 Found 67 hits of Enzyme Inhibition Constant Data
Found 67 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109327
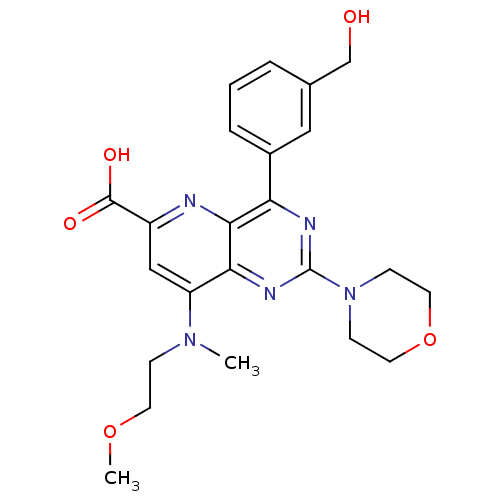
(US8609666, 47)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C23H27N5O5/c1-27(6-9-32-2)18-13-17(22(30)31)24-21-19(16-5-3-4-15(12-16)14-29)25-23(26-20(18)21)28-7-10-33-11-8-28/h3-5,12-13,29H,6-11,14H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109313
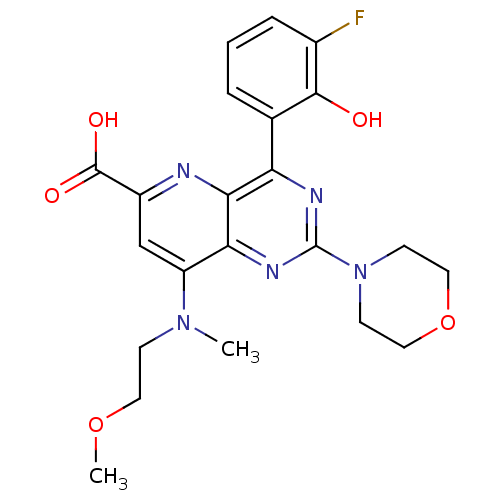
(US8609666, 33)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C22H24FN5O5/c1-27(6-9-32-2)16-12-15(21(30)31)24-19-17(13-4-3-5-14(23)20(13)29)25-22(26-18(16)19)28-7-10-33-11-8-28/h3-5,12,29H,6-11H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109317

(US8609666, 37)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C21H22FN5O5/c1-26(5-8-28)15-11-14(20(30)31)23-18-16(12-3-2-4-13(22)19(12)29)24-21(25-17(15)18)27-6-9-32-10-7-27/h2-4,11,28-29H,5-10H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109314
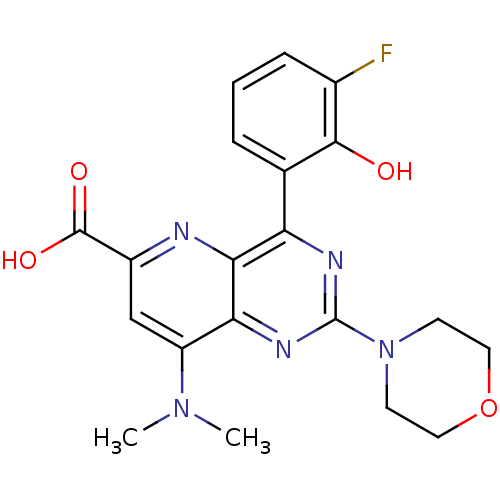
(US8609666, 34)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C20H20FN5O4/c1-25(2)14-10-13(19(28)29)22-17-15(11-4-3-5-12(21)18(11)27)23-20(24-16(14)17)26-6-8-30-9-7-26/h3-5,10,27H,6-9H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109341
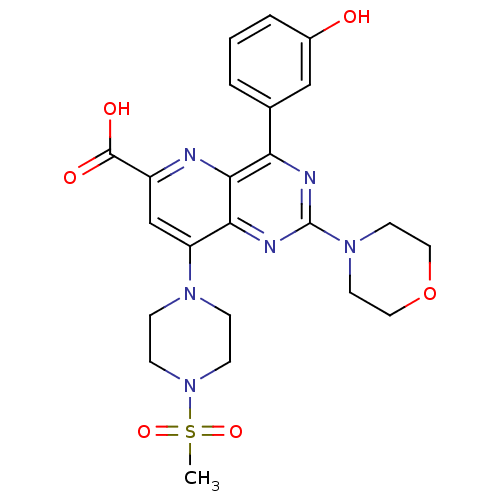
(US8609666, 61)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C23H26N6O6S/c1-36(33,34)29-7-5-27(6-8-29)18-14-17(22(31)32)24-21-19(15-3-2-4-16(30)13-15)25-23(26-20(18)21)28-9-11-35-12-10-28/h2-4,13-14,30H,5-12H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109336

(US8609666, 56)Show SMILES CS(=O)(=O)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(O)cc(F)c1)C(O)=O Show InChI InChI=1S/C19H17FN4O6S/c1-31(28,29)14-9-13(18(26)27)21-17-15(10-6-11(20)8-12(25)7-10)22-19(23-16(14)17)24-2-4-30-5-3-24/h6-9,25H,2-5H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109316

(US8609666, 36)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C21H22FN5O5/c1-31-8-5-23-14-11-15(20(29)30)24-18-16(12-3-2-4-13(22)19(12)28)25-21(26-17(14)18)27-6-9-32-10-7-27/h2-4,11,28H,5-10H2,1H3,(H,23,24)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109294
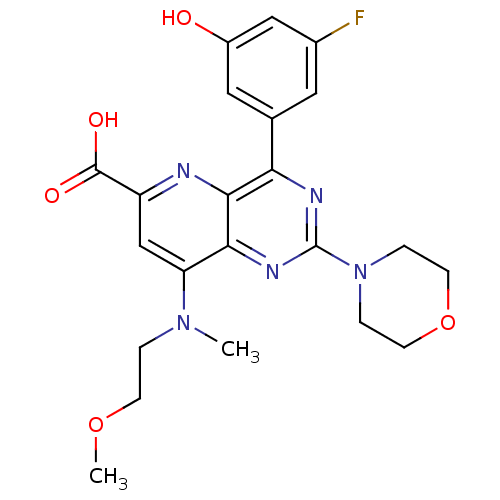
(US8609666, 14)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(O)cc(F)c1)C(O)=O Show InChI InChI=1S/C22H24FN5O5/c1-27(3-6-32-2)17-12-16(21(30)31)24-20-18(13-9-14(23)11-15(29)10-13)25-22(26-19(17)20)28-4-7-33-8-5-28/h9-12,29H,3-8H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109322
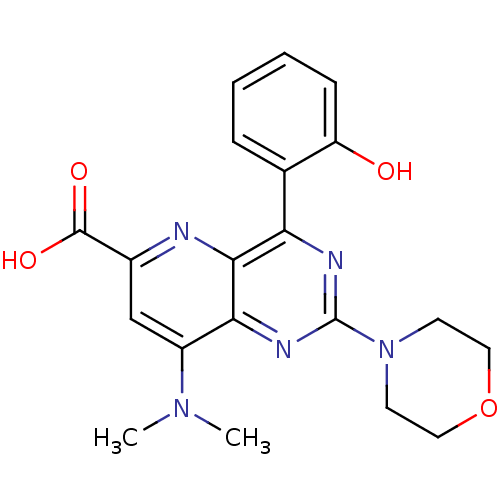
(US8609666, 42)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccccc1O)C(O)=O Show InChI InChI=1S/C20H21N5O4/c1-24(2)14-11-13(19(27)28)21-18-16(12-5-3-4-6-15(12)26)22-20(23-17(14)18)25-7-9-29-10-8-25/h3-6,11,26H,7-10H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109293
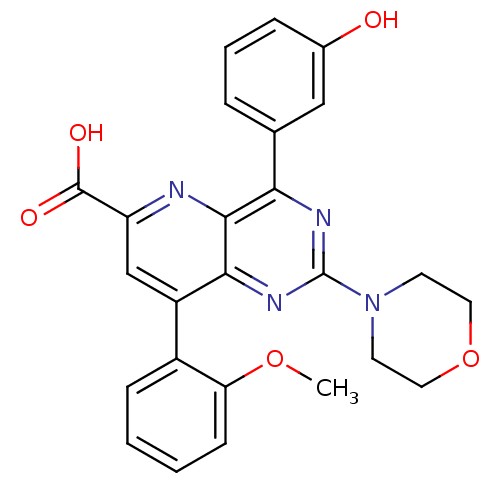
(US8609666, 13)Show SMILES COc1ccccc1-c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C25H22N4O5/c1-33-20-8-3-2-7-17(20)18-14-19(24(31)32)26-23-21(15-5-4-6-16(30)13-15)27-25(28-22(18)23)29-9-11-34-12-10-29/h2-8,13-14,30H,9-12H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109319
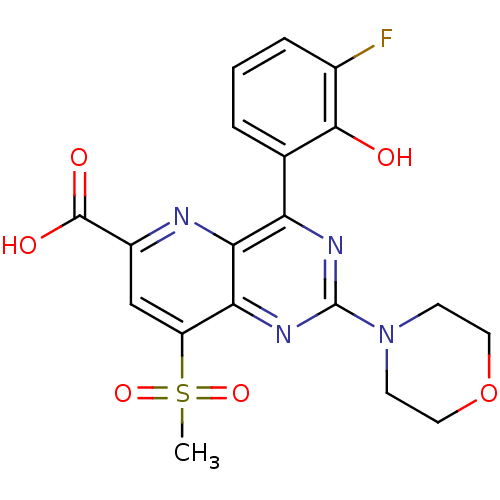
(US8609666, 39)Show SMILES CS(=O)(=O)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C19H17FN4O6S/c1-31(28,29)13-9-12(18(26)27)21-16-14(10-3-2-4-11(20)17(10)25)22-19(23-15(13)16)24-5-7-30-8-6-24/h2-4,9,25H,5-8H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109328
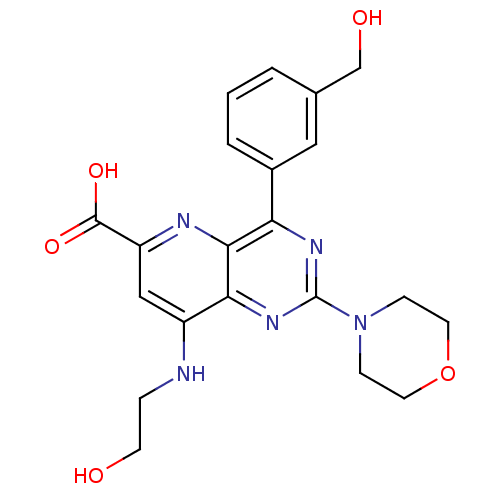
(US8609666, 48)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C21H23N5O5/c27-7-4-22-15-11-16(20(29)30)23-19-17(14-3-1-2-13(10-14)12-28)24-21(25-18(15)19)26-5-8-31-9-6-26/h1-3,10-11,27-28H,4-9,12H2,(H,22,23)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109290

(US8609666, 10)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C22H25N5O5/c1-26(6-9-31-2)17-13-16(21(29)30)23-20-18(14-4-3-5-15(28)12-14)24-22(25-19(17)20)27-7-10-32-11-8-27/h3-5,12-13,28H,6-11H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109347
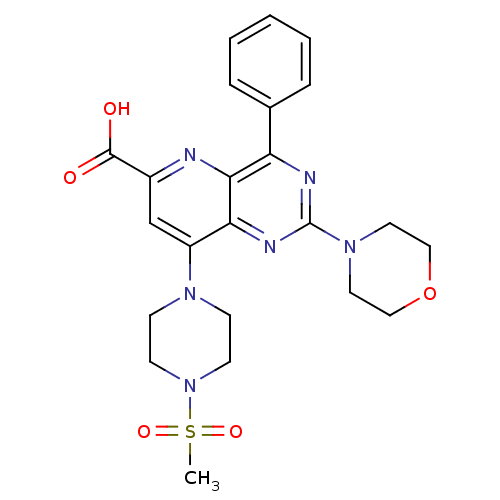
(US8609666, 67)Show SMILES CS(=O)(=O)N1CCN(CC1)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccccc1)C(O)=O Show InChI InChI=1S/C23H26N6O5S/c1-35(32,33)29-9-7-27(8-10-29)18-15-17(22(30)31)24-21-19(16-5-3-2-4-6-16)25-23(26-20(18)21)28-11-13-34-14-12-28/h2-6,15H,7-14H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109303
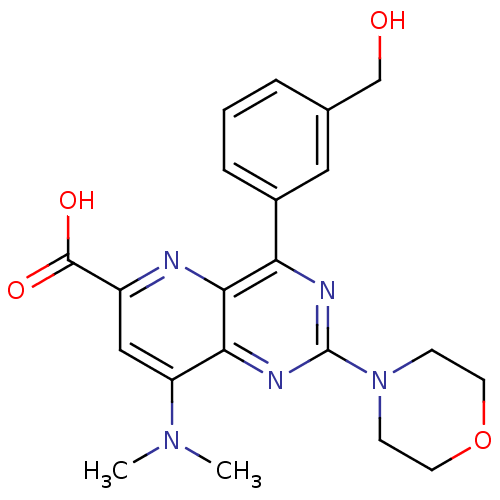
(US8609666, 23)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C21H23N5O4/c1-25(2)16-11-15(20(28)29)22-19-17(14-5-3-4-13(10-14)12-27)23-21(24-18(16)19)26-6-8-30-9-7-26/h3-5,10-11,27H,6-9,12H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109318
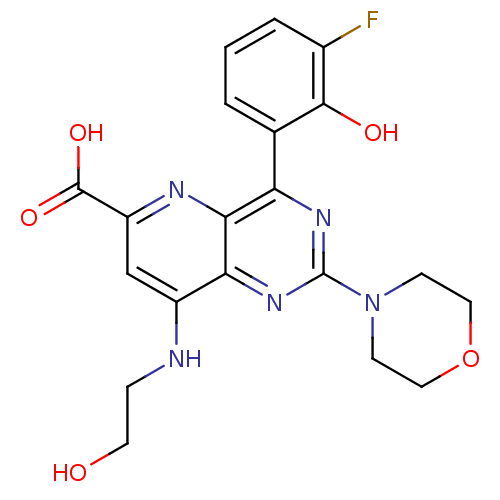
(US8609666, 38)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C20H20FN5O5/c21-12-3-1-2-11(18(12)28)15-17-16(25-20(24-15)26-5-8-31-9-6-26)13(22-4-7-27)10-14(23-17)19(29)30/h1-3,10,27-28H,4-9H2,(H,22,23)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109315
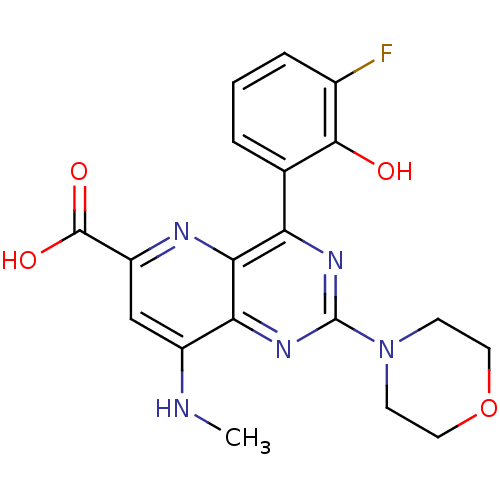
(US8609666, 35)Show SMILES CNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(F)c1O)C(O)=O Show InChI InChI=1S/C19H18FN5O4/c1-21-12-9-13(18(27)28)22-16-14(10-3-2-4-11(20)17(10)26)23-19(24-15(12)16)25-5-7-29-8-6-25/h2-4,9,26H,5-8H2,1H3,(H,21,22)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109343
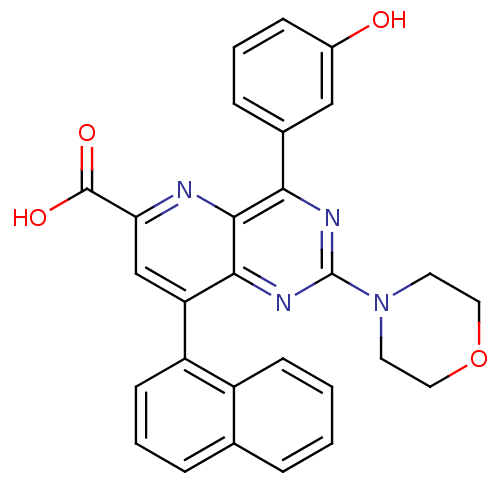
(US8609666, 63)Show SMILES OC(=O)c1cc(-c2cccc3ccccc23)c2nc(nc(-c3cccc(O)c3)c2n1)N1CCOCC1 Show InChI InChI=1S/C28H22N4O4/c33-19-8-3-7-18(15-19)24-26-25(31-28(30-24)32-11-13-36-14-12-32)22(16-23(29-26)27(34)35)21-10-4-6-17-5-1-2-9-20(17)21/h1-10,15-16,33H,11-14H2,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109331

