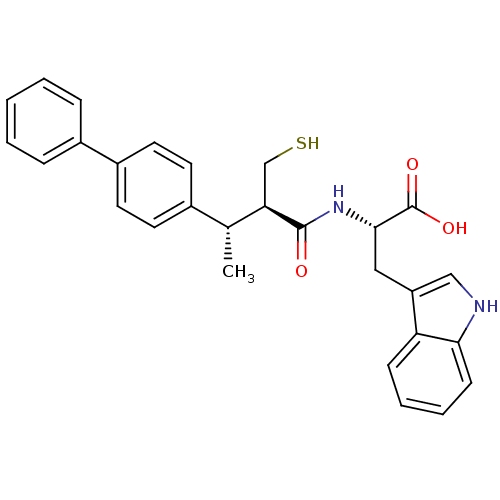 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3S)-3-(4-phenylpheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21637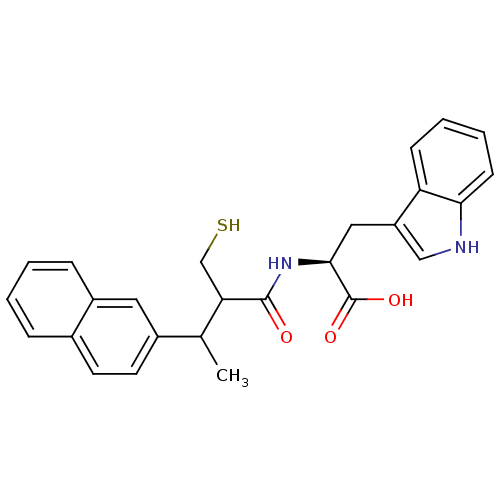 ((2S)-3-(1H-indol-3-yl)-2-[3-(naphthalen-2-yl)-2-(s...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.40 | -49.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21656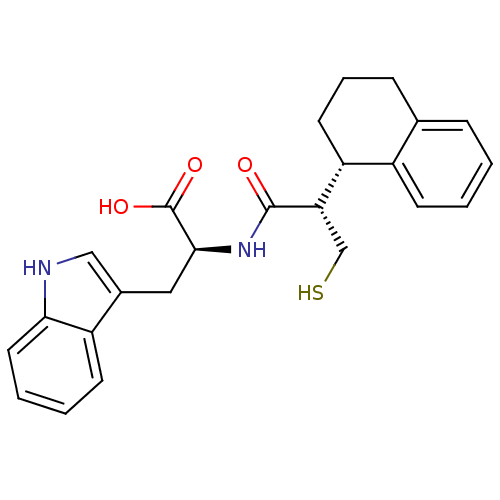 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21652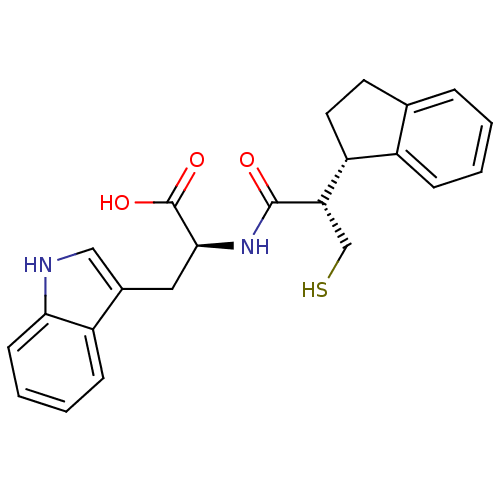 ((2S)-2-[(2S)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21654 ((2S)-2-[(2S)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21658 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1R)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21643 (CGS-31,447 | [3-(naphthalen-1-yl)-1-{[(1S)-2-(4-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21646 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3R)-3-phenyl-2-(sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21640 ((2S)-3-(1H-indol-3-yl)-2-[3-sulfanyl-2-(1,2,3,4-te...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21639 ((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21633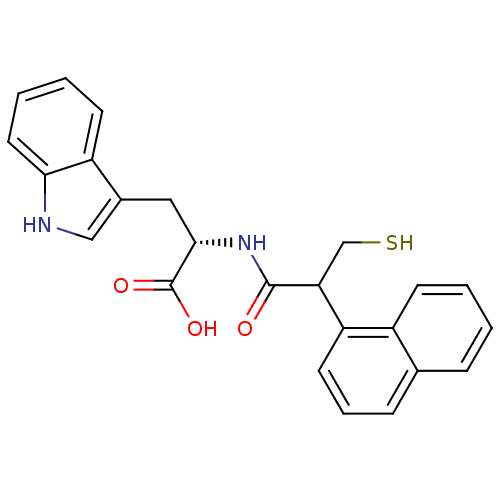 ((2S)-3-(1H-indol-3-yl)-2-[2-(naphthalen-1-yl)-3-su...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21656 ((2S)-3-(1H-indol-3-yl)-2-[(2S)-3-sulfanyl-2-[(1S)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21653 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21655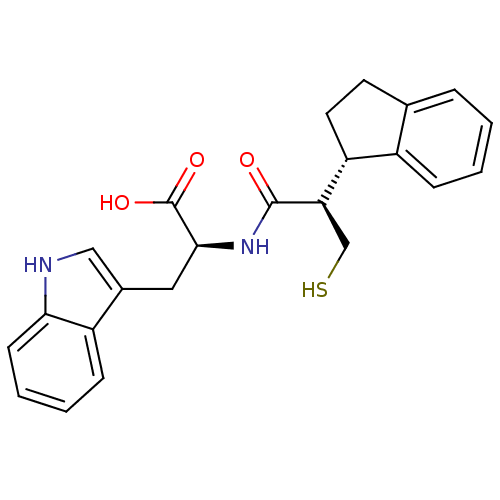 ((2S)-2-[(2R)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -44.