

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renin (Homo sapiens (Human)) | BDBM50036999 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036995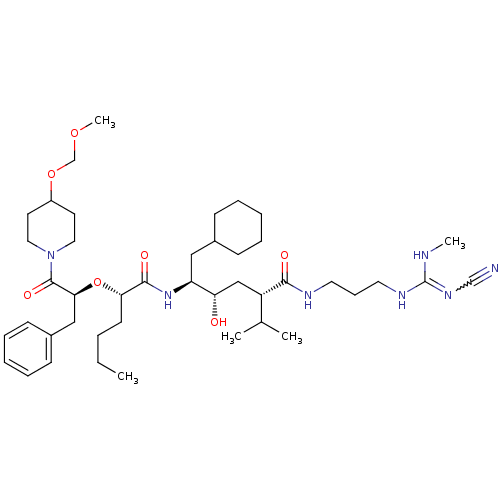 (1N-[3-methylamino(cyano imino)methylaminopropyl]-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036979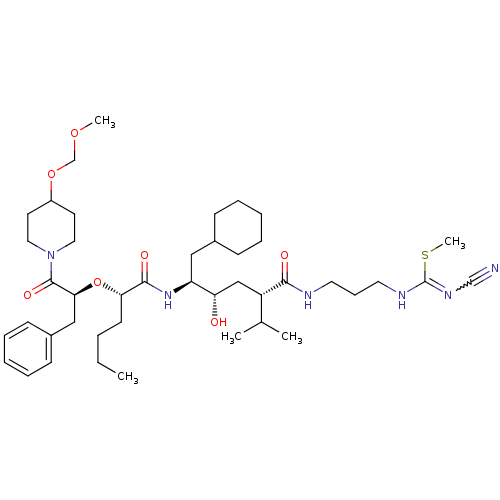 (1N-[3-cyanoimino(methylsulfanyl)methylaminopropyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036977 (1N-[3-amino(cyanoimino)methylaminopropyl]-5-[1-[1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036989 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036974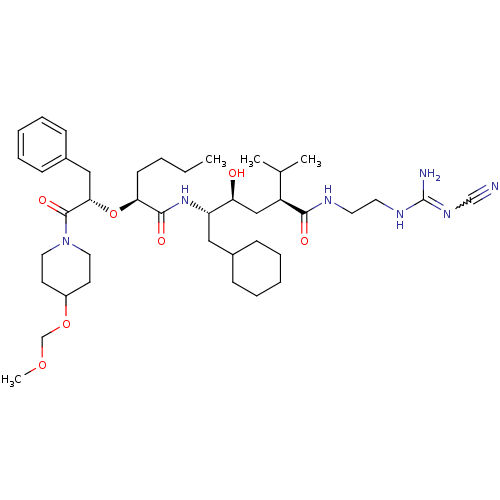 (1N-[2-amino(cyanoimino)methylaminoethyl]-5-[1-[1-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037007 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036985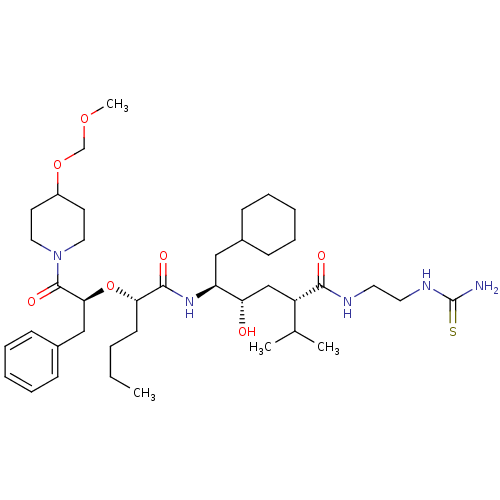 