

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000293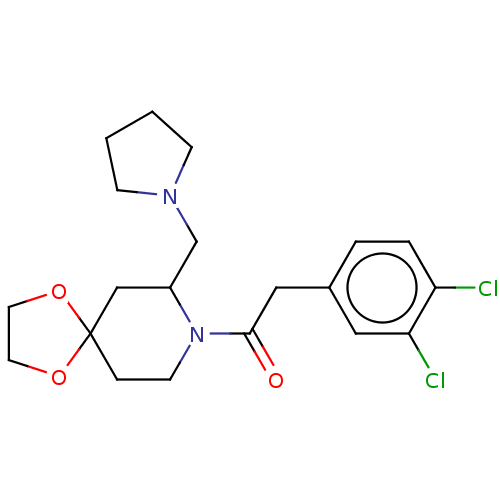 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000288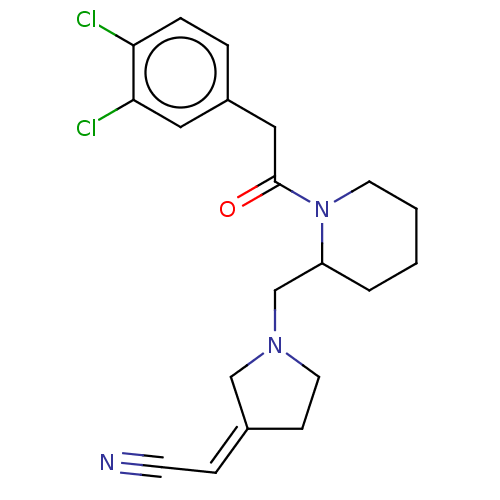 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000271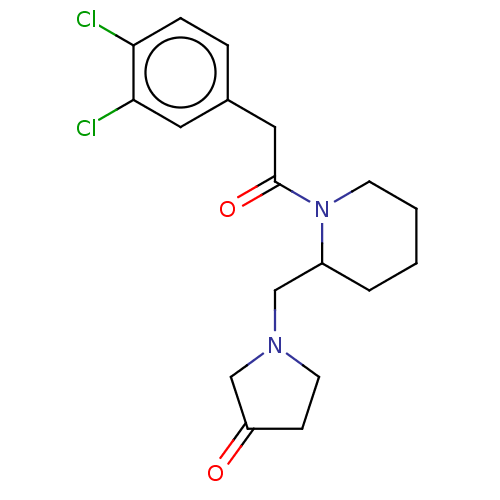 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000260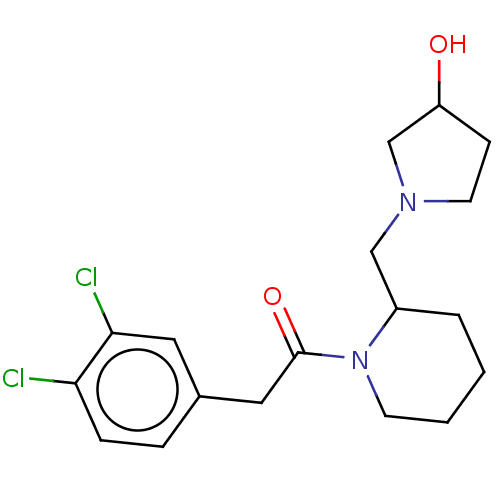 (2-(3,4-Dichloro-phenyl)-1-[2-(3-hydroxy-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000284 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000292 (2-(3,4-Dichloro-phenyl)-1-[2-(2,5-dihydro-pyrrol-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000274 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000286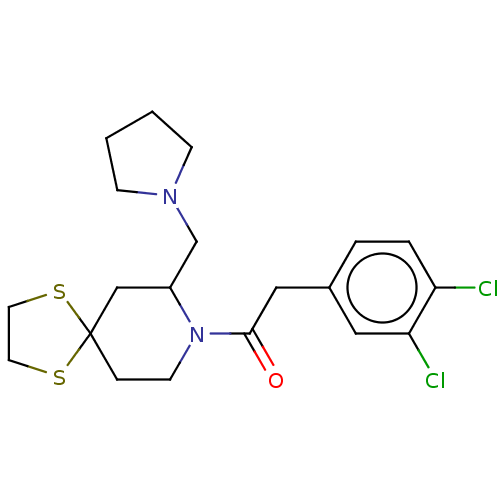 (2-(3,4-Dichloro-phenyl)-1-(7-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000297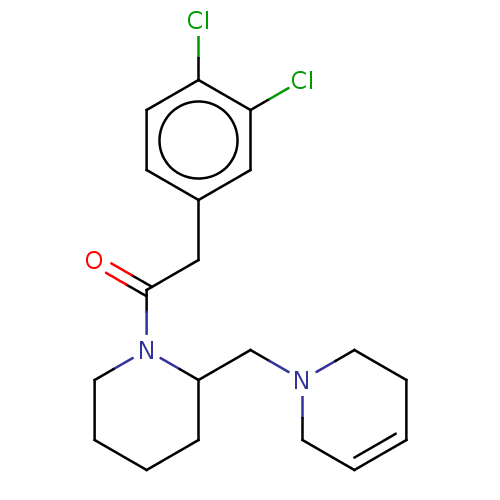 (2-(3,4-Dichloro-phenyl)-1-[2-(3,6-dihydro-2H-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000287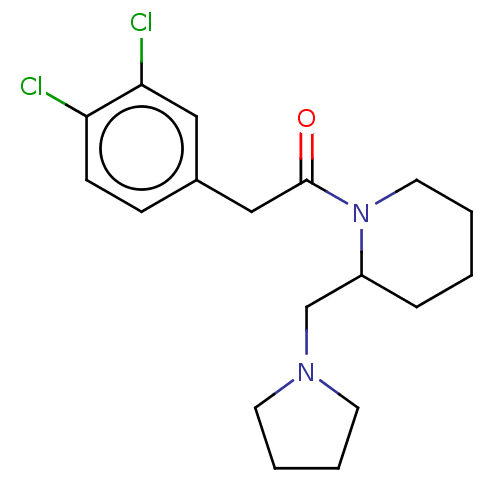 (2-(3,4-Dichloro-phenyl)-1-(2-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Kappa-opioid receptor agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000285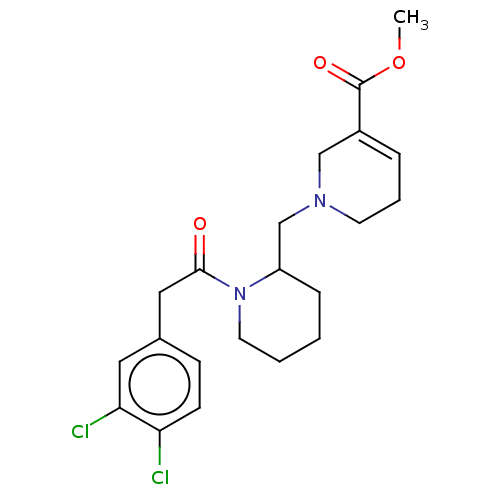 (1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000273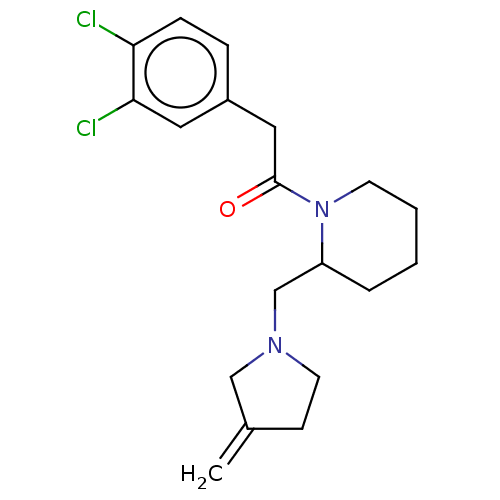 (2-(3,4-Dichloro-phenyl)-1-[2-(3-methylene-pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000275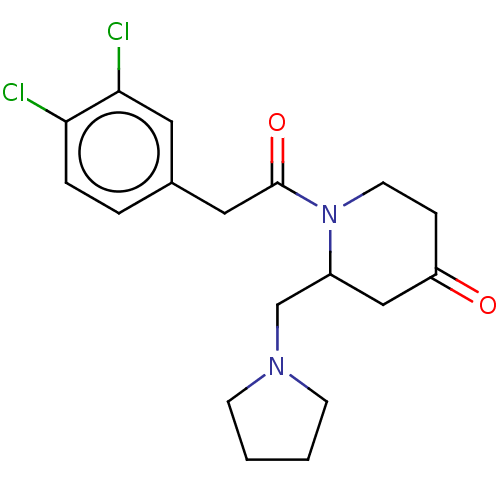 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-2-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000272 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-6-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000282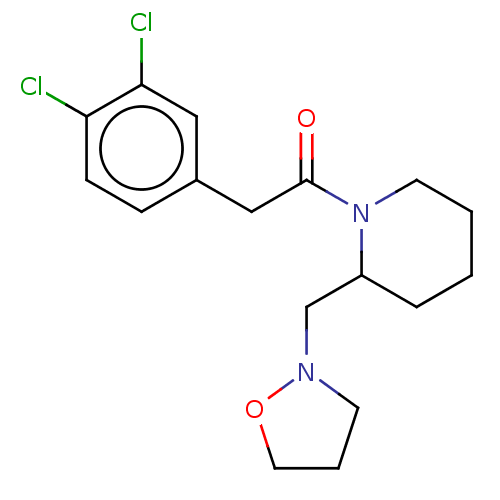 (2-(3,4-Dichloro-phenyl)-1-(2-isoxazolidin-2-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000267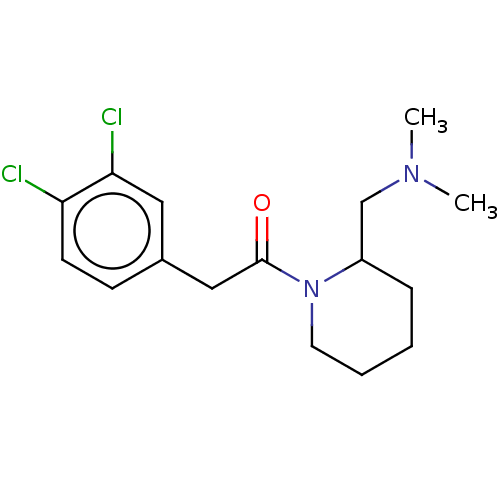 (2-(3,4-Dichloro-phenyl)-1-(2-dimethylaminomethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000289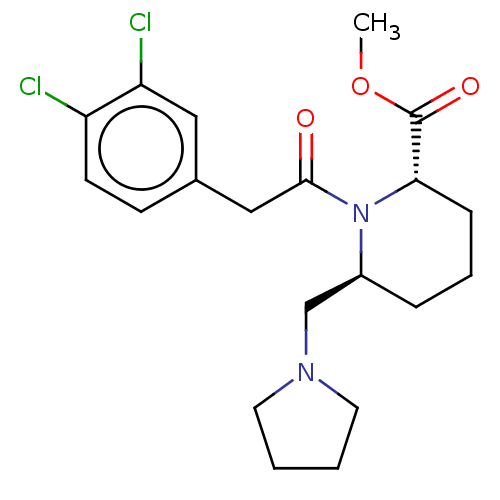 