

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50126761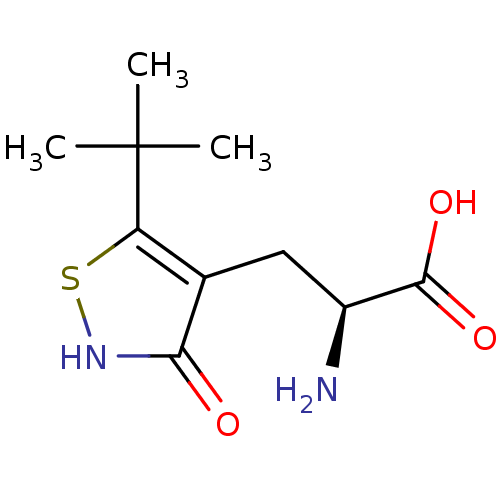 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GluK1 (unknown origin) | J Med Chem 56: 593-624 (2013) Article DOI: 10.1021/jm3011433 BindingDB Entry DOI: 10.7270/Q23B61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50126761 ((S)-2-AMINO-3-(3-HYDROXY-5-TERT-BUTYLISOTHIAZOL-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric kainate receptor (GluR5) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50166288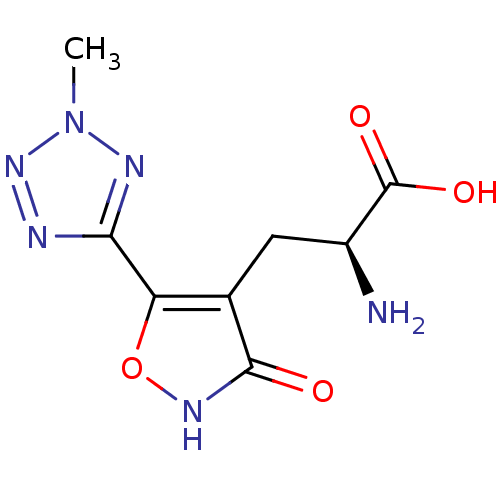 ((S)-2-Amino-3-[3-hydroxy-5-(2-methyl-2H-tetrazol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 110 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50126764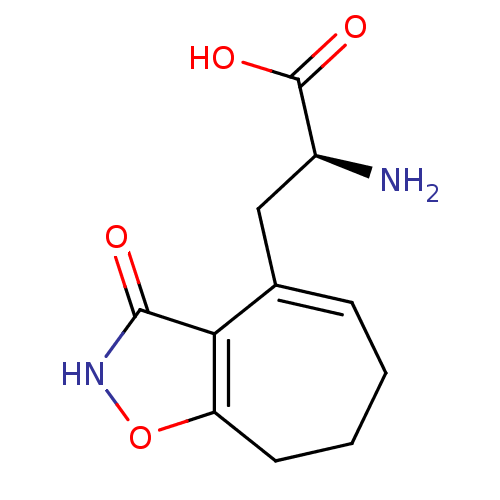 ((S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric Ionotropic glutamate receptor ionotropic kainate 1 (GluR5) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50126764 ((S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at ConA-treated rat GluR5(Q) RNA-edited isoform expressed in Xenopus laevis oocytes assessed as glutamic acid-induced maximal curren... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50060627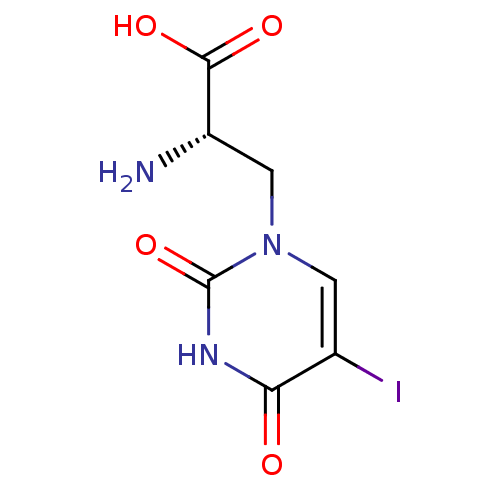 ((S)-2-Amino-3-(5-iodo-2,4-dioxo-3,4-dihydro-2H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Potency on Ionotropic glutamate receptor kainate in dorsal root ganglion cells | J Med Chem 40: 3645-50 (1997) Article DOI: 10.1021/jm9702387 BindingDB Entry DOI: 10.7270/Q28G8MCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50059672 ((S)-2-AMINO-3-[3-HYDROXY-5-(2-METHYL-2H-TETRAZOL-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 5 (Homo sapiens (Human)) | BDBM50126763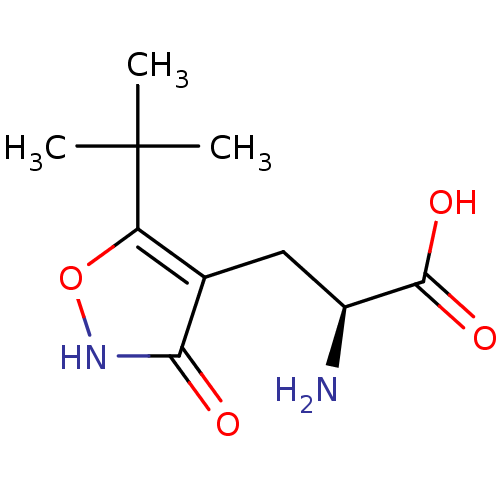 ((S)-2-amino-3-(3-hydroxy-5-tert-butyl-4-isoxazolyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 480 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Electrophysiological data on Xenopus oocytes, expressing homomeric Ionotropic glutamate receptor ionotropic kainate 1 (GluR5) | J Med Chem 46: 1350-8 (2003) Article DOI: 10.1021/jm0204441 BindingDB Entry DOI: 10.7270/Q2902346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50126764 ((S)-2-amino-3-(3-hydroxy-7,8-dihydro-6H-cyclohepta...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at rat recombinant GluR5(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50295042 ((S)-2-Amino-3-((RS)-3-hydroxy-8-methyl-7,8-dihydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at ConA-treated rat GluR5(Q) RNA-edited isoform expressed in Xenopus laevis oocytes assessed as glutamic acid-induced maximal curren... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50126763 ((S)-2-amino-3-(3-hydroxy-5-tert-butyl-4-isoxazolyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 660 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at ConA-treated rat GluR5(Q) RNA-edited isoform expressed in Xenopus laevis oocytes assessed as glutamic acid-induced maximal curren... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50002369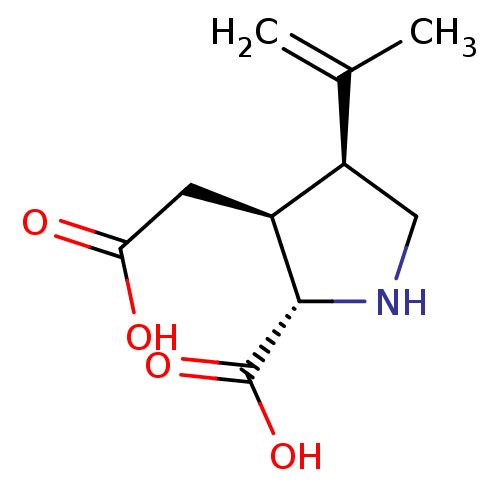 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50088222 ((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity on rat dorsal root ganglion neurons (thought to express GluR5 receptors) | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50295042 ((S)-2-Amino-3-((RS)-3-hydroxy-8-methyl-7,8-dihydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 860 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at rat recombinant GluR5(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50091477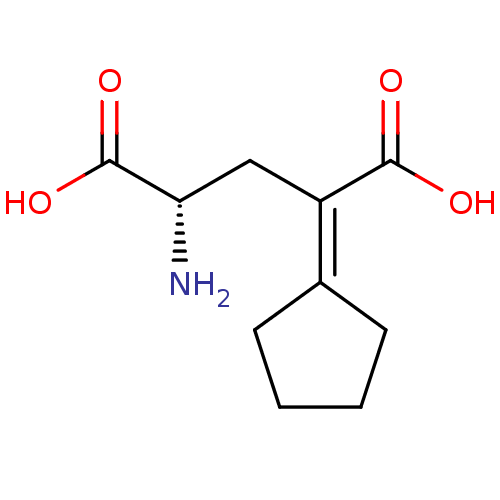 ((S)-2-Amino-4-cyclopentylidene-pentanedioic acid |...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity on rat dorsal root ganglion neurons (thought to express GluR5 receptors) | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088216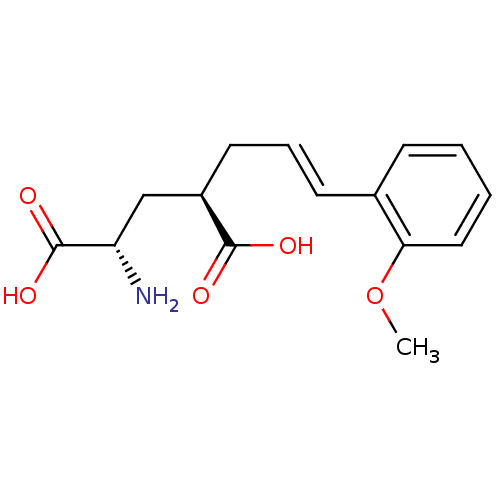 (CHEMBL59284 | E-2-Amino-4-[3-(2-methoxy-phenyl)-al...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Rattus norvegicus) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at rat recombinant GluR6(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088222 ((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088222 ((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against GluR5 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088222 ((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at GluK1 (unknown origin) | J Med Chem 56: 593-624 (2013) Article DOI: 10.1021/jm3011433 BindingDB Entry DOI: 10.7270/Q23B61GZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088197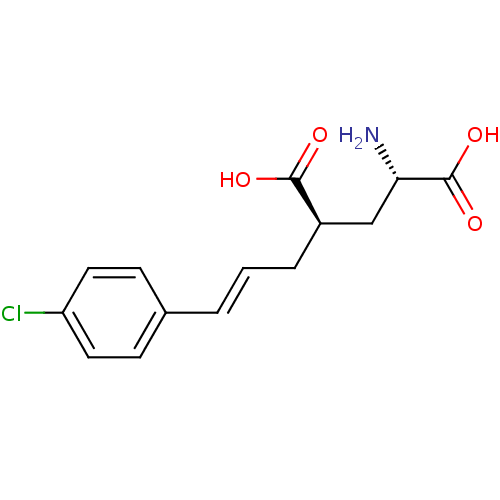 (CHEMBL58590 | E-2-Amino-4-[3-(4-chloro-phenyl)-all...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration of the compound required for evoking response in HEK293 cell | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088216 (CHEMBL59284 | E-2-Amino-4-[3-(2-methoxy-phenyl)-al...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against GluR5 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50166286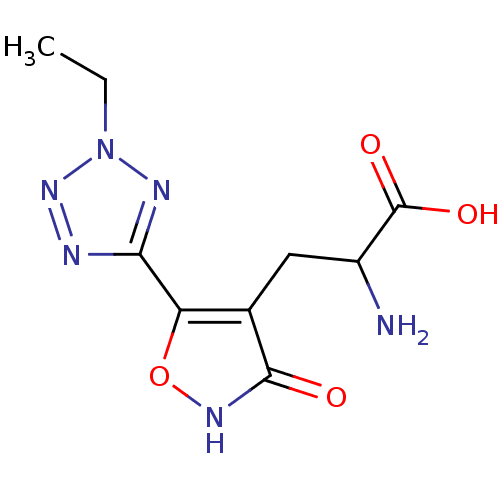 ((RS)-2-amino-3-[3-hydroxy-5-(2-ethyl-2H-5-tetrazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM17662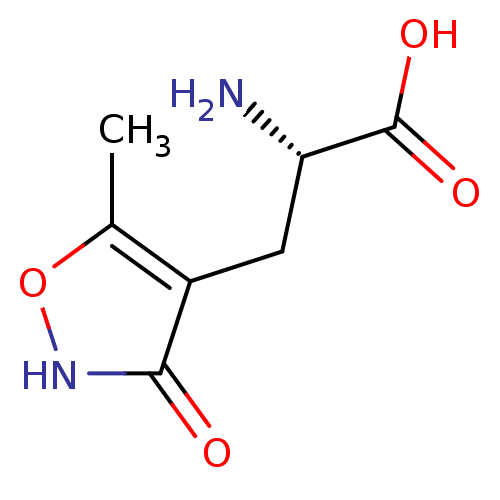 ((2S)-2-amino-3-(3-hydroxy-5-methyl-1,2-oxazol-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088213 ((2S,4R)-4-allyl glutamate | 2-Allyl-4-amino-pentan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration of the compound required for evoking response in HEK293 cell | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50088212 (2-Amino-4-but-2-enyl-pentanedioic acid(LY339180) |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration of the compound required for evoking response in HEK293 cell | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50091477 ((S)-2-Amino-4-cyclopentylidene-pentanedioic acid |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088213 ((2S,4R)-4-allyl glutamate | 2-Allyl-4-amino-pentan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity on rat dorsal root ganglion neurons (thought to express GluR5 receptors) | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50091481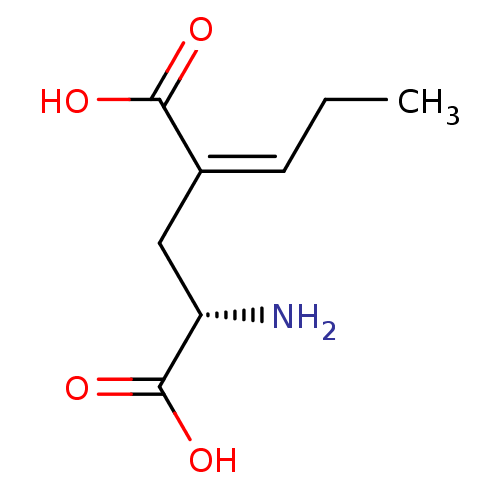 ((S)-2-Amino-4-propylidene-pentanedioicacid | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.42E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 5 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | n/a | n/a | 1.62E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against GluR5 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088213 ((2S,4R)-4-allyl glutamate | 2-Allyl-4-amino-pentan...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a |
