

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421091 (CHEMBL2088336) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421094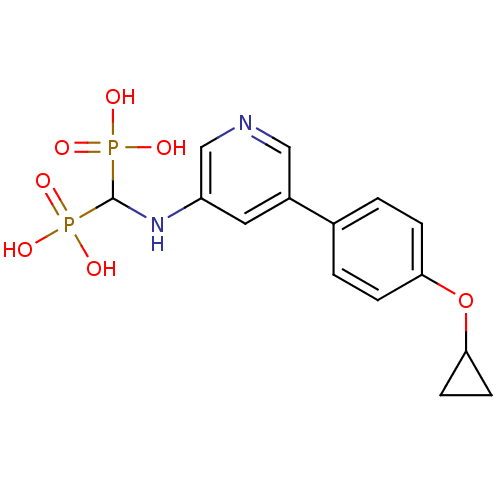 (CHEMBL2088339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50386555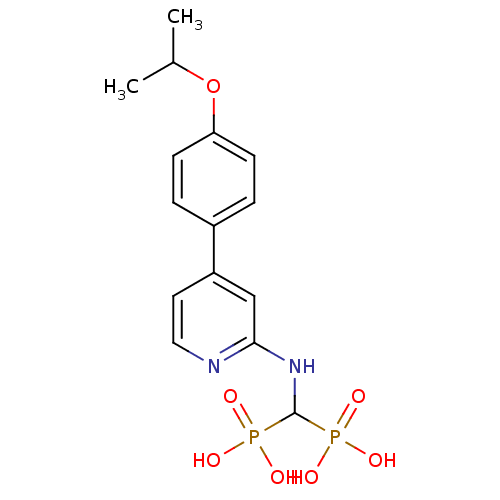 (CHEMBL2048241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50386552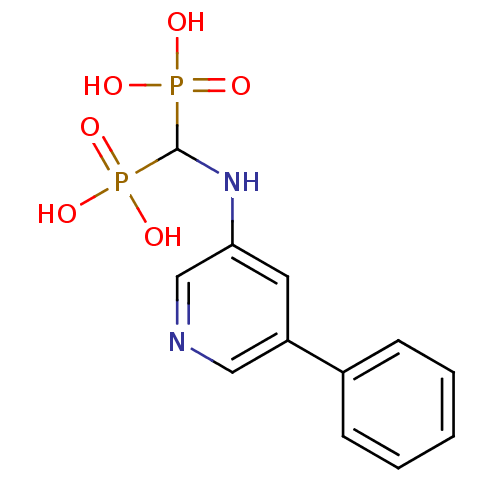 (CHEMBL2048238) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421093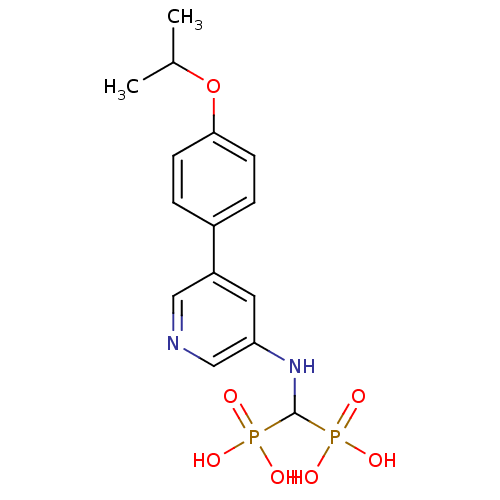 (CHEMBL2088338) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104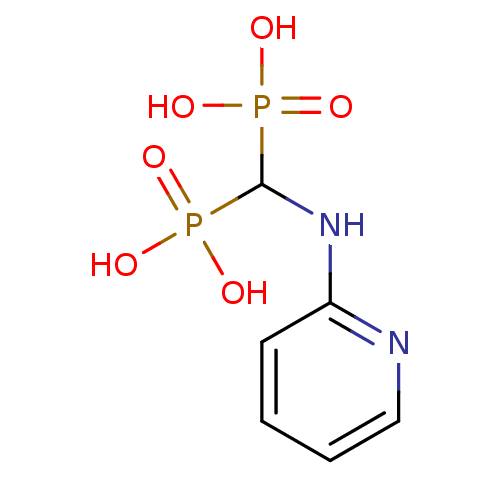 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25259 (3-(decyloxy)-1-(2-hydrogen phosphonato-2-phosphono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS using GPP and [3H]IPP as substrate by scintillation counting | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421090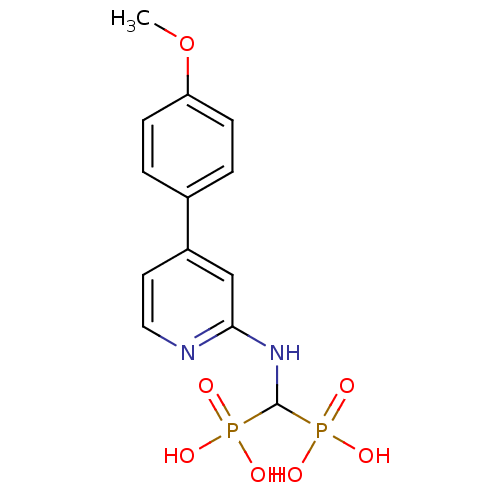 (CHEMBL2048247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50386553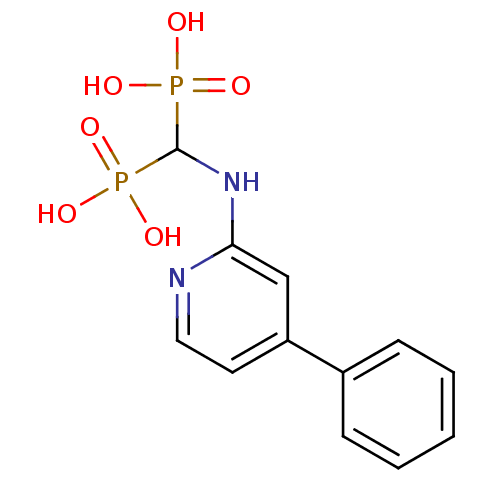 (CHEMBL2048239) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50386551 (CHEMBL2048237) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25259 (3-(decyloxy)-1-(2-hydrogen phosphonato-2-phosphono...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant GGPS using [14C]-IPP as substrate incubated for 15 mins prior to substrate addition measured after 20 mins by scintil... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421095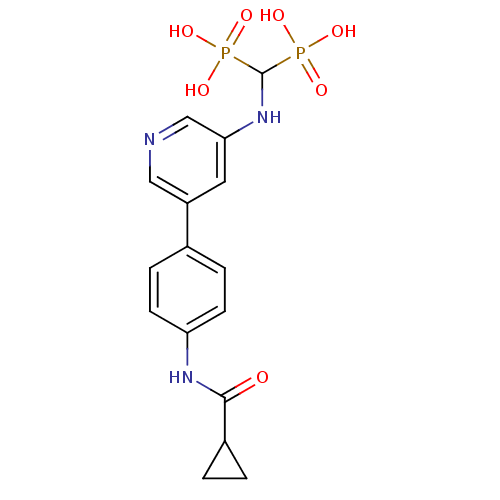 (CHEMBL2088342) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421089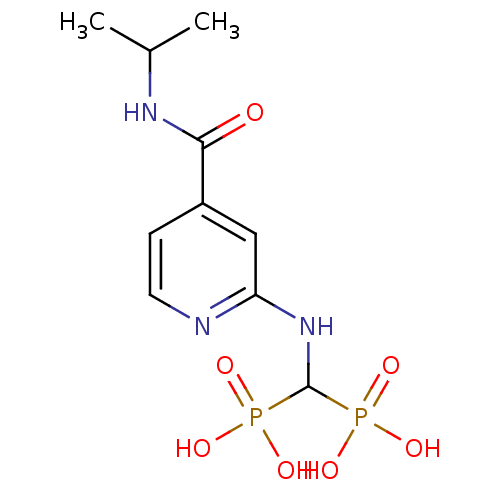 (CHEMBL2088344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50421092 (CHEMBL2088337) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human His6-tagged recombinant FPPS expressed in Escherichia coli BL21(DE3) using GPP and [3H]IPP as substrate incubated for 5 mins prio... | Bioorg Med Chem 20: 5583-91 (2012) Article DOI: 10.1016/j.bmc.2012.07.019 BindingDB Entry DOI: 10.7270/Q2J67J6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||