

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19423 (HDAC inhibitor, Compound 1 | N-[2-amino-5-(thiophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | <0.200 | <-55.4 | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178095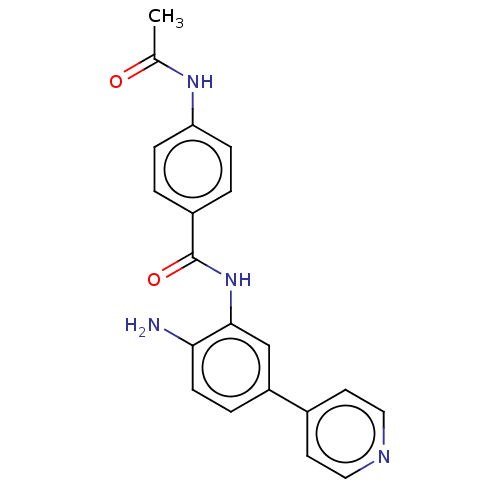 (BRD2492) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | 2 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24621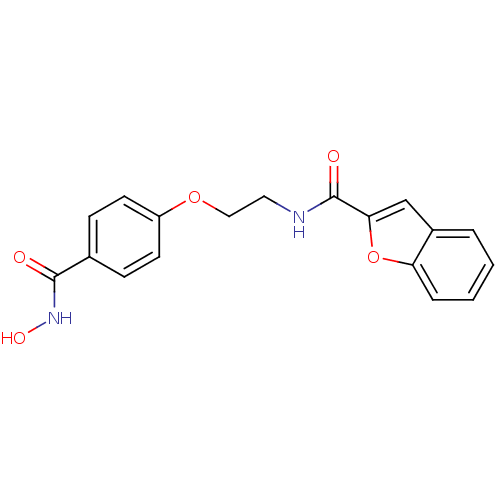 (CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24620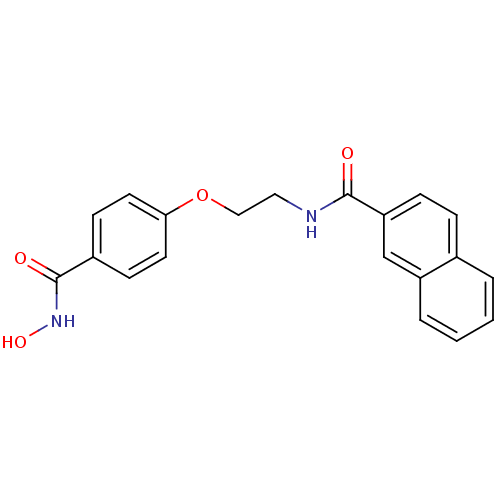 (CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24622 (3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19422 (4-(acetylamino)-N-(2-amino-phenyl) benzamide | CI-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | -42.4 | 41 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24618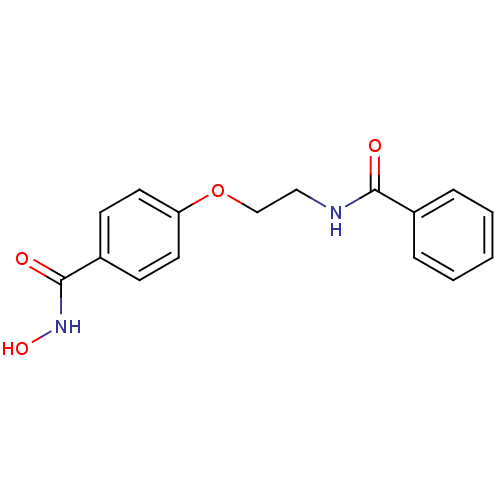 (CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM19149 (CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 48 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM22450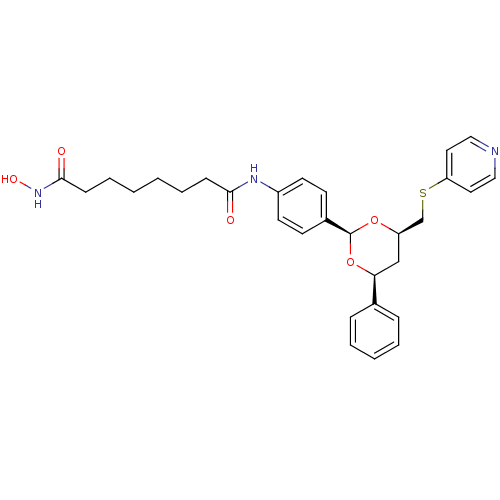 (N-hydroxy-N -(4-{(2R,4S,6R)-4-phenyl-6-[(pyridin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 88 | -39.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM22449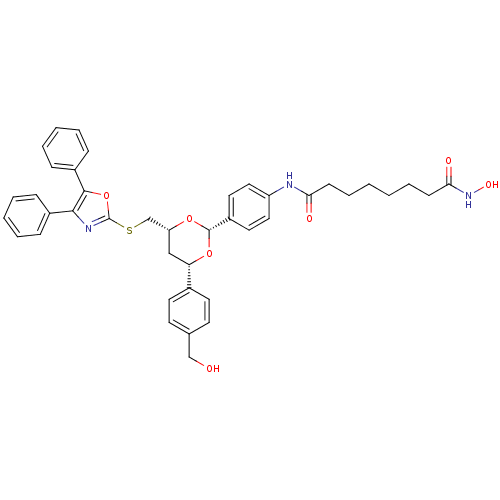 (CHEMBL356769 | N-(4-{(2R,4R,6S)-4-{[(4,5-diphenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 995 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Notre Dame | Assay Description To assess the effect of test compounds on histone deacetylase enzyme function in Vitro, a fluorometric assay was performed using HDAC, which incubate... | J Med Chem 51: 2898-906 (2008) Article DOI: 10.1021/jm7015254 BindingDB Entry DOI: 