(US8609666, 51)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)cc1O)C(O)=O Show InChI InChI=1S/C22H24FN5O5/c1-27(5-8-32-2)16-12-15(21(30)31)24-20-18(14-4-3-13(23)11-17(14)29)25-22(26-19(16)20)28-6-9-33-10-7-28/h3-4,11-12,29H,5-10H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109321
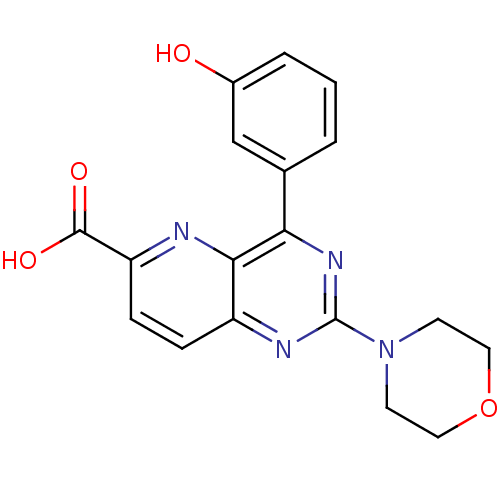
(US8609666, 41)Show SMILES OC(=O)c1ccc2nc(nc(-c3cccc(O)c3)c2n1)N1CCOCC1 Show InChI InChI=1S/C18H16N4O4/c23-12-3-1-2-11(10-12)15-16-13(4-5-14(19-16)17(24)25)20-18(21-15)22-6-8-26-9-7-22/h1-5,10,23H,6-9H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109296
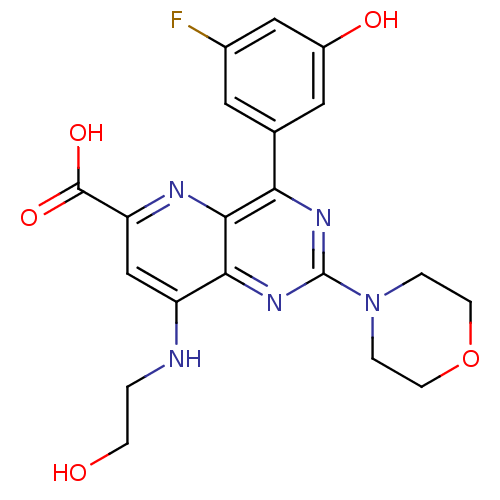
(US8609666, 16)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(O)cc(F)c1)C(O)=O Show InChI InChI=1S/C20H20FN5O5/c21-12-7-11(8-13(28)9-12)16-18-17(25-20(24-16)26-2-5-31-6-3-26)14(22-1-4-27)10-15(23-18)19(29)30/h7-10,27-28H,1-6H2,(H,22,23)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109309

(US8609666, 29)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)ccc1O)C(O)=O Show InChI InChI=1S/C20H20FN5O4/c1-25(2)14-10-13(19(28)29)22-18-16(12-9-11(21)3-4-15(12)27)23-20(24-17(14)18)26-5-7-30-8-6-26/h3-4,9-10,27H,5-8H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109311

(US8609666, 31)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)ccc1O)C(O)=O Show InChI InChI=1S/C21H22FN5O5/c1-31-7-4-23-14-11-15(20(29)30)24-19-17(13-10-12(22)2-3-16(13)28)25-21(26-18(14)19)27-5-8-32-9-6-27/h2-3,10-11,28H,4-9H2,1H3,(H,23,24)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109304
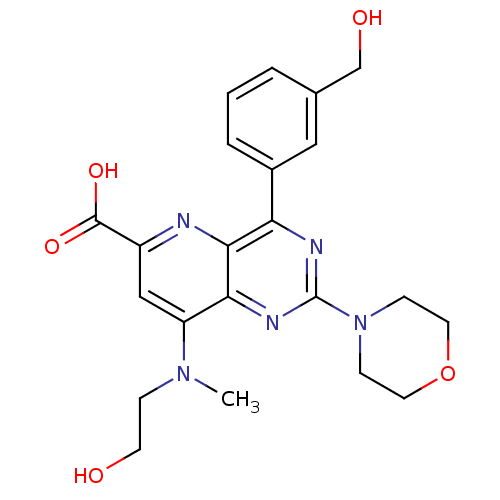
(US8609666, 24)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C22H25N5O5/c1-26(5-8-28)17-12-16(21(30)31)23-20-18(15-4-2-3-14(11-15)13-29)24-22(25-19(17)20)27-6-9-32-10-7-27/h2-4,11-12,28-29H,5-10,13H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109292
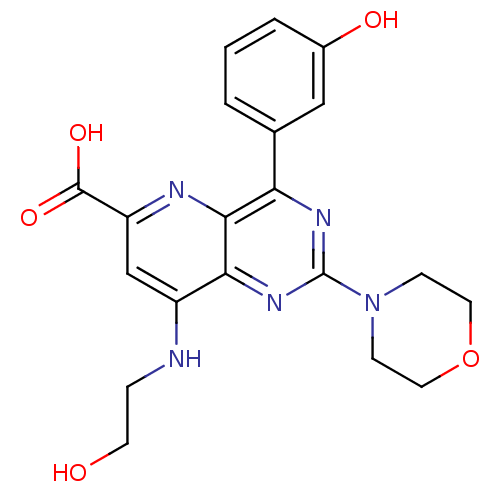
(US8609666, 12)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C20H21N5O5/c26-7-4-21-14-11-15(19(28)29)22-18-16(12-2-1-3-13(27)10-12)23-20(24-17(14)18)25-5-8-30-9-6-25/h1-3,10-11,26-27H,4-9H2,(H,21,22)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109300
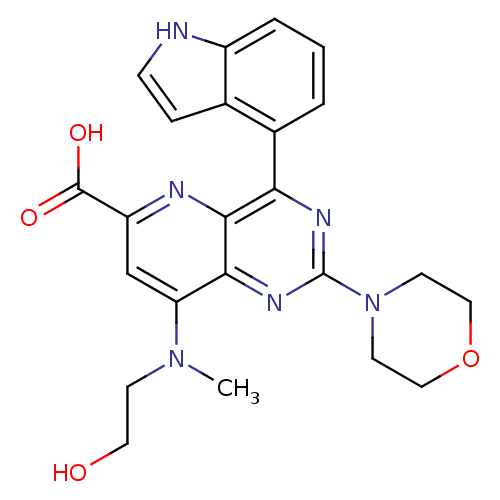
(US8609666, 20)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc2[nH]ccc12)C(O)=O Show InChI InChI=1S/C23H24N6O4/c1-28(7-10-30)18-13-17(22(31)32)25-21-19(15-3-2-4-16-14(15)5-6-24-16)26-23(27-20(18)21)29-8-11-33-12-9-29/h2-6,13,24,30H,7-12H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109340
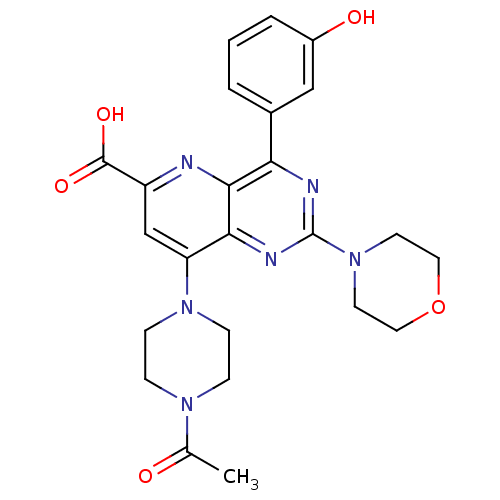
(US8609666, 60)Show SMILES CC(=O)N1CCN(CC1)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C24H26N6O5/c1-15(31)28-5-7-29(8-6-28)19-14-18(23(33)34)25-22-20(16-3-2-4-17(32)13-16)26-24(27-21(19)22)30-9-11-35-12-10-30/h2-4,13-14,32H,5-12H2,1H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109324
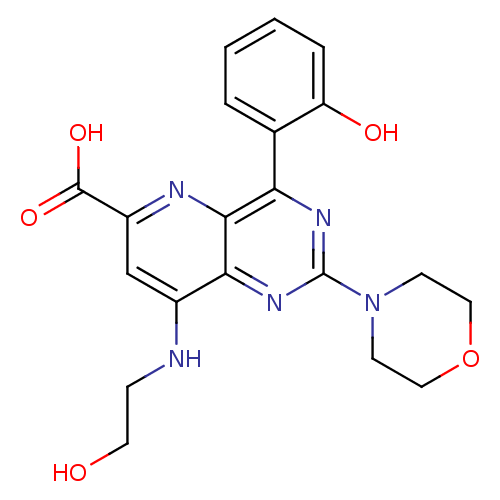
(US8609666, 44)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccccc1O)C(O)=O Show InChI InChI=1S/C20H21N5O5/c26-8-5-21-13-11-14(19(28)29)22-18-16(12-3-1-2-4-15(12)27)23-20(24-17(13)18)25-6-9-30-10-7-25/h1-4,11,26-27H,5-10H2,(H,21,22)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109332

(US8609666, 52)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)cc1O)C(O)=O Show InChI InChI=1S/C20H20FN5O4/c1-25(2)14-10-13(19(28)29)22-18-16(12-4-3-11(21)9-15(12)27)23-20(24-17(14)18)26-5-7-30-8-6-26/h3-4,9-10,27H,5-8H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109334
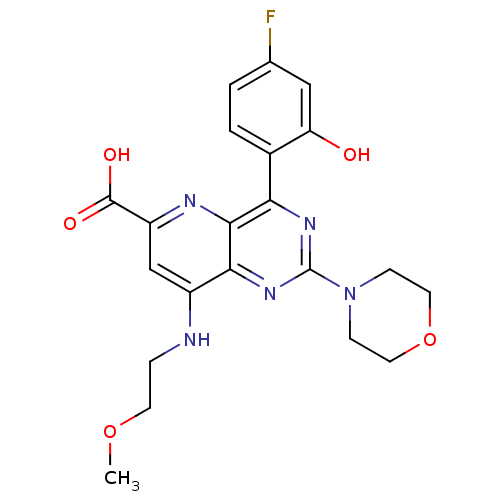
(US8609666, 54)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)cc1O)C(O)=O Show InChI InChI=1S/C21H22FN5O5/c1-31-7-4-23-14-11-15(20(29)30)24-19-17(13-3-2-12(22)10-16(13)28)25-21(26-18(14)19)27-5-8-32-9-6-27/h2-3,10-11,28H,4-9H2,1H3,(H,23,24)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109295

(US8609666, 15)Show SMILES COCCOc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(O)cc(F)c1)C(O)=O Show InChI InChI=1S/C21H21FN4O6/c1-30-6-7-32-16-11-15(20(28)29)23-19-17(12-8-13(22)10-14(27)9-12)24-21(25-18(16)19)26-2-4-31-5-3-26/h8-11,27H,2-7H2,1H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109289

(US8609666, 9)Show SMILES OC(=O)c1cc(Cl)c2nc(nc(-c3cccc(O)c3)c2n1)N1CCOCC1 Show InChI InChI=1S/C18H15ClN4O4/c19-12-9-13(17(25)26)20-16-14(10-2-1-3-11(24)8-10)21-18(22-15(12)16)23-4-6-27-7-5-23/h1-3,8-9,24H,4-7H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109299

(US8609666, 19)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc2[nH]ccc12)C(O)=O Show InChI InChI=1S/C23H24N6O4/c1-32-10-7-25-17-13-18(22(30)31)26-21-19(15-3-2-4-16-14(15)5-6-24-16)27-23(28-20(17)21)29-8-11-33-12-9-29/h2-6,13,24H,7-12H2,1H3,(H,25,26)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109297
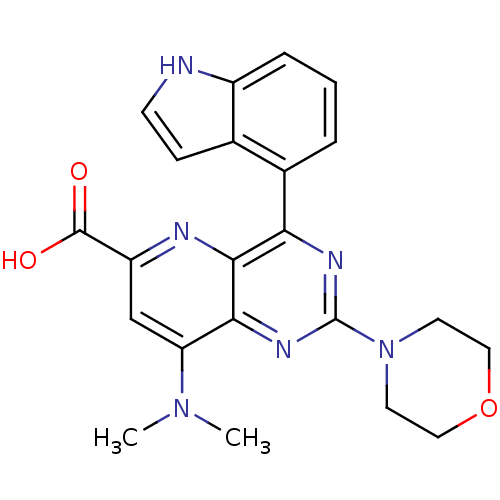
(US8609666, 17)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc2[nH]ccc12)C(O)=O Show InChI InChI=1S/C22H22N6O3/c1-27(2)17-12-16(21(29)30)24-20-18(14-4-3-5-15-13(14)6-7-23-15)25-22(26-19(17)20)28-8-10-31-11-9-28/h3-7,12,23H,8-11H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109337

(US8609666, 57)Show SMILES CN(C)CCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc2[nH]ccc12)C(O)=O Show InChI InChI=1S/C25H29N7O3/c1-30(2)9-10-31(3)20-15-19(24(33)34)27-23-21(17-5-4-6-18-16(17)7-8-26-18)28-25(29-22(20)23)32-11-13-35-14-12-32/h4-8,15,26H,9-14H2,1-3H3,(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109335
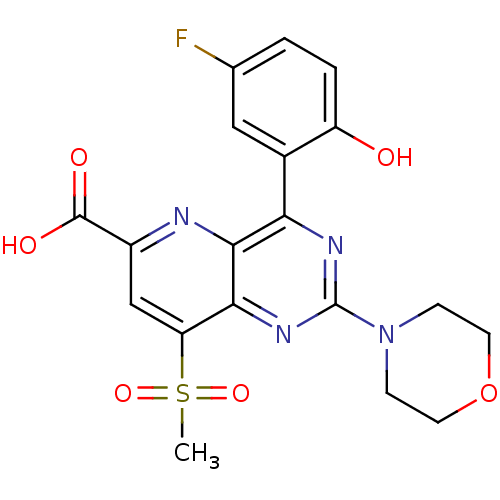
(US8609666, 55)Show SMILES CS(=O)(=O)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)ccc1O)C(O)=O Show InChI InChI=1S/C19H17FN4O6S/c1-31(28,29)14-9-12(18(26)27)21-17-15(11-8-10(20)2-3-13(11)25)22-19(23-16(14)17)24-4-6-30-7-5-24/h2-3,8-9,25H,4-7H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 139 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109320

(US8609666, 40)Show SMILES CNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C19H19N5O4/c1-20-13-10-14(18(26)27)21-17-15(11-3-2-4-12(25)9-11)22-19(23-16(13)17)24-5-7-28-8-6-24/h2-4,9-10,25H,5-8H2,1H3,(H,20,21)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109301
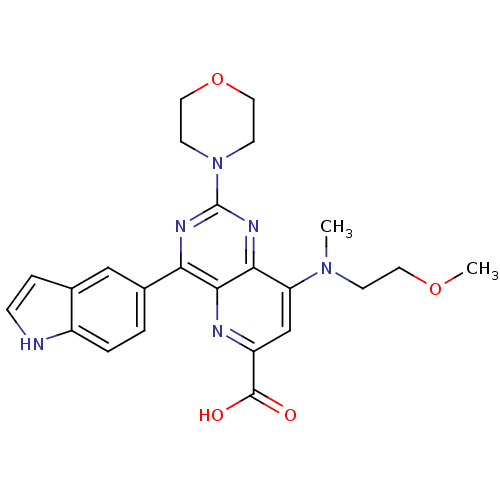
(US8609666, 21)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc2[nH]ccc2c1)C(O)=O Show InChI InChI=1S/C24H26N6O4/c1-29(7-10-33-2)19-14-18(23(31)32)26-22-20(16-3-4-17-15(13-16)5-6-25-17)27-24(28-21(19)22)30-8-11-34-12-9-30/h3-6,13-14,25H,7-12H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109339
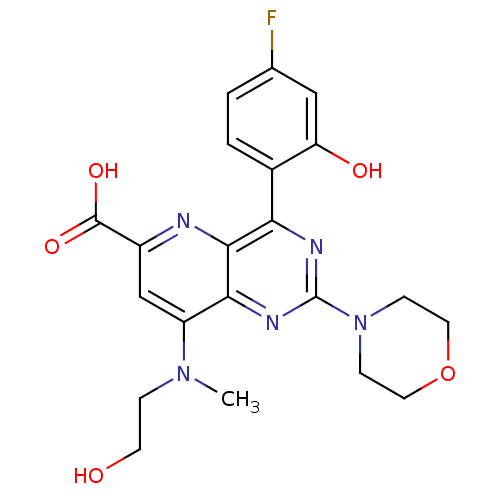
(US8609666, 59)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)cc1O)C(O)=O Show InChI InChI=1S/C21H22FN5O5/c1-26(4-7-28)15-11-14(20(30)31)23-19-17(13-3-2-12(22)10-16(13)29)24-21(25-18(15)19)27-5-8-32-9-6-27/h2-3,10-11,28-29H,4-9H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109342

(US8609666, 62)Show SMILES Cc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C19H18N4O4/c1-11-9-14(18(25)26)20-17-15(11)21-19(23-5-7-27-8-6-23)22-16(17)12-3-2-4-13(24)10-12/h2-4,9-10,24H,5-8H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 225 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109291
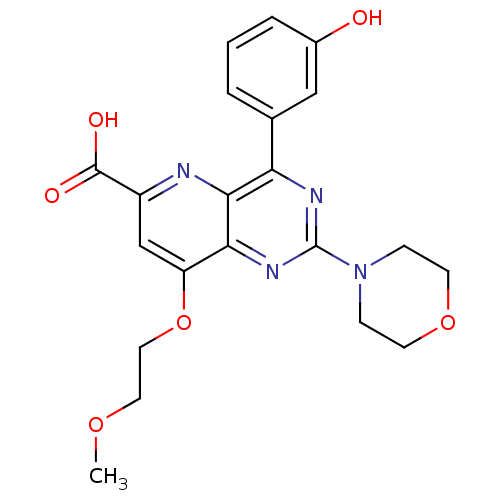
(US8609666, 11)Show SMILES COCCOc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(O)c1)C(O)=O Show InChI InChI=1S/C21H22N4O6/c1-29-9-10-31-16-12-15(20(27)28)22-19-17(13-3-2-4-14(26)11-13)23-21(24-18(16)19)25-5-7-30-8-6-25/h2-4,11-12,26H,5-10H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109283
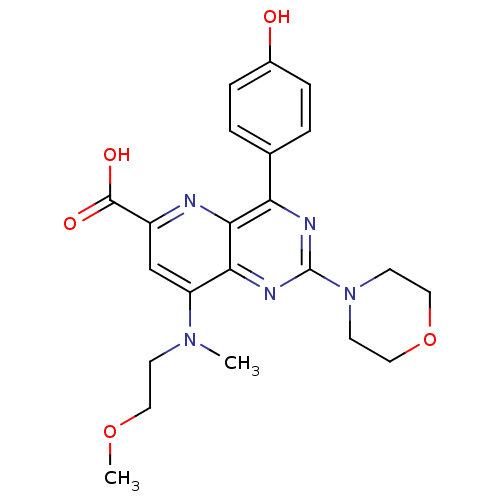
(US8609666, 3)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C22H25N5O5/c1-26(7-10-31-2)17-13-16(21(29)30)23-20-18(14-3-5-15(28)6-4-14)24-22(25-19(17)20)27-8-11-32-12-9-27/h3-6,13,28H,7-12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109330
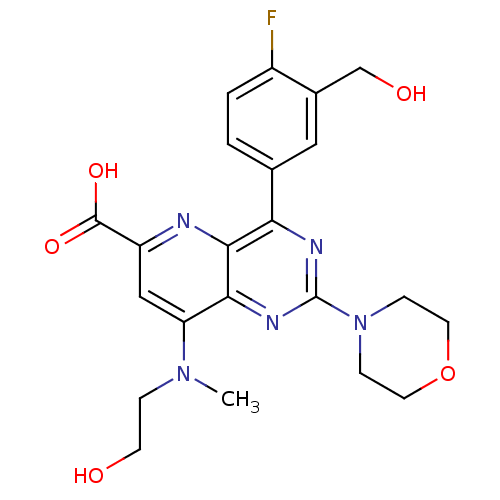
(US8609666, 50)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)c(CO)c1)C(O)=O Show InChI InChI=1S/C22H24FN5O5/c1-27(4-7-29)17-11-16(21(31)32)24-20-18(13-2-3-15(23)14(10-13)12-30)25-22(26-19(17)20)28-5-8-33-9-6-28/h2-3,10-11,29-30H,4-9,12H2,1H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 303 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109310

(US8609666, 30)Show SMILES CNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)ccc1O)C(O)=O Show InChI InChI=1S/C19H18FN5O4/c1-21-12-9-13(18(27)28)22-17-15(11-8-10(20)2-3-14(11)26)23-19(24-16(12)17)25-4-6-29-7-5-25/h2-3,8-9,26H,4-7H2,1H3,(H,21,22)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109329

(US8609666, 49)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)c(CO)c1)C(O)=O Show InChI InChI=1S/C21H22FN5O4/c1-26(2)16-10-15(20(29)30)23-19-17(12-3-4-14(22)13(9-12)11-28)24-21(25-18(16)19)27-5-7-31-8-6-27/h3-4,9-10,28H,5-8,11H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 329 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109325
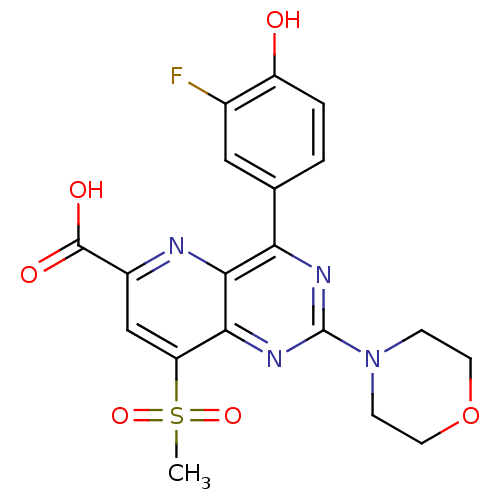
(US8609666, 45)Show SMILES CS(=O)(=O)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)c(F)c1)C(O)=O Show InChI InChI=1S/C19H17FN4O6S/c1-31(28,29)14-9-12(18(26)27)21-17-15(10-2-3-13(25)11(20)8-10)22-19(23-16(14)17)24-4-6-30-7-5-24/h2-3,8-9,25H,4-7H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 335 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
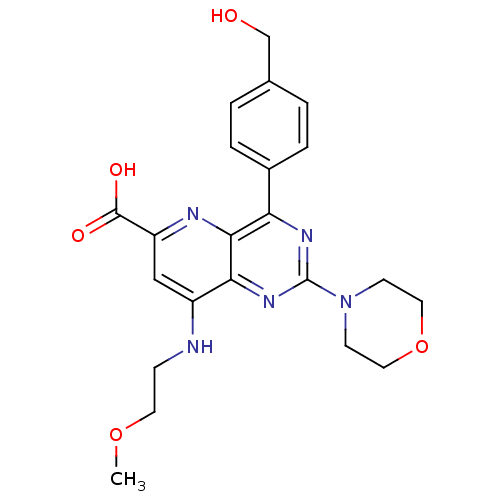
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109326
(US8609666, 46)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(CO)cc1)C(O)=O Show InChI InChI=1S/C22H25N5O5/c1-31-9-6-23-16-12-17(21(29)30)24-20-18(15-4-2-14(13-28)3-5-15)25-22(26-19(16)20)27-7-10-32-11-8-27/h2-5,12,28H,6-11,13H2,1H3,(H,23,24)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 346 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109284

(US8609666, 4)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H21N5O4/c1-24(2)15-11-14(19(27)28)21-18-16(12-3-5-13(26)6-4-12)22-20(23-17(15)18)25-7-9-29-10-8-25/h3-6,11,26H,7-10H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109333

(US8609666, 53)Show SMILES CNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(F)cc1O)C(O)=O Show InChI InChI=1S/C19H18FN5O4/c1-21-12-9-13(18(27)28)22-17-15(11-3-2-10(20)8-14(11)26)23-19(24-16(12)17)25-4-6-29-7-5-25/h2-3,8-9,26H,4-7H2,1H3,(H,21,22)(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109323
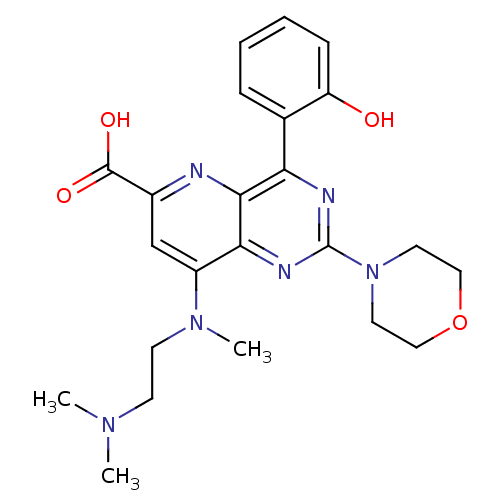
(US8609666, 43)Show SMILES CN(C)CCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccccc1O)C(O)=O Show InChI InChI=1S/C23H28N6O4/c1-27(2)8-9-28(3)17-14-16(22(31)32)24-21-19(15-6-4-5-7-18(15)30)25-23(26-20(17)21)29-10-12-33-13-11-29/h4-7,14,30H,8-13H2,1-3H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 459 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109285
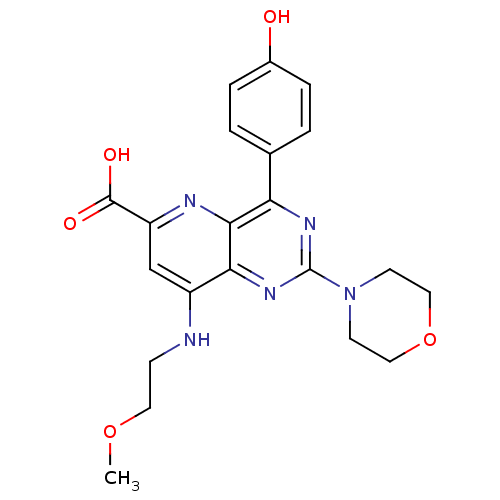
(US8609666, 5)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C21H23N5O5/c1-30-9-6-22-15-12-16(20(28)29)23-19-17(13-2-4-14(27)5-3-13)24-21(25-18(15)19)26-7-10-31-11-8-26/h2-5,12,27H,6-11H2,1H3,(H,22,23)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 504 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109302
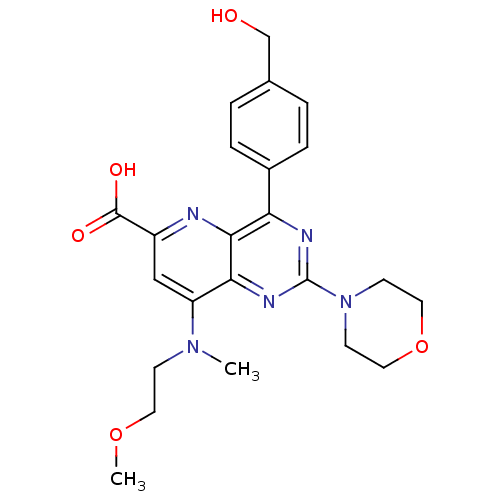
(US8609666, 22)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(CO)cc1)C(O)=O Show InChI InChI=1S/C23H27N5O5/c1-27(7-10-32-2)18-13-17(22(30)31)24-21-19(16-5-3-15(14-29)4-6-16)25-23(26-20(18)21)28-8-11-33-12-9-28/h3-6,13,29H,7-12,14H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 546 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109312
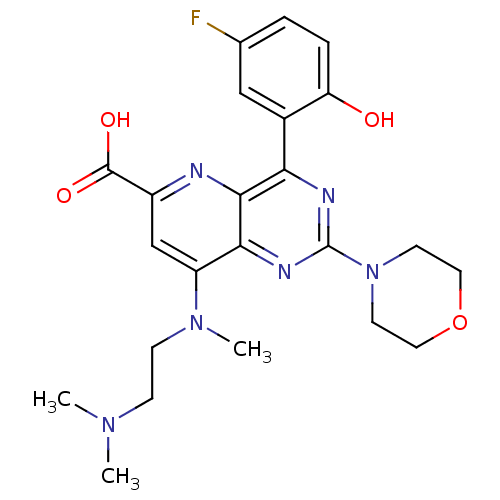
(US8609666, 32)Show SMILES CN(C)CCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)ccc1O)C(O)=O Show InChI InChI=1S/C23H27FN6O4/c1-28(2)6-7-29(3)17-13-16(22(32)33)25-21-19(15-12-14(24)4-5-18(15)31)26-23(27-20(17)21)30-8-10-34-11-9-30/h4-5,12-13,31H,6-11H2,1-3H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 564 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109344
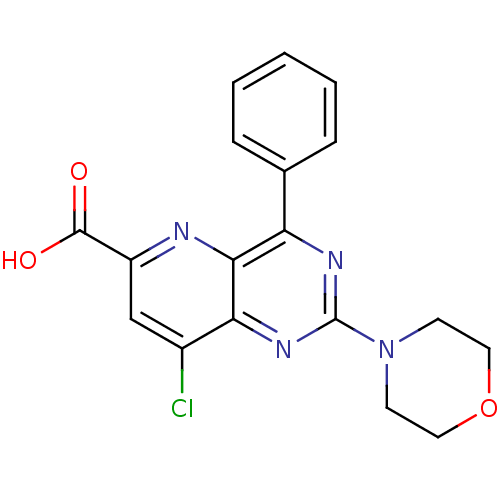
(US8609666, 64)Show SMILES OC(=O)c1cc(Cl)c2nc(nc(-c3ccccc3)c2n1)N1CCOCC1 Show InChI InChI=1S/C18H15ClN4O3/c19-12-10-13(17(24)25)20-16-14(11-4-2-1-3-5-11)21-18(22-15(12)16)23-6-8-26-9-7-23/h1-5,10H,6-9H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 678 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109287
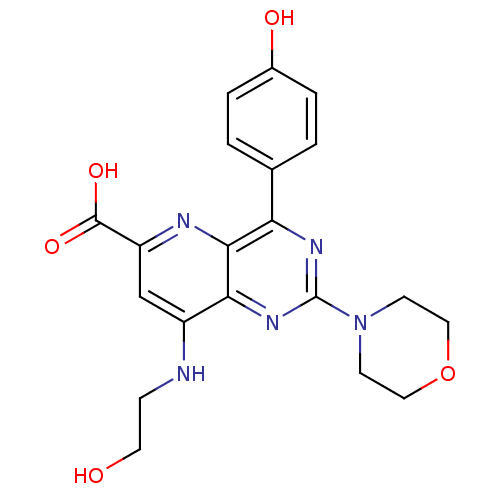
(US8609666, 7)Show SMILES OCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C20H21N5O5/c26-8-5-21-14-11-15(19(28)29)22-18-16(12-1-3-13(27)4-2-12)23-20(24-17(14)18)25-6-9-30-10-7-25/h1-4,11,26-27H,5-10H2,(H,21,22)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 732 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109298

(US8609666, 18)Show SMILES CNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc2[nH]ccc12)C(O)=O Show InChI InChI=1S/C21H20N6O3/c1-22-15-11-16(20(28)29)24-19-17(13-3-2-4-14-12(13)5-6-23-14)25-21(26-18(15)19)27-7-9-30-10-8-27/h2-6,11,23H,7-10H2,1H3,(H,22,24)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109288

(US8609666, 8)Show SMILES OC(=O)c1ccc2nc(nc(-c3ccc(O)cc3)c2n1)N1CCOCC1 Show InChI InChI=1S/C18H16N4O4/c23-12-3-1-11(2-4-12)15-16-13(5-6-14(19-16)17(24)25)20-18(21-15)22-7-9-26-10-8-22/h1-6,23H,7-10H2,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109338
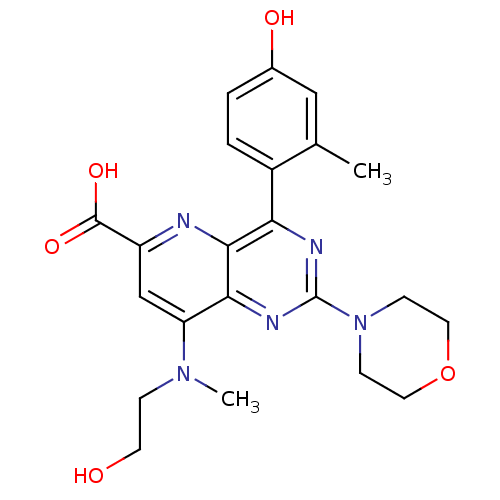
(US8609666, 58)Show SMILES CN(CCO)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1C)C(O)=O Show InChI InChI=1S/C22H25N5O5/c1-13-11-14(29)3-4-15(13)18-20-19(25-22(24-18)27-6-9-32-10-7-27)17(26(2)5-8-28)12-16(23-20)21(30)31/h3-4,11-12,28-29H,5-10H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 936 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109281
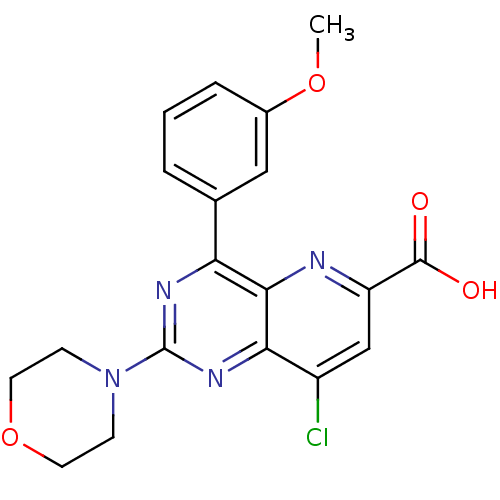
(US8609666, 1)Show SMILES COc1cccc(c1)-c1nc(nc2c(Cl)cc(nc12)C(O)=O)N1CCOCC1 Show InChI InChI=1S/C19H17ClN4O4/c1-27-12-4-2-3-11(9-12)15-17-16(13(20)10-14(21-17)18(25)26)23-19(22-15)24-5-7-28-8-6-24/h2-4,9-10H,5-8H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109346

(US8609666, 66)Show SMILES CN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(c1)C#N)C(O)=O Show InChI InChI=1S/C21H20N6O3/c1-26(2)16-11-15(20(28)29)23-19-17(14-5-3-4-13(10-14)12-22)24-21(25-18(16)19)27-6-8-30-9-7-27/h3-5,10-11H,6-9H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109345
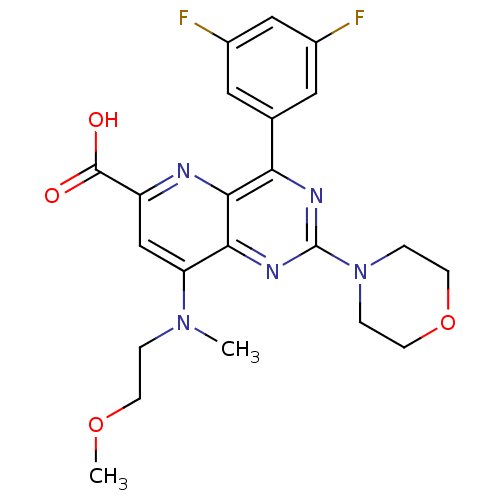
(US8609666, 65)Show SMILES COCCN(C)c1cc(nc2c(nc(nc12)N1CCOCC1)-c1cc(F)cc(F)c1)C(O)=O Show InChI InChI=1S/C22H23F2N5O4/c1-28(3-6-32-2)17-12-16(21(30)31)25-20-18(13-9-14(23)11-15(24)10-13)26-22(27-19(17)20)29-4-7-33-8-5-29/h9-12H,3-8H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109306
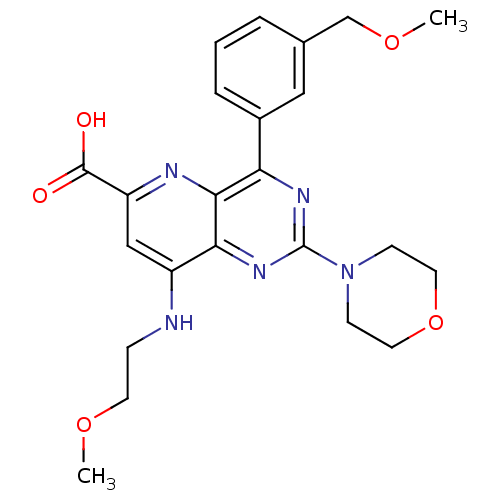
(US8609666, 26)Show SMILES COCCNc1cc(nc2c(nc(nc12)N1CCOCC1)-c1cccc(COC)c1)C(O)=O Show InChI InChI=1S/C23H27N5O5/c1-31-9-6-24-17-13-18(22(29)30)25-21-19(16-5-3-4-15(12-16)14-32-2)26-23(27-20(17)21)28-7-10-33-11-8-28/h3-5,12-13H,6-11,14H2,1-2H3,(H,24,25)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109307

(US8609666, 27)Show SMILES COCc1cccc(c1)-c1nc(nc2c(cc(nc12)C(O)=O)N(C)CCO)N1CCOCC1 Show InChI InChI=1S/C23H27N5O5/c1-27(6-9-29)18-13-17(22(30)31)24-21-19(16-5-3-4-15(12-16)14-32-2)25-23(26-20(18)21)28-7-10-33-11-8-28/h3-5,12-13,29H,6-11,14H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109308
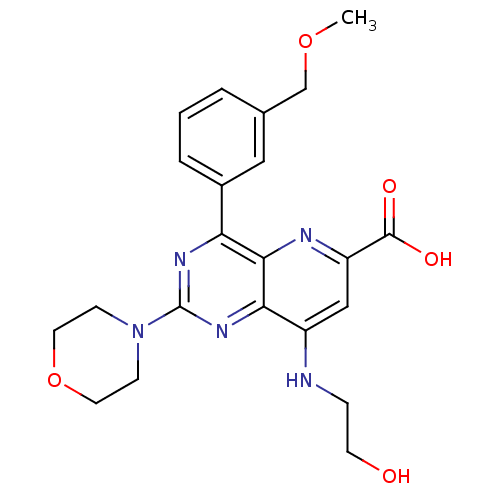
(US8609666, 28)Show SMILES COCc1cccc(c1)-c1nc(nc2c(NCCO)cc(nc12)C(O)=O)N1CCOCC1 Show InChI InChI=1S/C22H25N5O5/c1-31-13-14-3-2-4-15(11-14)18-20-19(26-22(25-18)27-6-9-32-10-7-27)16(23-5-8-28)12-17(24-20)21(29)30/h2-4,11-12,28H,5-10,13H2,1H3,(H,23,24)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109286
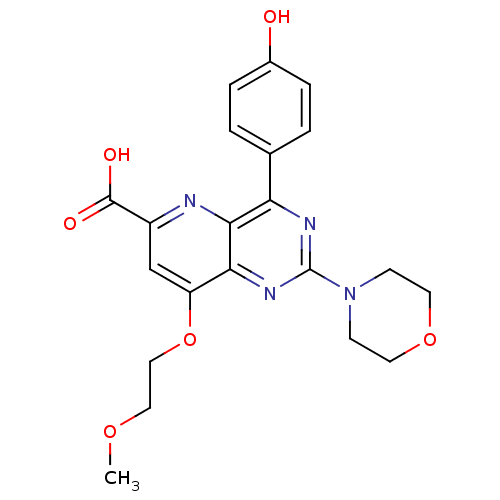
(US8609666, 6)Show SMILES COCCOc1cc(nc2c(nc(nc12)N1CCOCC1)-c1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C21H22N4O6/c1-29-10-11-31-16-12-15(20(27)28)22-19-17(13-2-4-14(26)5-3-13)23-21(24-18(16)19)25-6-8-30-9-7-25/h2-5,12,26H,6-11H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109282

(US8609666, 2)Show SMILES COc1cccc(c1)-c1nc(nc2ccc(nc12)C(O)=O)N1CCOCC1 Show InChI InChI=1S/C19H18N4O4/c1-26-13-4-2-3-12(11-13)16-17-14(5-6-15(20-17)18(24)25)21-19(22-16)23-7-9-27-10-8-23/h2-6,11H,7-10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM109305
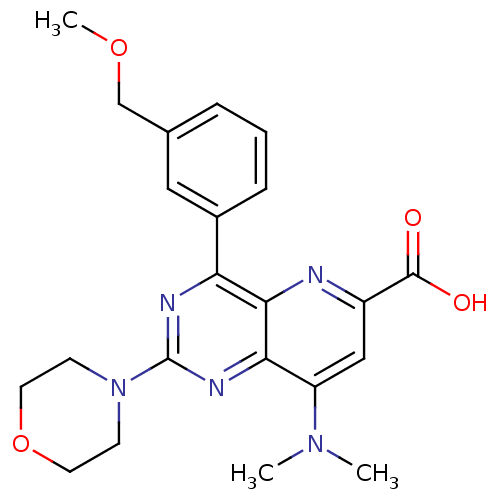
(US8609666, 25)Show SMILES COCc1cccc(c1)-c1nc(nc2c(cc(nc12)C(O)=O)N(C)C)N1CCOCC1 Show InChI InChI=1S/C22H25N5O4/c1-26(2)17-12-16(21(28)29)23-20-18(15-6-4-5-14(11-15)13-30-3)24-22(25-19(17)20)27-7-9-31-10-8-27/h4-6,11-12H,7-10,13H2,1-3H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono SA
US Patent
| Assay Description
The efficacy of compounds of the invention in inhibiting the PI3K induced-lipid phosphorylation may be tested in the following binding assay. The ass... |
US Patent US8609666 (2013)
BindingDB Entry DOI: 10.7270/Q26M35G5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data