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21644 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-phenyl-2-(sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21648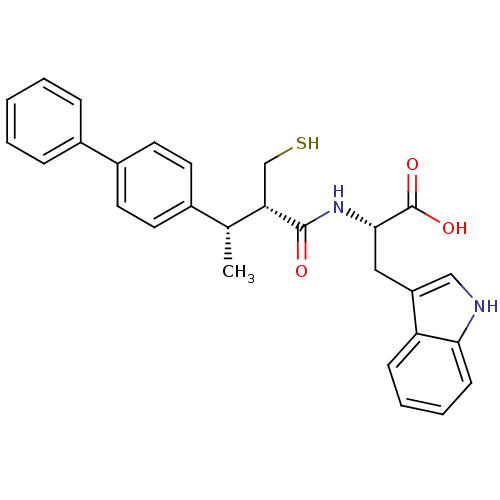 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-(4-phenylpheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21645 ((2S)-3-(1H-indol-3-yl)-2-[(2R,3R)-3-phenyl-2-(sulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21650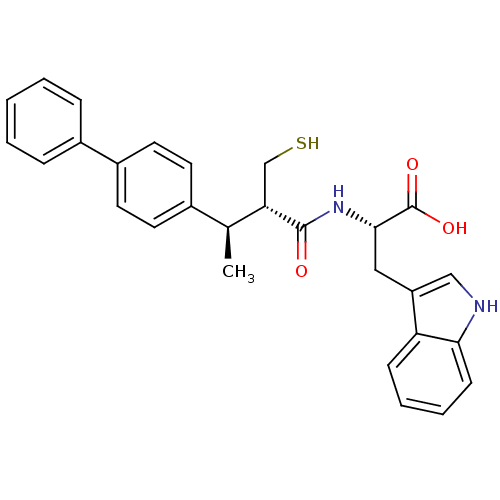 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3R)-3-(4-phenylpheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21632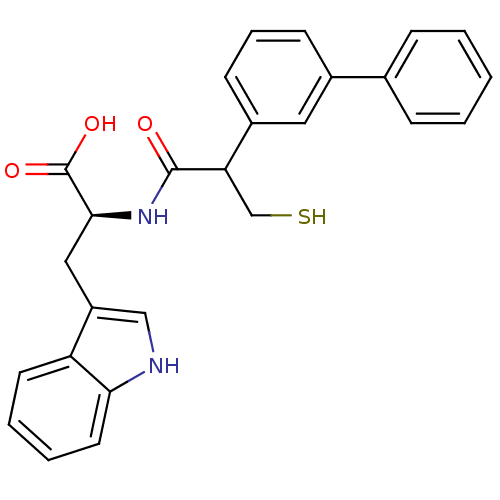 ((2S)-3-(1H-indol-3-yl)-2-[2-(3-phenylphenyl)-3-sul...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21650 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3R)-3-(4-phenylpheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21635 ((2S)-3-(1H-indol-3-yl)-2-[3-phenyl-2-(sulfanylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21652 ((2S)-2-[(2S)-2-[(1S)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21659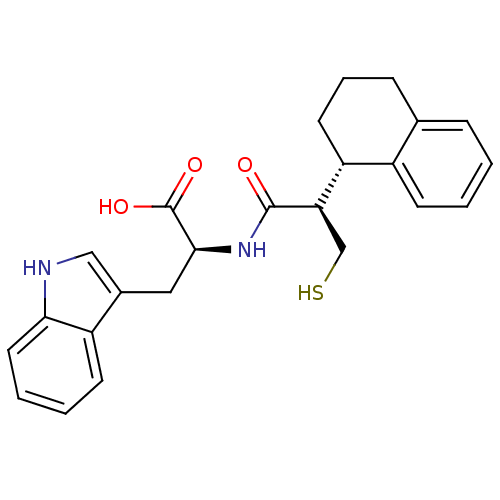 ((2S)-3-(1H-indol-3-yl)-2-[(2R)-3-sulfanyl-2-[(1S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21653 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21637 ((2S)-3-(1H-indol-3-yl)-2-[3-(naphthalen-2-yl)-2-(s...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21638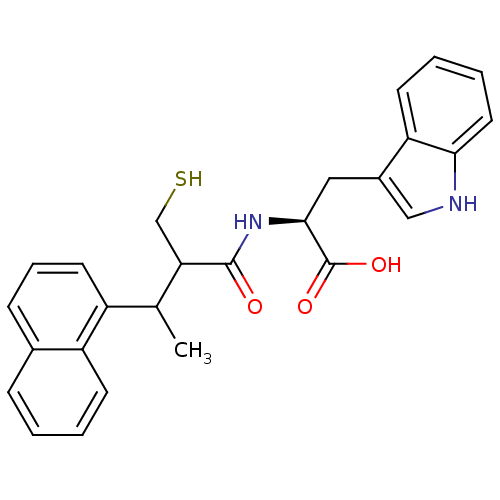 ((2S)-3-(1H-indol-3-yl)-2-[3-(naphthalen-1-yl)-2-(s...