

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135806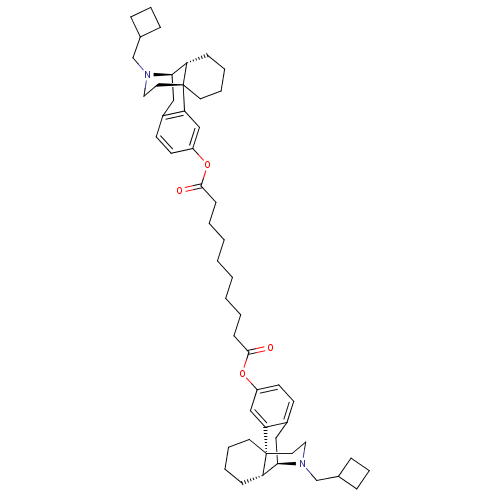 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300372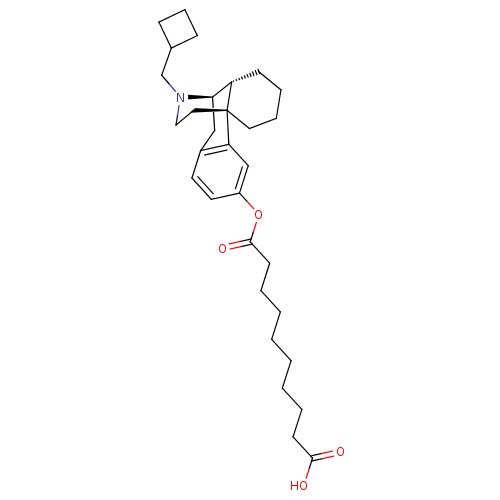 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300378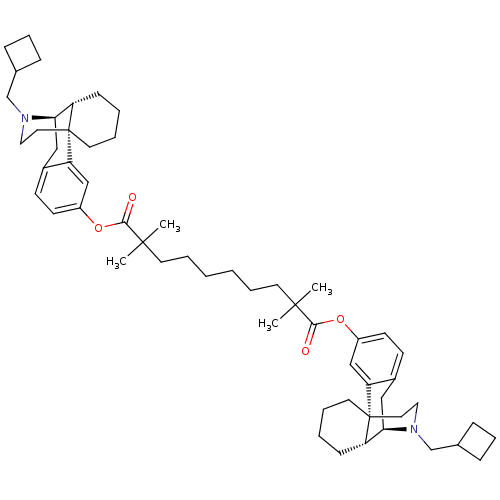 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300378 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300377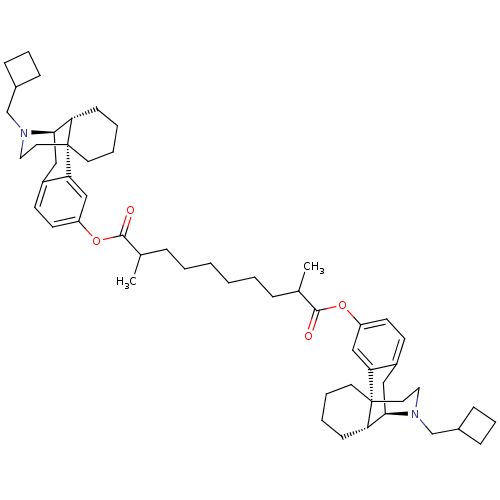 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]U69593 from human kappa opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300372 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300374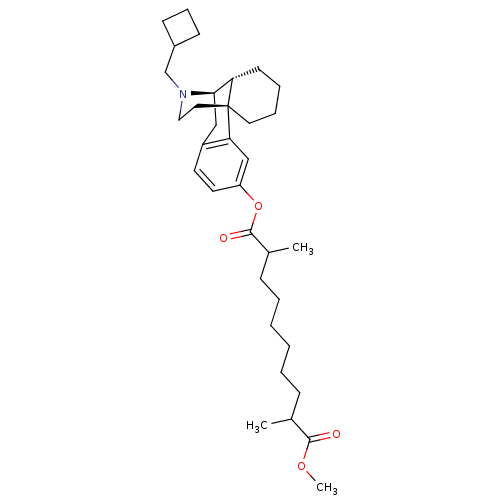 (CHEMBL566762 | Methyl 17-((-)-N-Cyclobutylmethyl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300374 (CHEMBL566762 | Methyl 17-((-)-N-Cyclobutylmethyl)m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300379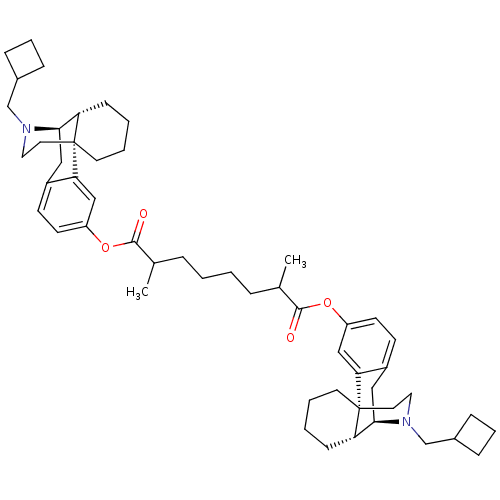 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,7-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300379 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,7-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300381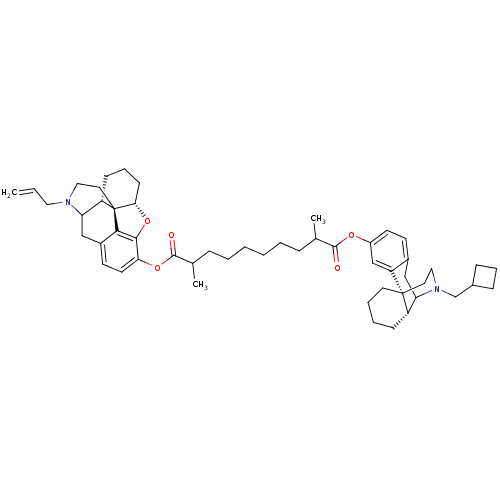 ((5alpha)-17-Allyl-14-hydroxy-6-oxo-4,5-epoxymorphi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300381 ((5alpha)-17-Allyl-14-hydroxy-6-oxo-4,5-epoxymorphi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300376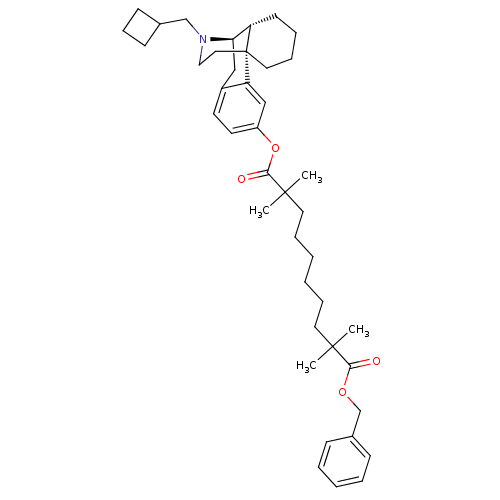 (Benzyl 17-((-)-N-Cyclobutylmethyl)morphinan-3-yl 2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300376 (Benzyl 17-((-)-N-Cyclobutylmethyl)morphinan-3-yl 2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300378 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300372 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300375 (2,2,9,9-Tetramethyldecanedioic Acid 10-((-)-N-Cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300374 (CHEMBL566762 | Methyl 