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000295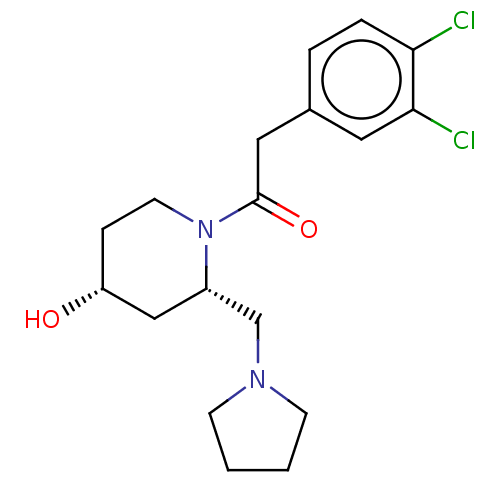 (2-(3,4-Dichloro-phenyl)-1-(4-hydroxy-2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000263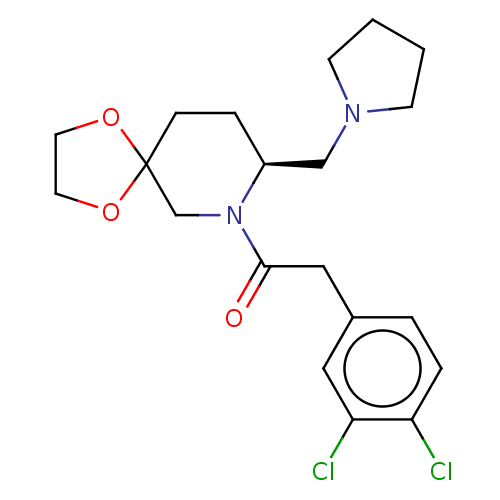 (2-(3,4-Dichloro-phenyl)-1-(8-pyrrolidin-1-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000268 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-2-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000278 (2-(3,4-Dichloro-phenyl)-1-(2-piperidin-1-ylmethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000269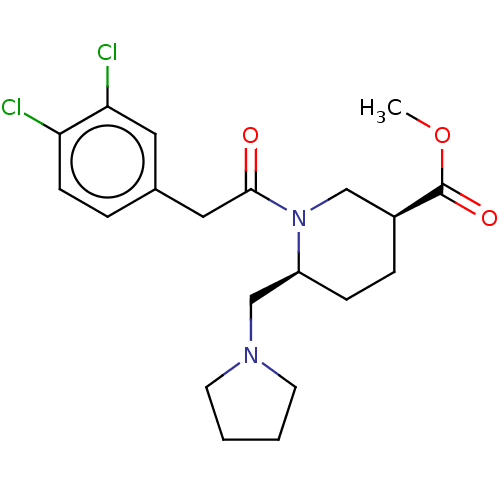 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000283 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000280 (2-(3,4-Dichloro-phenyl)-1-[2-(4-methylene-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000270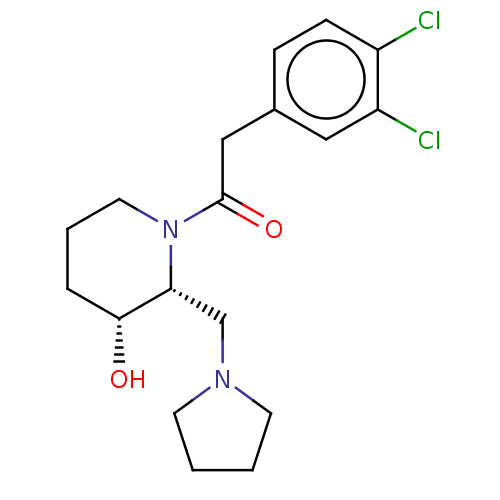 (2-(3,4-Dichloro-phenyl)-1-(3-hydroxy-2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000276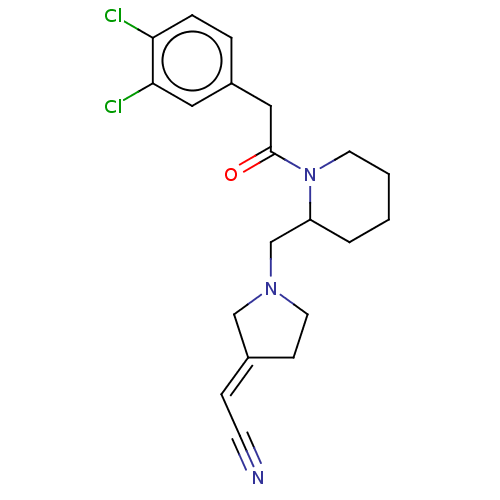 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000264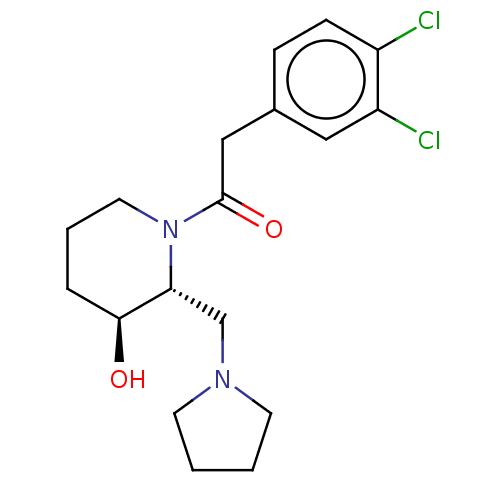 (2-(3,4-Dichloro-phenyl)-1-(3-hydroxy-2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000265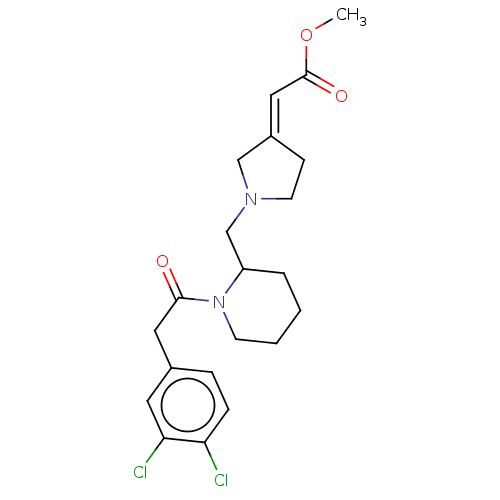 ((1-{1-[2-(3,4-Dichloro-phenyl)-acetyl]-piperidin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000262 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-2-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000290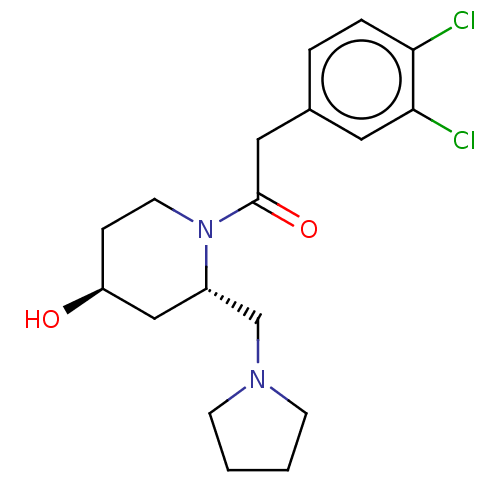 (2-(3,4-Dichloro-phenyl)-1-(4-hydroxy-2-pyrrolidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000294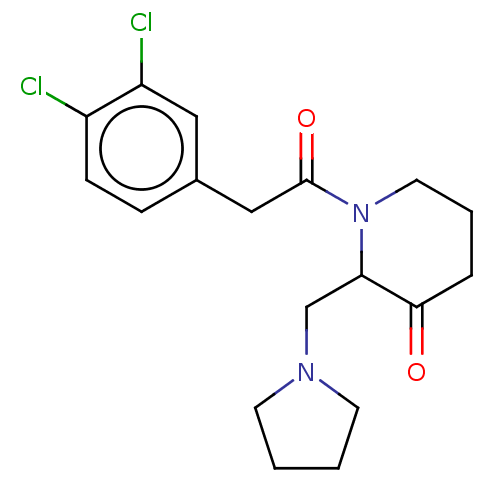 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-2-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000261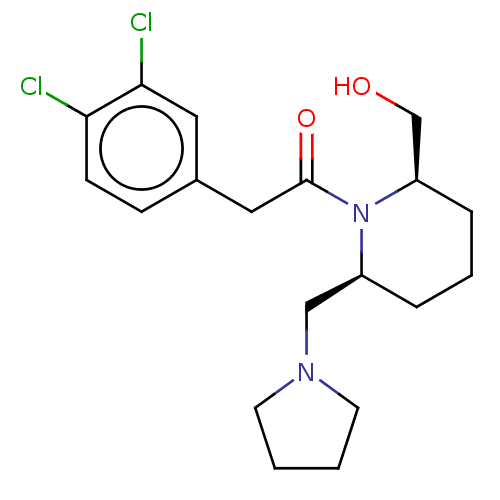 (2-(3,4-Dichloro-phenyl)-1-(2-hydroxymethyl-6-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency of the