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036988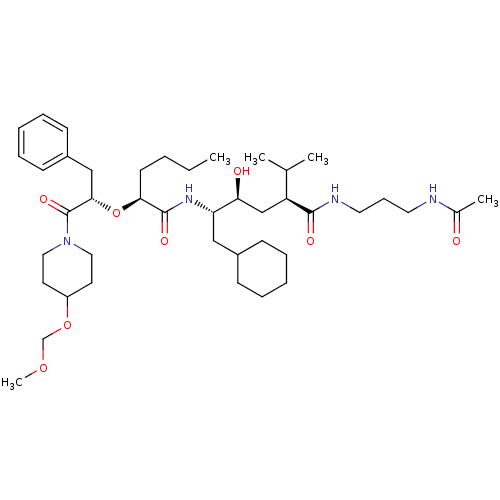 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036978 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037013 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036972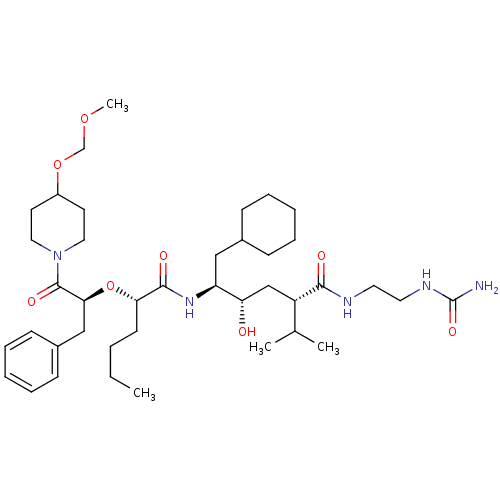 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006187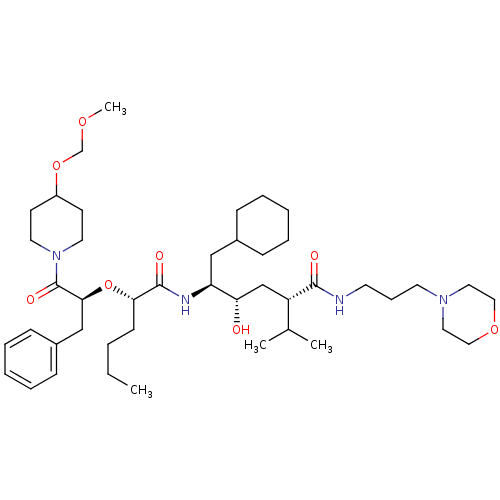 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036986 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037016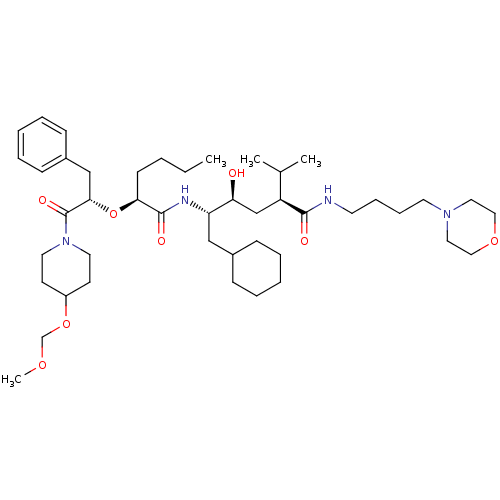 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037006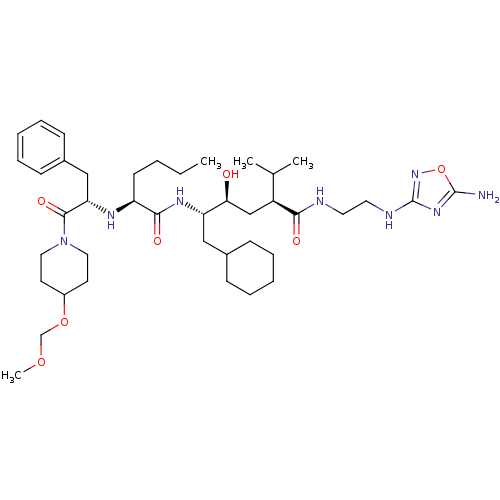 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036973 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037020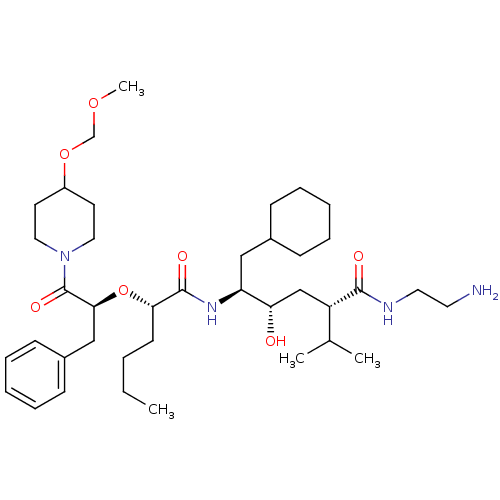 