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 51 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS | Assay Description NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21636 ((2S)-3-(1H-indol-3-yl)-2-[3-(4-phenylphenyl)-2-(su...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21648 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-(4-phenylpheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21657 ((2S)-3-(1H-indol-3-yl)-2-[(2R)-3-sulfanyl-2-[(1R)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21630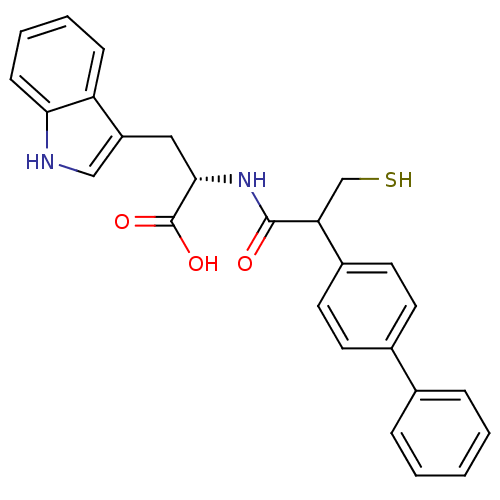 ((2S)-3-(1H-indol-3-yl)-2-[2-(4-phenylphenyl)-3-sul...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21638 ((2S)-3-(1H-indol-3-yl)-2-[3-(naphthalen-1-yl)-2-(s...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description Specific activity of ACE was assayed in black 96-well microplates with or without various concentrations of inhibitors. N-Cbz-Phe-His-Leu was added, ... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21639 ((2S)-2-[2-(2,3-dihydro-1H-inden-1-yl)-3-sulfanylpr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM21644 ((2S)-3-(1H-indol-3-yl)-2-[(2S,3S)-3-phenyl-2-(sulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS | Assay Description ECE activity was performed in black 96-well microplates with recombinant human ECE-1c (hECE-1c). The hECE-1c was preincubated with or without increa... | J Med Chem 45: 1477-86 (2002) Article DOI: 10.1021/jm0005454 BindingDB Entry DOI: 10.7270/Q2T72FQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87 total ) | Next | Last >> |