University of California Berkeley Curated by ChEMBL | Assay Description Agonist activity at iGluR6 L439C mutant expressed in human HEK293 cells assessed as increase in intracellular Ca2+ levels by FURA-2-M staining based ... | Nat Chem Biol 2: 47-52 (2006) Article DOI: 10.1038/nchembio756 BindingDB Entry DOI: 10.7270/Q2JS9T8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM26431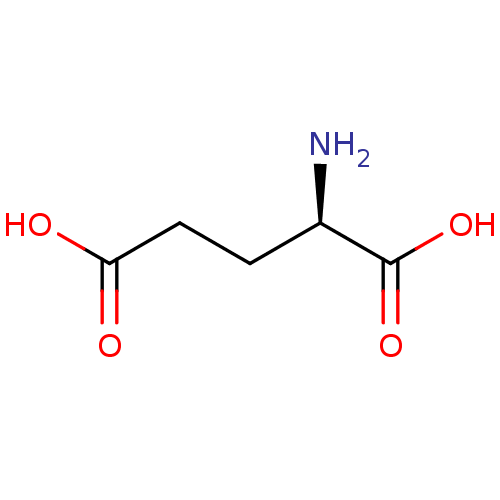 ((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a |
University Walk Curated by ChEMBL | Assay Description Induction of calcium influx in HEK293 cells expressing human GLUK5 by FLIPR assay | J Med Chem 49: 2579-92 (2006) Article DOI: 10.1021/jm051086f BindingDB Entry DOI: 10.7270/Q2FQ9W76 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM26431 ((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50166285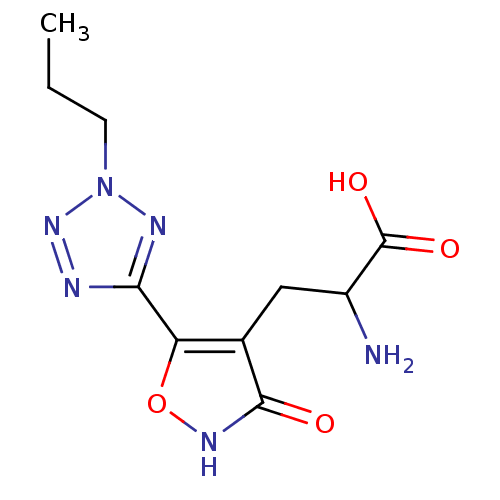 ((RS)-2-amino-3-[3-hydroxy-5-(2-propyl-2H-5-tetrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 3.52E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity on rat dorsal root ganglion neurons (thought to express GluR5 receptors) | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50002369 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at rat recombinant GluR5(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088212 (2-Amino-4-but-2-enyl-pentanedioic acid(LY339180) |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.83E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Rattus norvegicus) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Activity at rat cloned iGluR6 expressed in human HEK293 cells by calcium imaging assay | J Med Chem 51: 2311-5 (2008) Article DOI: 10.1021/jm701394a BindingDB Entry DOI: 10.7270/Q2Z320HT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088197 (CHEMBL58590 | E-2-Amino-4-[3-(4-chloro-phenyl)-all...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.08E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 3 (Rattus norvegicus) | BDBM50166287 ((RS)-2-amino-3-[3-hydroxy-5-(2-isopropyl-2H-5-tetr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a |