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036982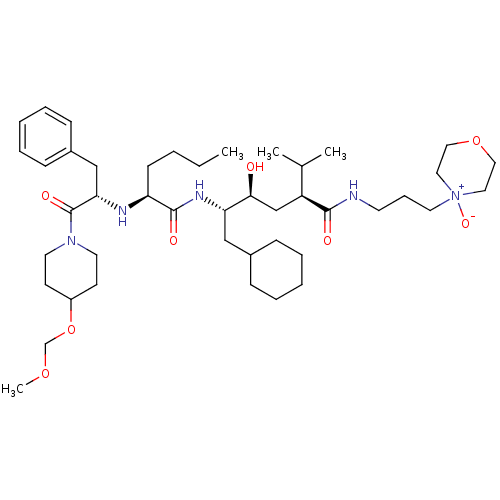 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036983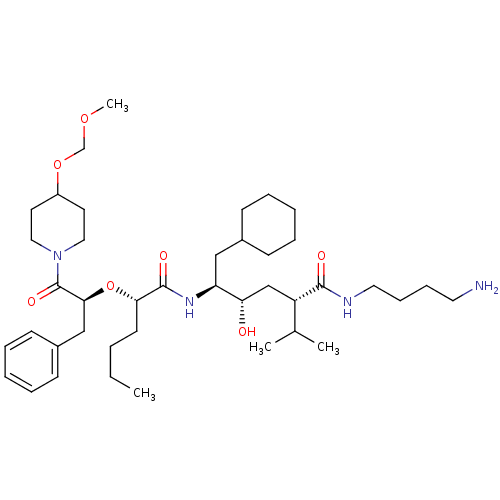 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037010 (CHEMBL100949 | [3-((2S,4S,5S)-5-{(S)-2-[(S)-1-Benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036975 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036984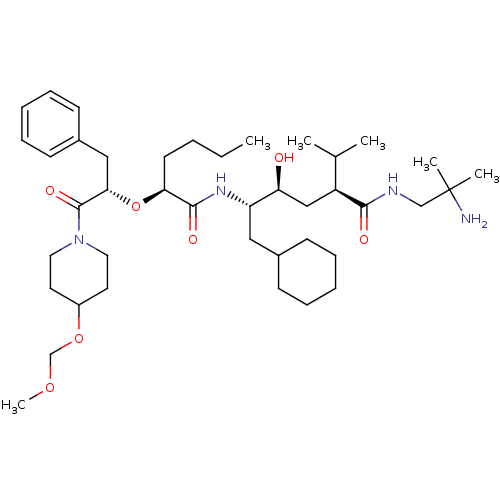 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036992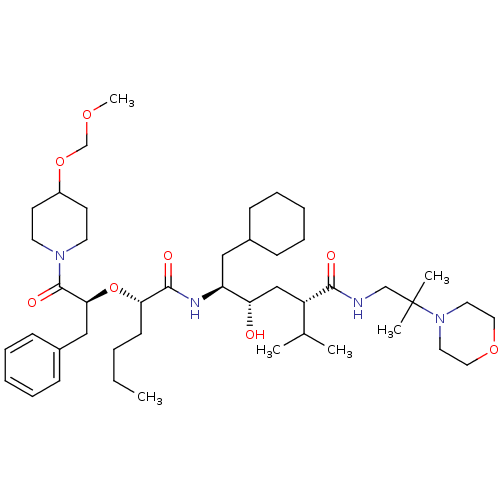 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037003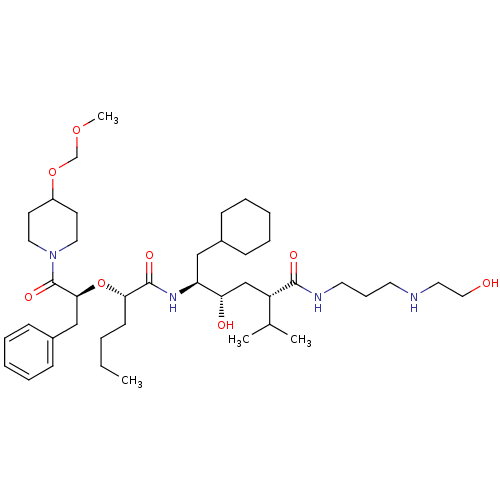 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036996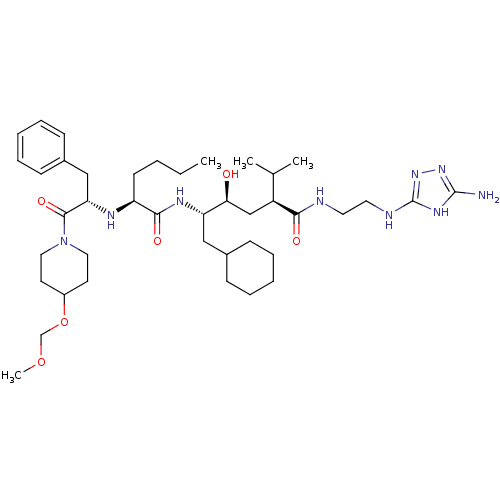 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037018 