10.7270/Q2NC5ZG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM178100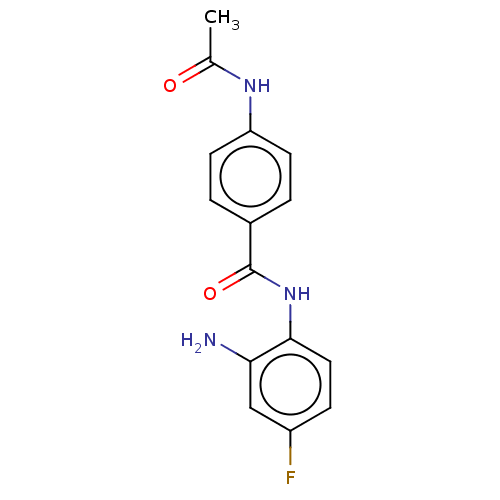 (BRD3308 | US11377423, Cmpd 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 5.10E+3 | -30.2 | 1.08E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Broad Institute of Harvard and MIT | Assay Description Purified HDAC1-9 (0.5~5 nM) were incubated with 2 μM carboxyfluorescein (FAM)-labeled acetylated peptide substrate A or B and test compound for ... | ACS Chem Biol 11: 363-74 (2016) Article DOI: 10.1021/acschembio.5b00640 BindingDB Entry DOI: 10.7270/Q2BZ64T2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM24623 (4-[2-({3-[(dimethylamino)methyl]-1-benzofuran-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics | Assay Description HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... | Mol Cancer Ther 5: 1309-17 (2006) Article DOI: 10.1158/1535-7163.MCT-05-0442 BindingDB Entry DOI: 10.7270/Q2DF6PJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM182756 (US9145412, Table 1, Compound 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | n/a |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9145412 (2015) BindingDB Entry DOI: 10.7270/Q28S4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM182757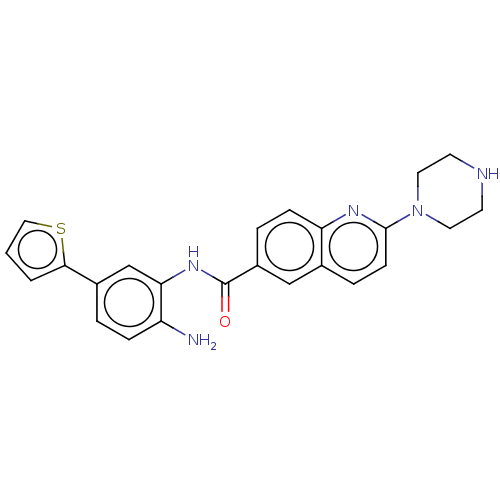 (US9145412, Table 1, Compound 23) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9145412 (2015) BindingDB Entry DOI: 10.7270/Q28S4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM182758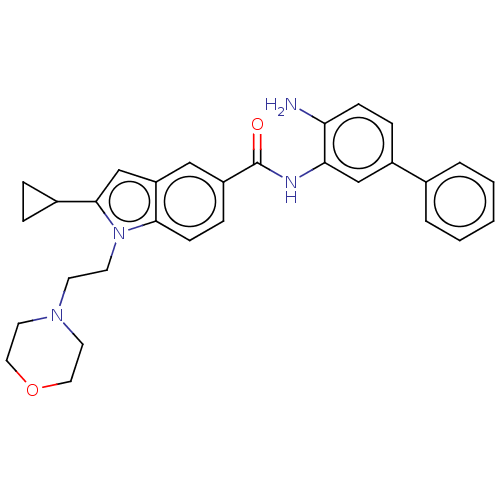 (US9145412, Table 1, Compound 33) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | n/a |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9145412 (2015) BindingDB Entry DOI: 10.7270/Q28S4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM182760 (US9145412, Table 1, Compound 47) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9145412 (2015) BindingDB Entry DOI: 10.7270/Q28S4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM182759 (US9145412, Table 1, Compound 48) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.4 | n/a |
ACETYLON PHARMACEUTICALS, INC. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9145412 (2015) BindingDB Entry DOI: 10.7270/Q28S4NQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM49152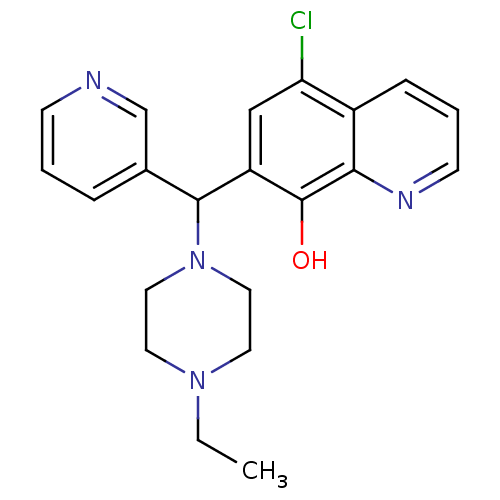 (5-chloranyl-7-[(4-ethylpiperazin-1-yl)-pyridin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.04E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Broad Institute | Assay Description Briefly, this fluorogenic assays uses an acetylated lysine tripeptide substrate, amide-linked to a fluorescently quenched aminocoumarin (AMC). Enzyme... | ACS Chem Biol 11: 1844-51 (2016) Article DOI: 10.1021/acschembio.6b00012 BindingDB Entry DOI: 10.7270/Q2QF8RPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213154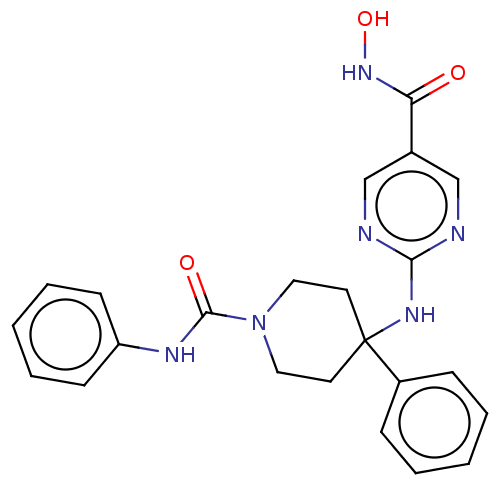 (US9278963, 001) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213155 (US9278963, 002) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213156 (US9278963, 003) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 346 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213157 (US9278963, 004) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 275 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213158 (US9278963, 005) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213159 (US9278963, 006) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 697 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213182 (US9278963, 007) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 119 