compound was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000281 (2-(3,4-Dichloro-phenyl)-1-(2-methyl-2-pyrrolidin-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000291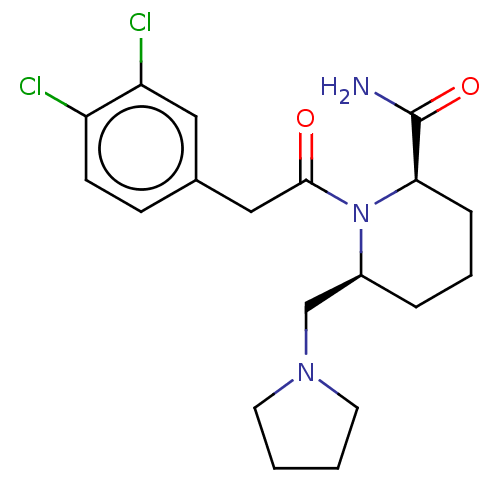 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000279 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000277 (1-(2-Allyl-2-pyrrolidin-1-ylmethyl-piperidin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa agonist potency was determined in vitro using rabbit vas deferens(LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000266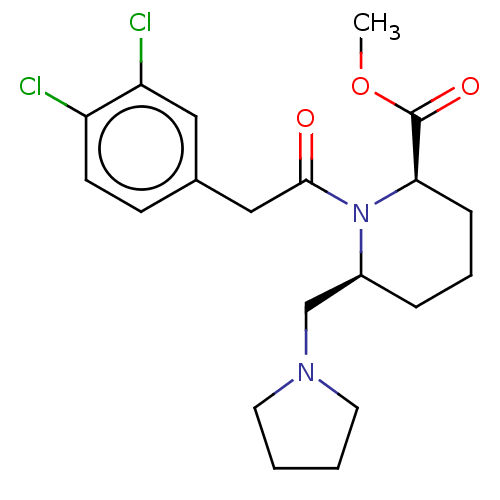 (1-[2-(3,4-Dichloro-phenyl)-acetyl]-6-pyrrolidin-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Group Research Ltd. Curated by ChEMBL | Assay Description Opioid receptor kappa 1 agonist potency was determined in vitro using rabbit vas deferens (LVD) preparation | J Med Chem 35: 490-501 (1992) BindingDB Entry DOI: 10.7270/Q2TQ60GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||