The Danish University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description In vitro activity of the compound at Ionotropic glutamate receptor using cortical wedge assays in rat brain synaptosoma | J Med Chem 48: 3438-42 (2005) Article DOI: 10.1021/jm050014l BindingDB Entry DOI: 10.7270/Q2H70FBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Rattus norvegicus) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Agonist activity at rat recombinant GluR6(Q) RNA-edited isoform expressed in HEK293 cells assessed as increase in intracellular calcium level by Fluo... | J Med Chem 52: 4911-22 (2009) Checked by Author Article DOI: 10.1021/jm900565c BindingDB Entry DOI: 10.7270/Q2R49RP9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM17657 ((2S)-2-aminopentanedioic acid | (S)-Glu | D-Glutam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Compound was tested for agonistic activity at Glutamate receptor 6 using HEK293 cells | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (Homo sapiens (Human)) | BDBM26431 ((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against GluR5 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Rattus norvegicus) | BDBM50373180 (CHEMBL260328) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Activity at rat cloned iGluR6 expressed in human HEK293 cells by calcium imaging assay | J Med Chem 51: 2311-5 (2008) Article DOI: 10.1021/jm701394a BindingDB Entry DOI: 10.7270/Q2Z320HT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50091477 ((S)-2-Amino-4-cyclopentylidene-pentanedioic acid |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd Curated by ChEMBL | Assay Description Binding affinity of compound was determined against Ionotropic glutamate receptor ionotropic kainate 2 using cell membranes prepared from HEK293 cell... | Bioorg Med Chem Lett 10: 1807-10 (2000) BindingDB Entry DOI: 10.7270/Q2765FVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 2 (Homo sapiens (Human)) | BDBM50088222 ((2S,4R)-2-Amino-4-((E)-3-naphthalen-2-yl-allyl)-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
Lilly, S.A. Curated by ChEMBL | Assay Description Effective concentration against human GluR6 expressed in HEK293 cells | J Med Chem 43: 1958-68 (2000) BindingDB Entry DOI: 10.7270/Q2FX78QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1291 total ) | Next | Last >> |