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213161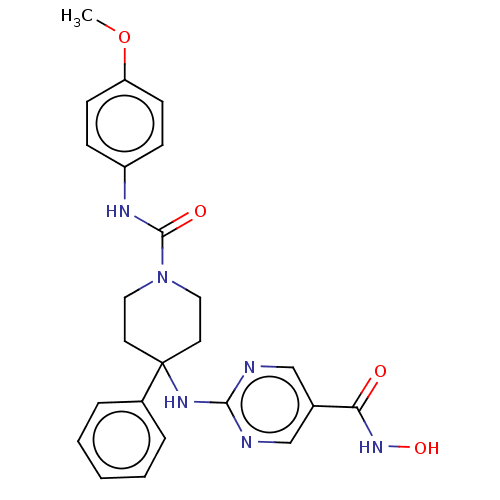 (US9278963, 008) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213162 (US9278963, 009) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 356 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213163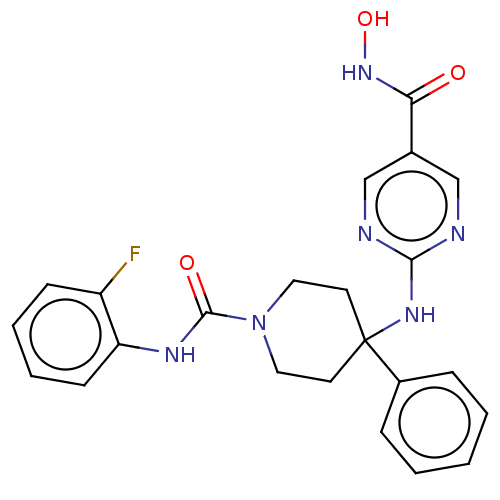 (US9278963, 010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213164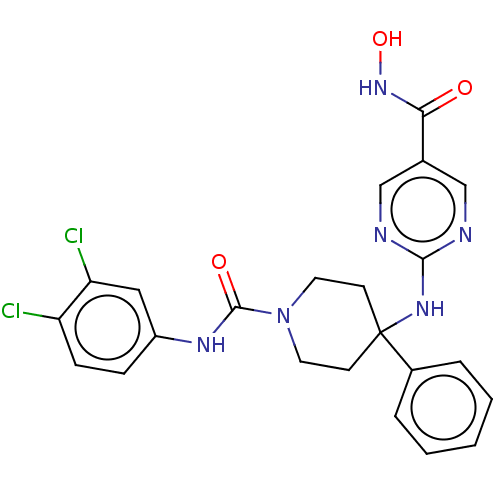 (US9278963, 011) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213165 (US9278963, 012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 266 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213166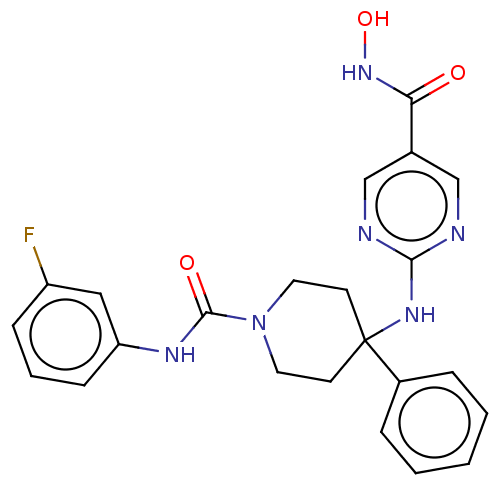 (US9278963, 013) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213167 (US9278963, 014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213168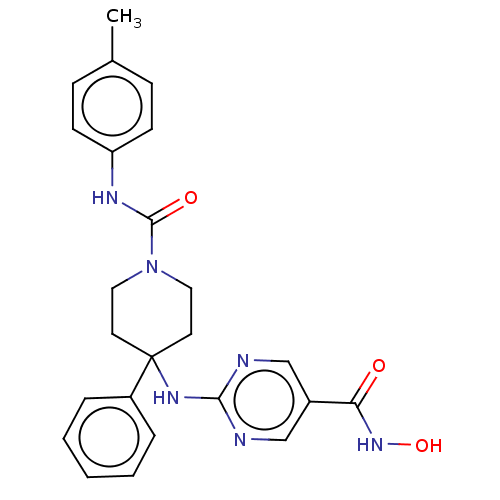 (US9278963, 015) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213169 (US9278963, 016) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213170 (US9278963, 017) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213171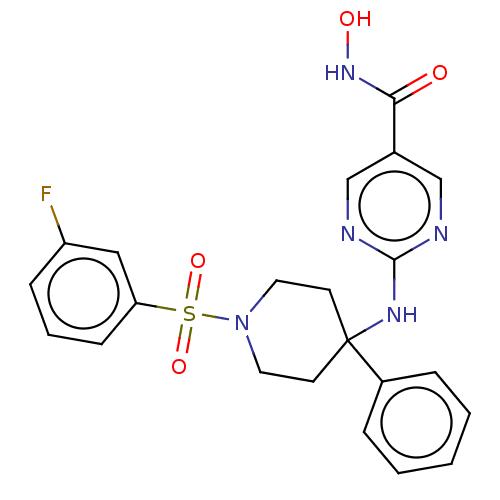 (US9278963, 018) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 551 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213172 (US9278963, 019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 854 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213173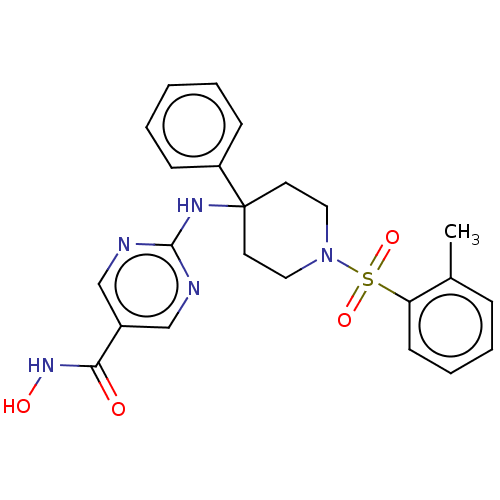 (US9278963, 020) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 372 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213174 (US9278963, 021) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 570 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213175 (US9278963, 022) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 704 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213176 (US9278963, 023) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 844 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213177 (US9278963, 024) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213183 (US9278963, 025) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213178 (US9278963, 026) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 206 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213184 (US9278963, 027) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213179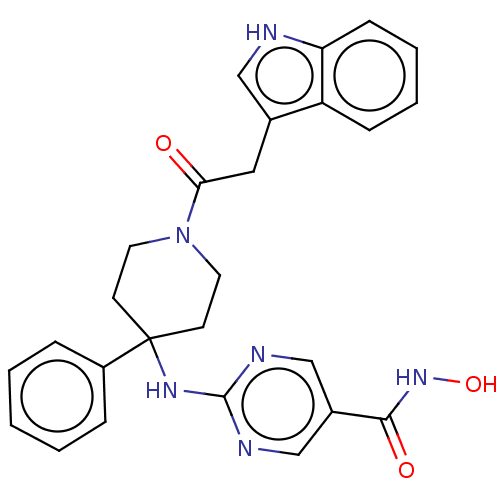 (US9278963, 028) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213180 (US9278963, 029) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 102 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213185 (US9278963, 030) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM213181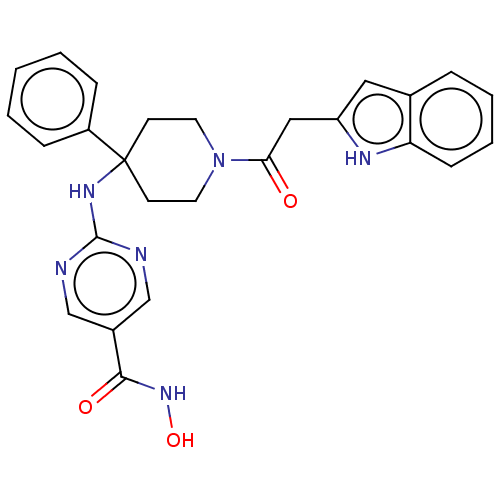 (US9278963, 031) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description The compounds were diluted in assay buffer (50 mM HEPES, pH 7.4, 100 mM KCl, 0.001% Tween-20, 0.05% BSA, 20 uM TCEP) to 6 fold their final concentrat... | US Patent US9278963 (2016) BindingDB Entry DOI: 10.7270/Q2GT5M0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM249364 (US9464073, 001 | US9884850, Compound 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Acetylon Pharmaceuticals, Inc. US Patent | Assay Description Compounds for testing were diluted in DMSO to 50 fold the final concentration and a ten point three fold dilution series was made. The compounds were... | US Patent US9464073 (2016) BindingDB Entry DOI: 10.7270/Q2TD9W8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 809 total ) | Next | Last >> |