17-((-)-N-Cyclobutylmethyl)m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300375 (2,2,9,9-Tetramethyldecanedioic Acid 10-((-)-N-Cycl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300379 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,7-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300381 ((5alpha)-17-Allyl-14-hydroxy-6-oxo-4,5-epoxymorphi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300376 (Benzyl 17-((-)-N-Cyclobutylmethyl)morphinan-3-yl 2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50300375 (2,2,9,9-Tetramethyldecanedioic Acid 10-((-)-N-Cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Displacement of [3H]naltrindole from human delta opioid receptor expressed in CHO cells after 3 hrs by liquid scintillation counting | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300378 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells assessed as inhibition of DAMGO-stimulated [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300380 ((1R,10R)-17-(cyclobutylmethyl)-17-azatetracyclo[7....) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300377 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,9-Dimet...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50135806 (CHEMBL147511 | MCL-144 | di[17-cyclobutylmethyl-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300373 (2,9-Dimethyldecanedioic Acid 10-((-)-N-Cyclobutylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300372 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50300372 (10-{[(1R,9R,10R)-17-(cyclobutylmethyl)-17-azatetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human kappa opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50300378 (Bis((-)-N-cyclobutylmethylmorphinan-3-yl)2,2,9,9-T...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as stimulation of [35S]GTPgammaS binding | J Med Chem 52: 7389-96 (2009) Article DOI: 10.1021/jm900379p BindingDB Entry DOI: 10.7270/Q29P31Q8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |