

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50271083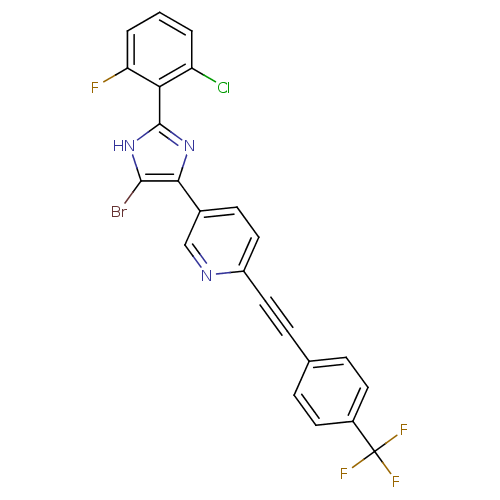 (5-(5-bromo-2-(2-chloro-6-fluorophenyl)-1H-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50227631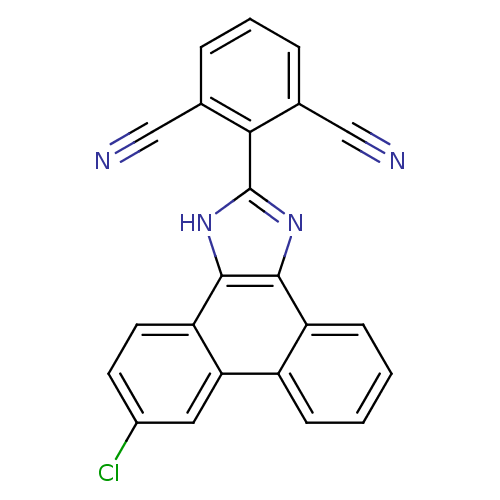 (2-(6-chloro-1H-phenanthro[9,10-d]imidazol-2-yl)iso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313205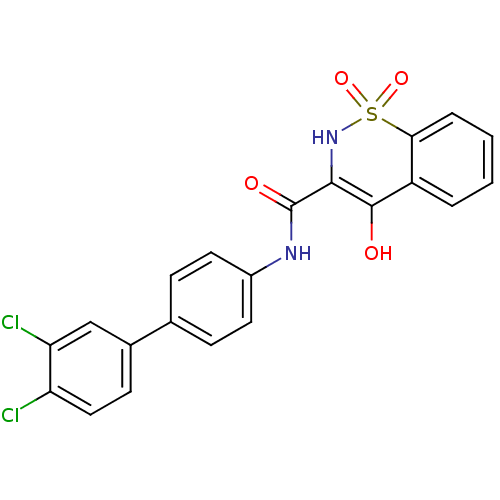 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of FLAP | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313223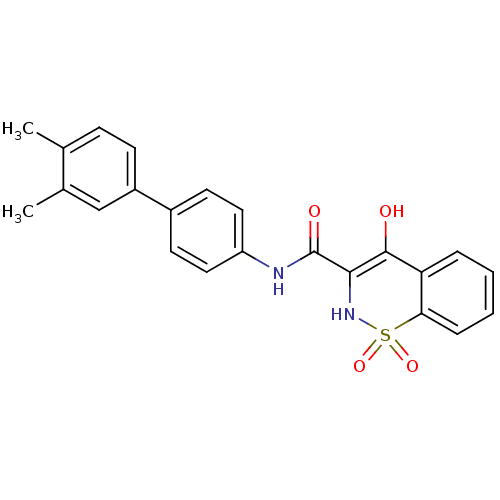 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313206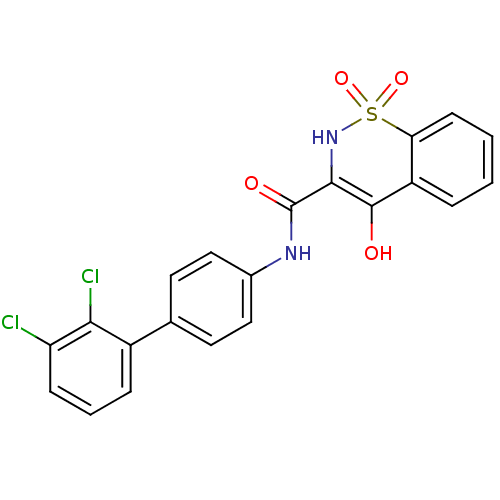 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313207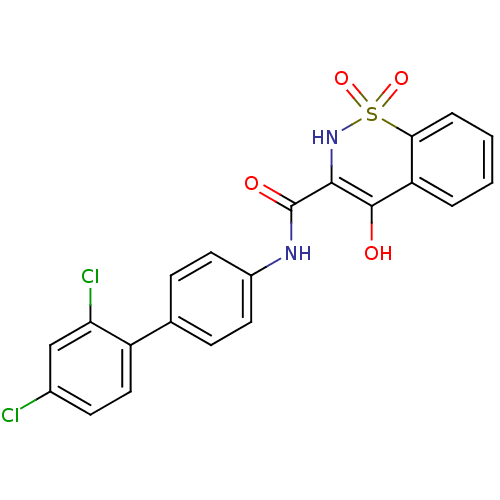 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313224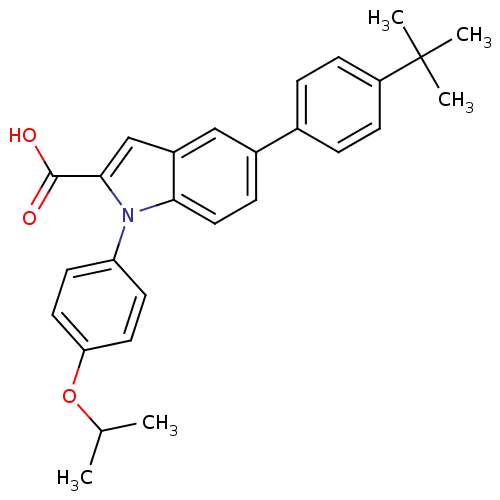 (5-(4-tert-butylphenyl)-1-(4-isopropoxyphenyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761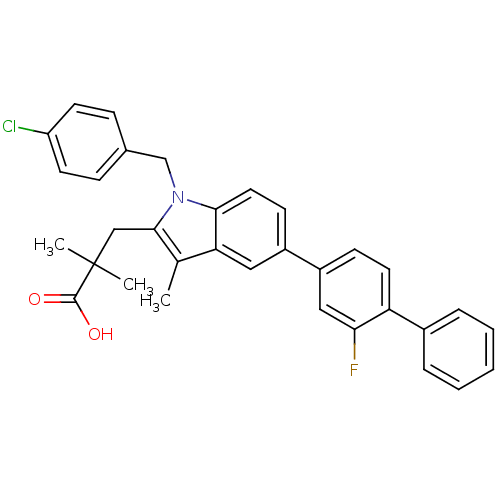 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313222 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313215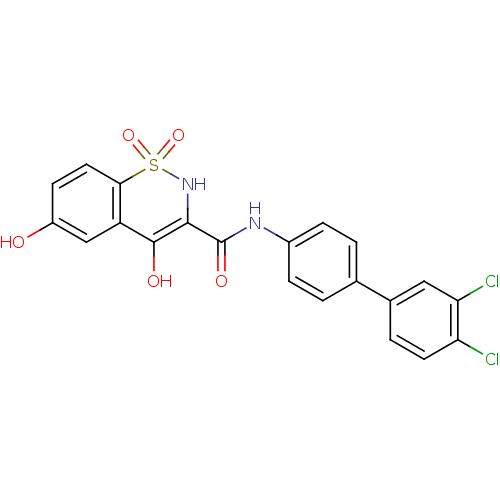 (4,6-Dihydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313196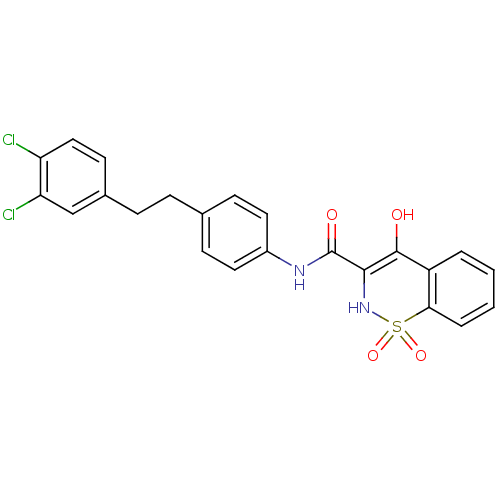 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313200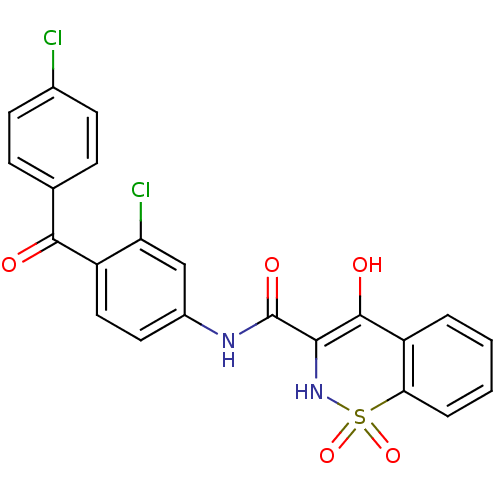 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313199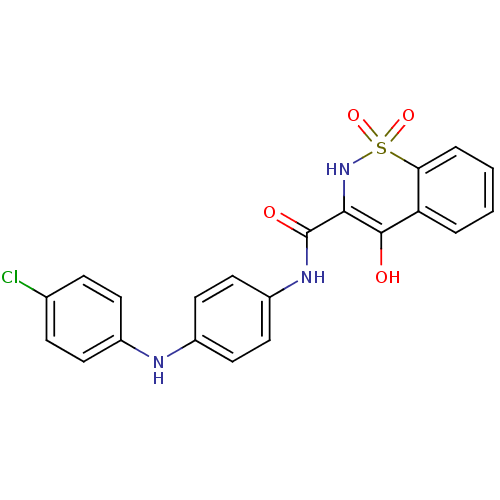 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313210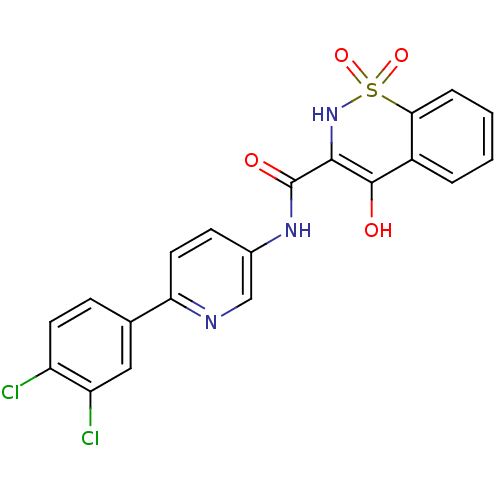 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313217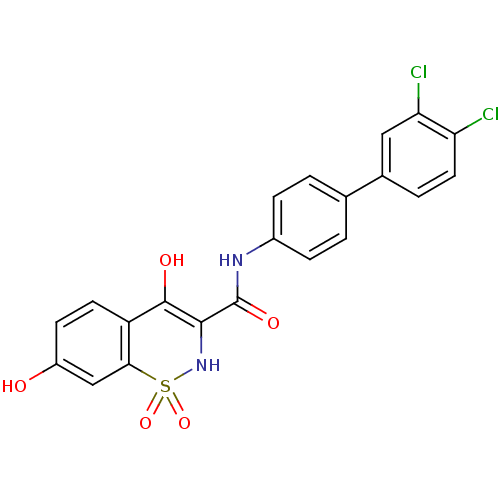 (4,7-Dihydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313219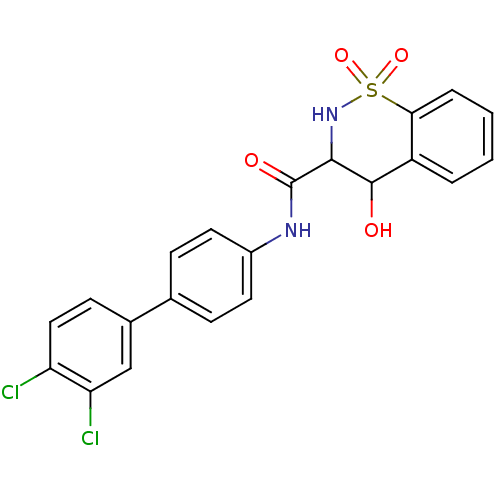 (4-Hydroxy-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313218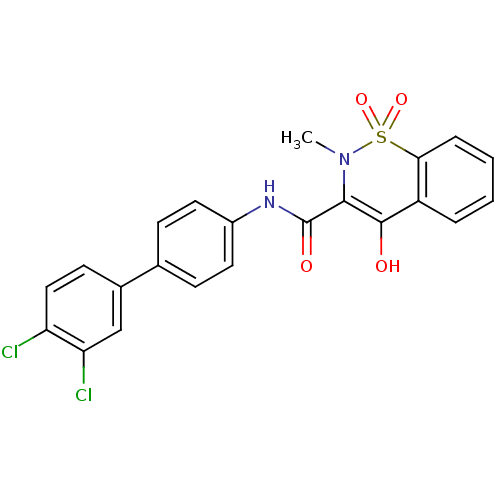 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313197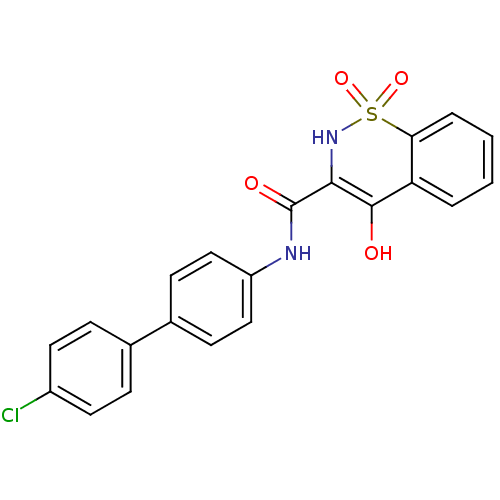 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50270594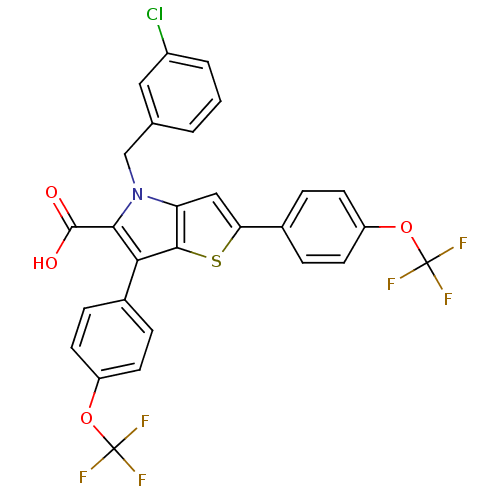 (4-(3-chlorobenzyl)-2,6-bis(4-(trifluoromethoxy)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313196 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta-stimulated human RASF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313198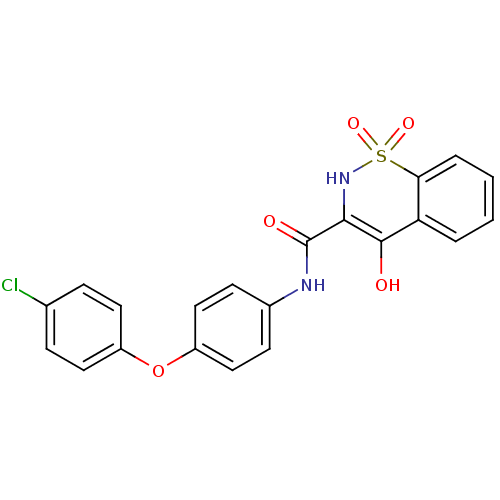 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313219 (4-Hydroxy-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313214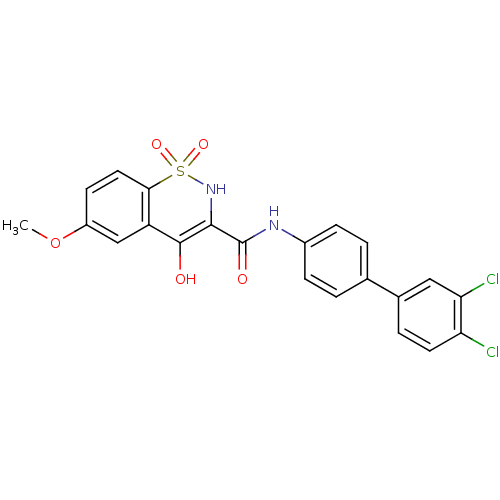 (4-Hydroxy-6-methoxy-1,1-dioxo-1,2-dihydro-1lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313210 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313223 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <750 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313207 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <760 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313202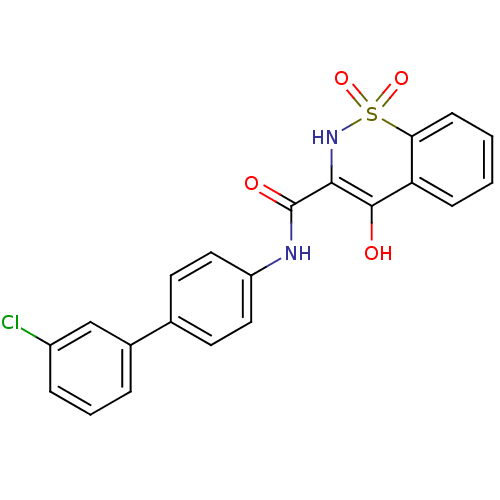 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313206 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <920 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313208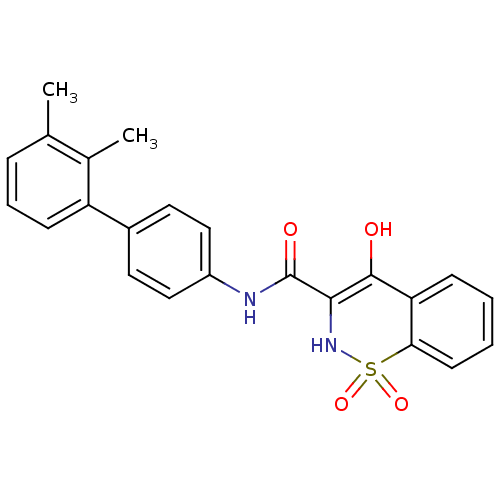 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50270616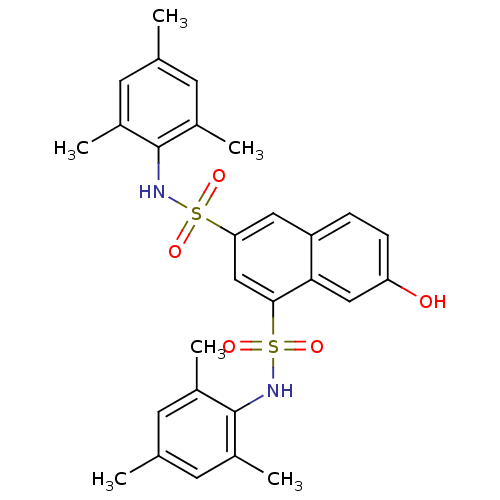 (7-hydroxy-N1,N3-dimesitylnaphthalene-1,3-disulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313200 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313214 (4-Hydroxy-6-methoxy-1,1-dioxo-1,2-dihydro-1lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50270615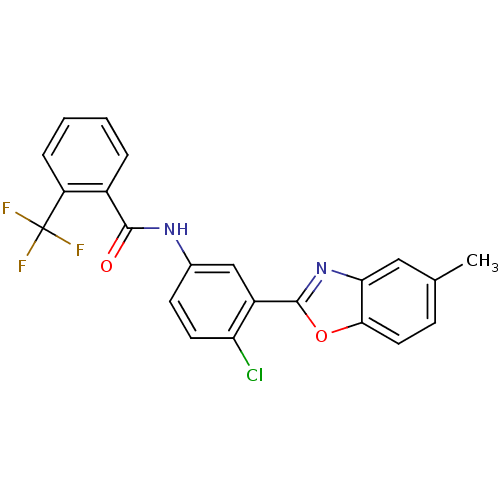 (CHEMBL521286 | N-(4-chloro-3-(5-methylbenzo[d]oxaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of mPGES1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313215 (4,6-Dihydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313197 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313225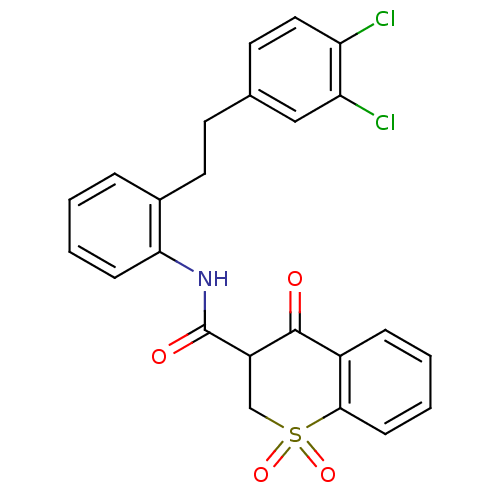 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-thiochr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313218 (4-Hydroxy-2-methyl-1,1-dioxo-1,2-dihydro-1lambda*6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313201 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313222 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313216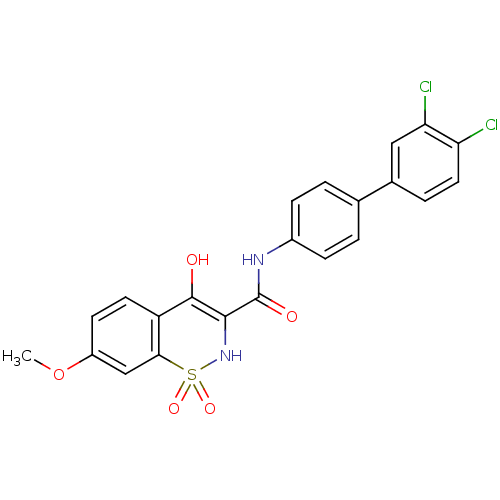 (4-Hydroxy-7-methoxy-1,1-dioxo-1,2-dihydro-1lambda*...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313204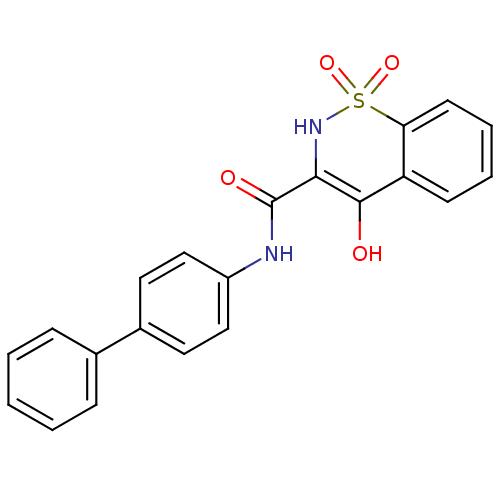 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313225 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-thiochr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313202 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313201 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313203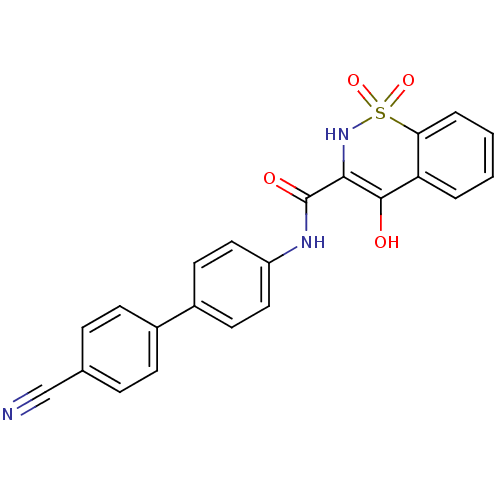 (CHEMBL1087266 | N-(4'-cyano-1,1'-biphenyl-4-yl)-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313199 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in LPS-stimulated human whole blood assessed as PGE2 level after 24 hrs by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313220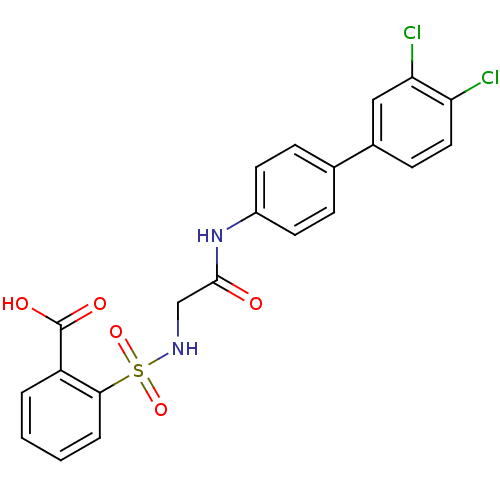 (2-{[(3',4'-Dichloro-biphenyl-4-ylcarbamoyl)-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313208 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313198 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313204 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313213 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313211 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313221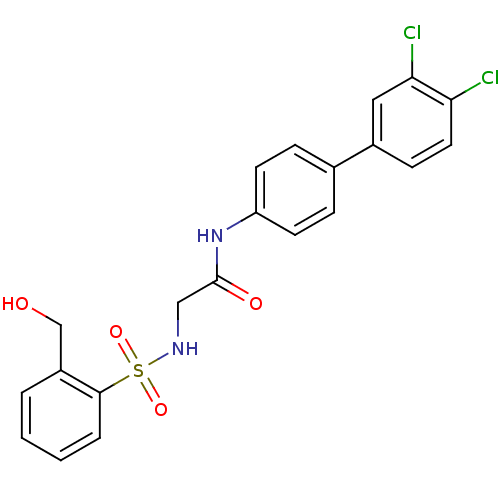 (CHEMBL1076568 | N-(3',4'-Dichloro-biphenyl-4-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313209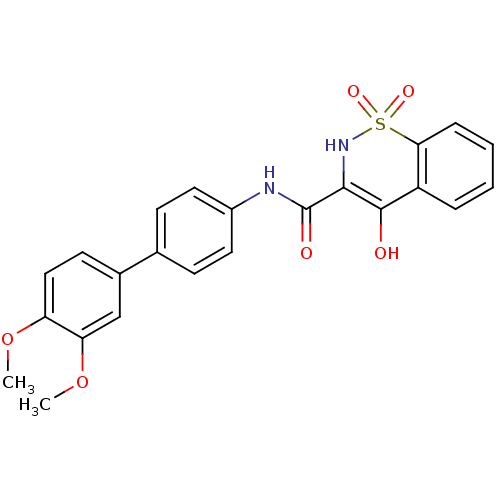 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313210 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313197 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313220 (2-{[(3',4'-Dichloro-biphenyl-4-ylcarbamoyl)-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 in IL-1beta treated human FF cells assessed as blockade of PGH2 to PGE2 conversion after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313206 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313219 (4-Hydroxy-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50313219 (4-Hydroxy-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313223 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50313212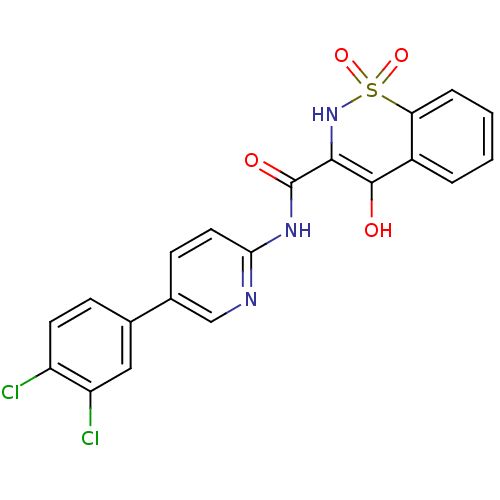 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human mPGES1 assessed as PGE2 level after 41 sec by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313196 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313198 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313202 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313207 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313225 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-thiochr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313204 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313208 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313201 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313200 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.56E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human thromboxane A synthase in IL-1-beta-stimulated human RASF cells assessed as TXB2 levels after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313222 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313215 (4,6-Dihydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313220 (2-{[(3',4'-Dichloro-biphenyl-4-ylcarbamoyl)-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313199 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin synthase (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human prostacyclin synthase in IL-1-beta-stimulated human RASF cells assessed as PGF1alpha levels after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313219 (4-Hydroxy-1,1-dioxo-1,2,3,4-tetrahydro-1lambda*6*-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1-beta-stimulated human RASF cells assessed as PGF2alpha levels after 50 mins by ELISA | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313214 (4-Hydroxy-6-methoxy-1,1-dioxo-1,2-dihydro-1lambda*...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human COX2 in IL-1beta treated human FF cells assessed as blockade of SnCl2-mediated PGH2 to PGF2alpha conversion after 50 mins by ELIS... | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.18E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313205 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50313196 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50313196 (4-Hydroxy-1,1-dioxo-1,2-dihydro-1lambda*6*-benzo[e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. St. Louis Laboratory Curated by ChEMBL | Assay Description Inhibition of human recombinant COX1 | Bioorg Med Chem Lett 20: 1604-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.060 BindingDB Entry DOI: 10.7270/Q25X292V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||