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037015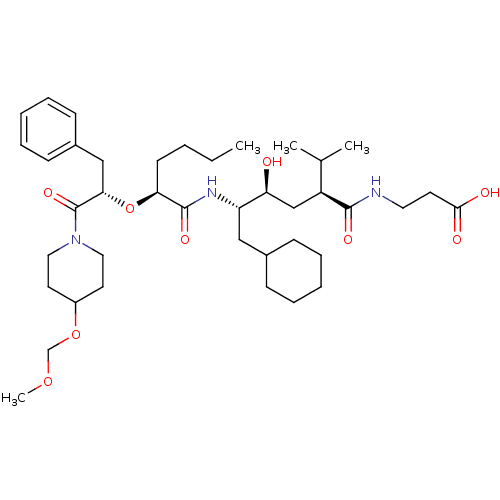 (3-((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006198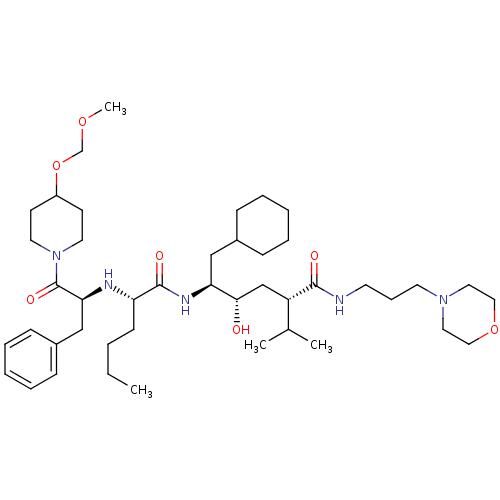 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50006179 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036971 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037011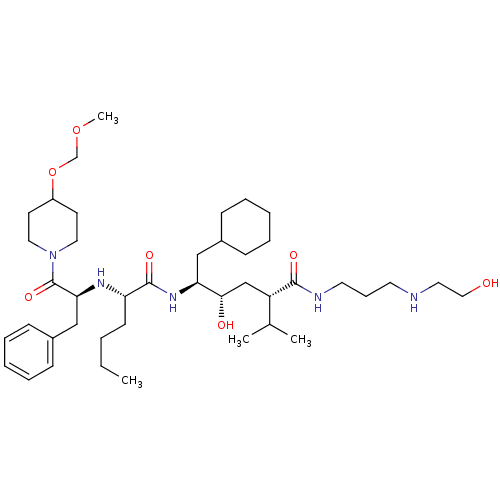 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037009 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036991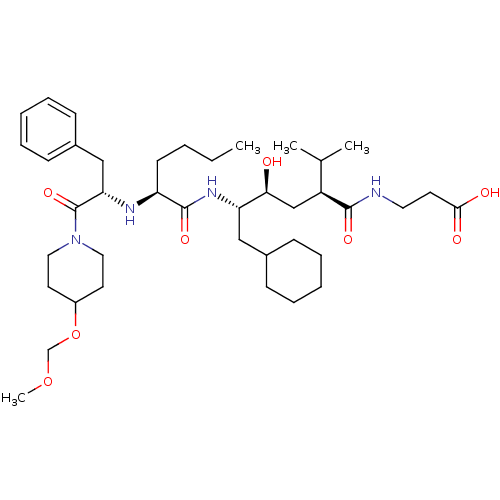 (3-((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036980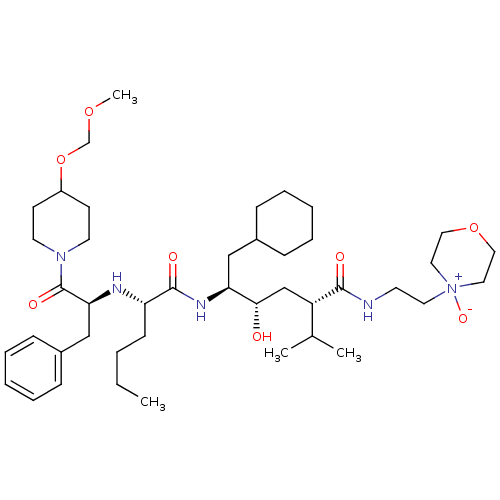 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036981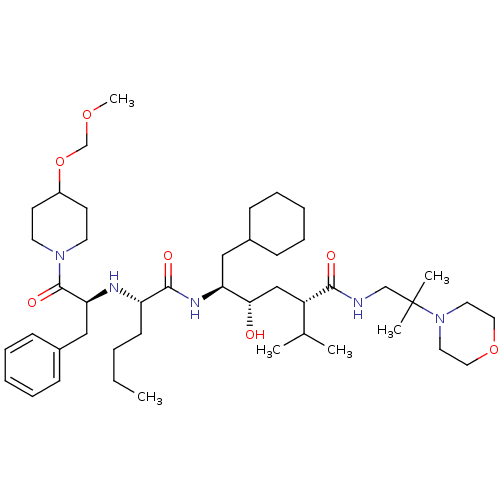 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037001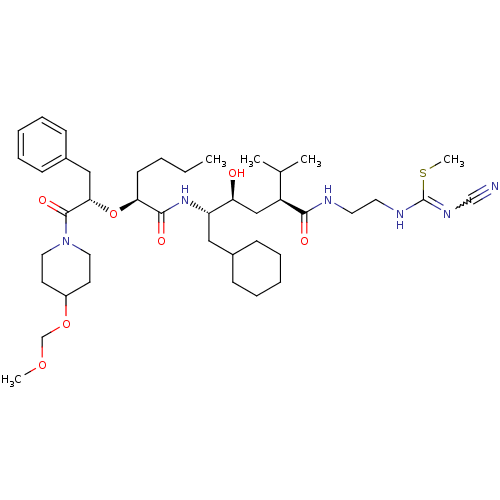 (1N-[2-cyanoimino(methylsulfanyl)methylaminoethyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036976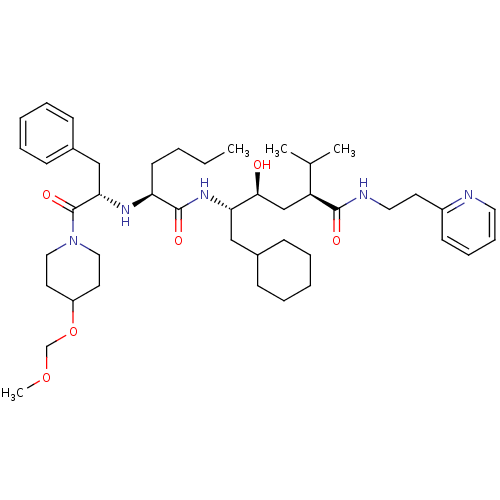 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037014 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036993 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037000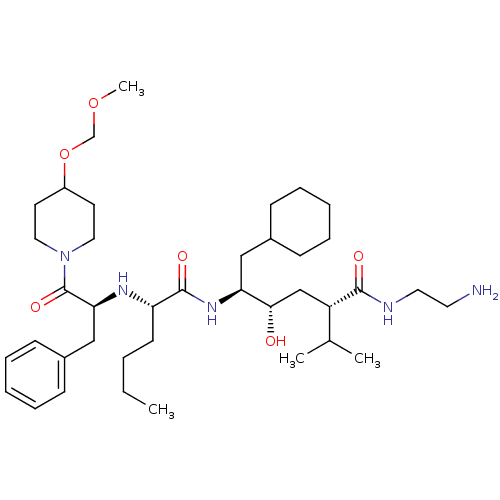 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037002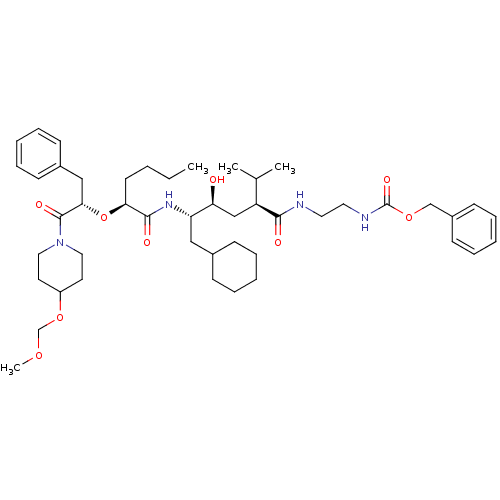 (CHEMBL329981 | [2-((2S,4S,5S)-5-{(S)-2-[(S)-1-Benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036994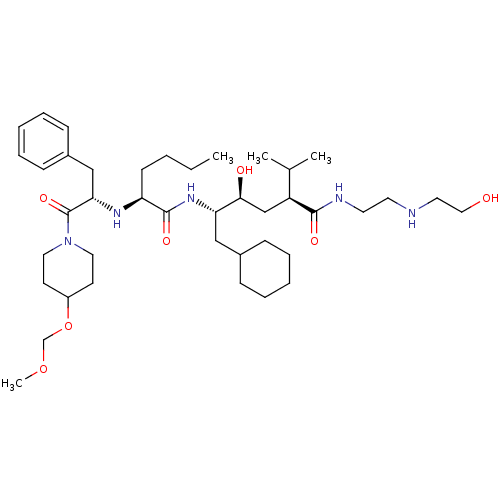 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037012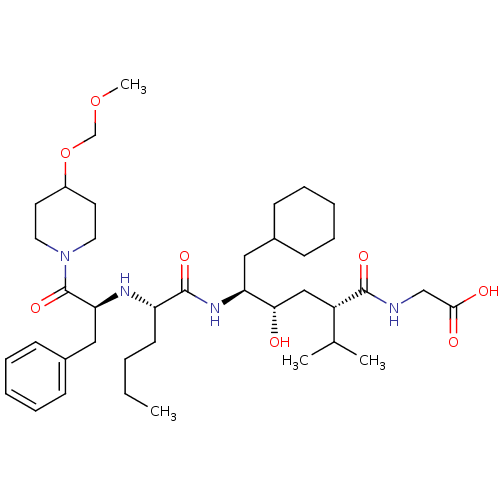 (((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036987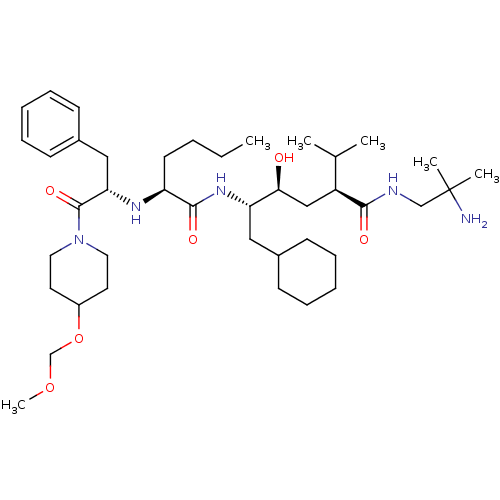 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50037004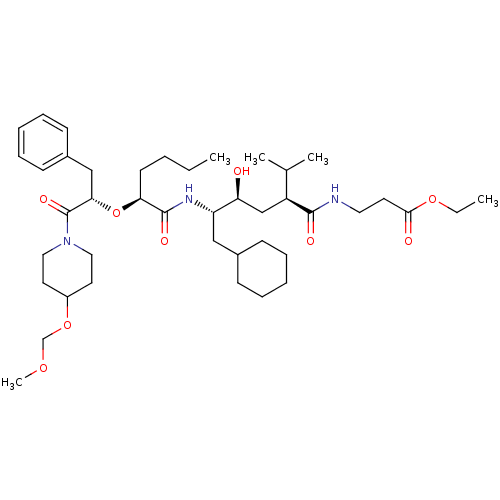 (3-((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin at pH of 7.4 | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036990 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036998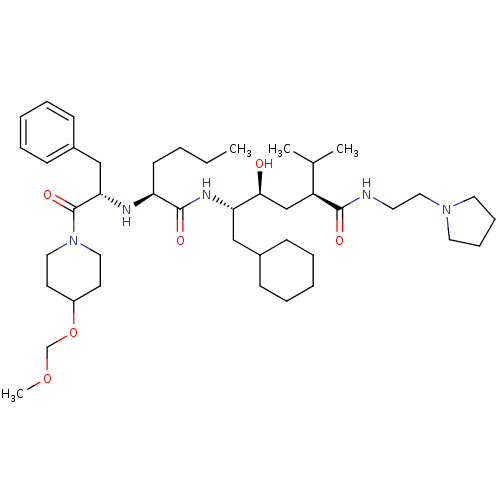 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the human plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036973 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the dog plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50036981 ((2S,4S,5S)-5-{(S)-2-[(S)-1-Benzyl-2-(4-methoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Tested in vitro for its ability to inhibit the dog plasma renin | J Med Chem 37: 2991-3007 (1994) BindingDB Entry DOI: 10.7270/Q26H4GFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 81 total ) | Next | Last >> |