

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457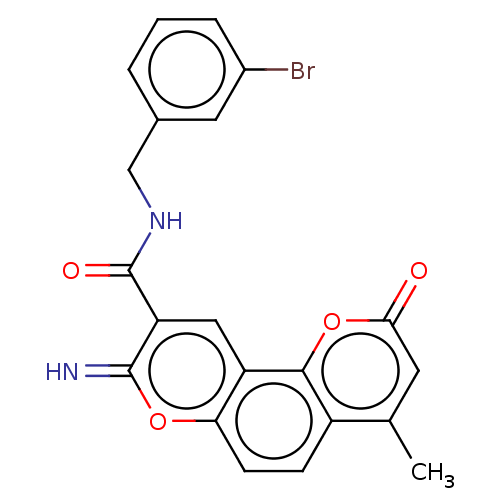 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as free enzyme preincubated for 5 mins followed by su... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Mixed-type inhibition of electric eel AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate complex preincubated for 5 mins f... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500457 (CHEMBL3746815) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM189377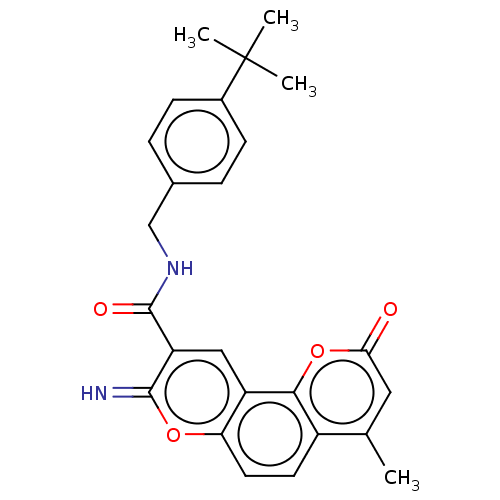 (N-(4-(tert-butyl)benzyl)-8-imino-4-methyl-2-oxo-2H...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500467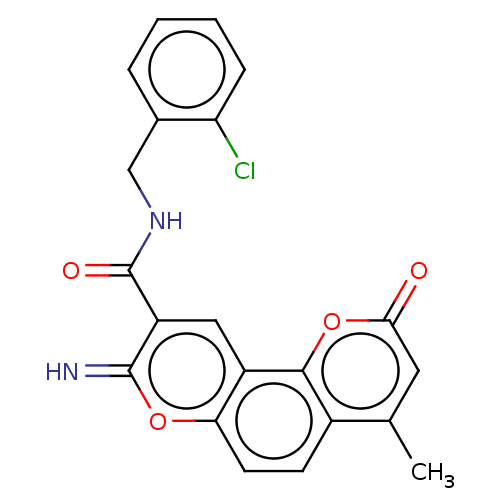 (CHEMBL3745757) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500458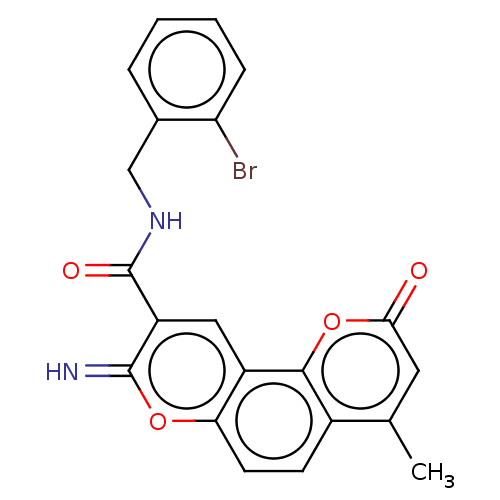 (CHEMBL3746565) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500456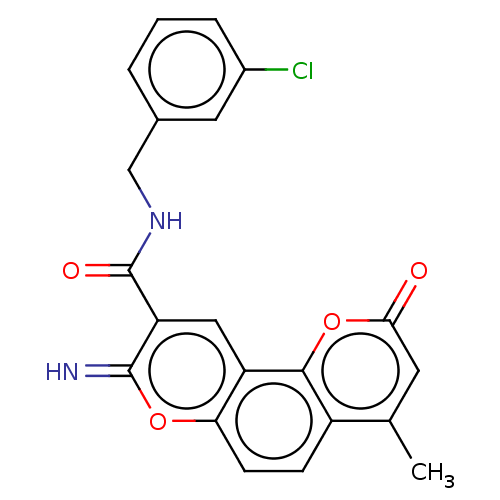 (CHEMBL3747774) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500455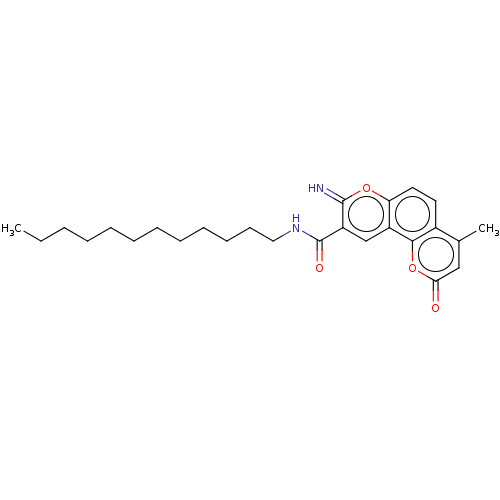 (CHEMBL3746856) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500464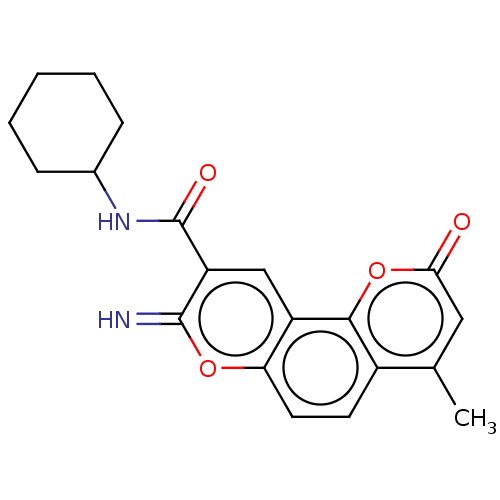 (CHEMBL3745983) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500469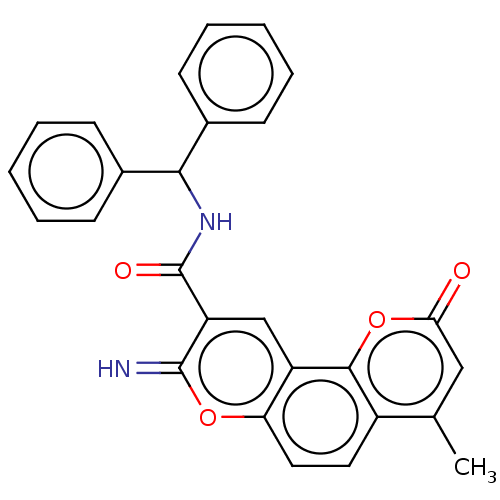 (CHEMBL3746981) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500462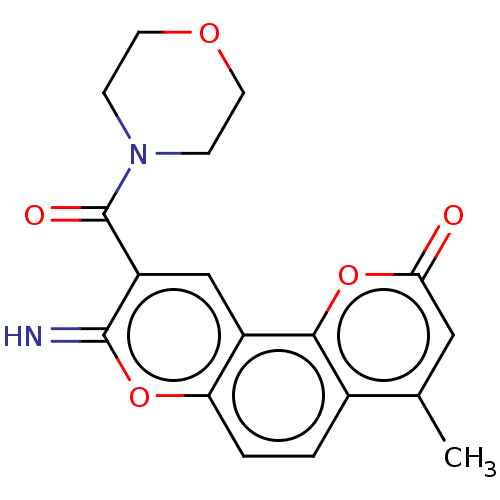 (CHEMBL3747348) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500466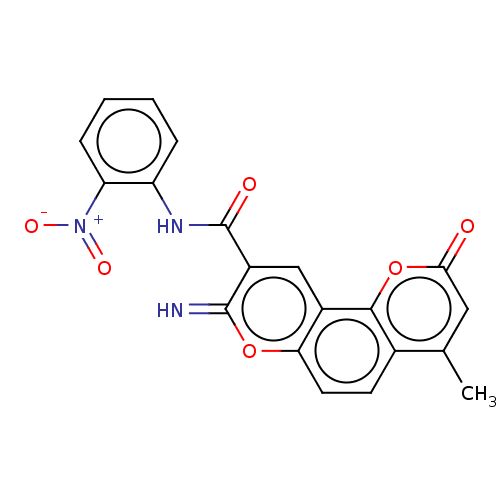 (CHEMBL3747027) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500468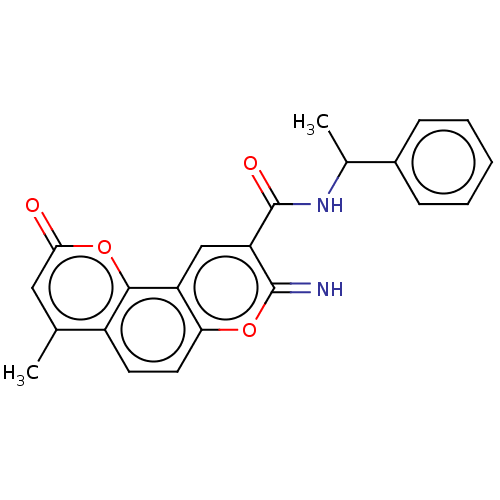 (CHEMBL3745771) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500460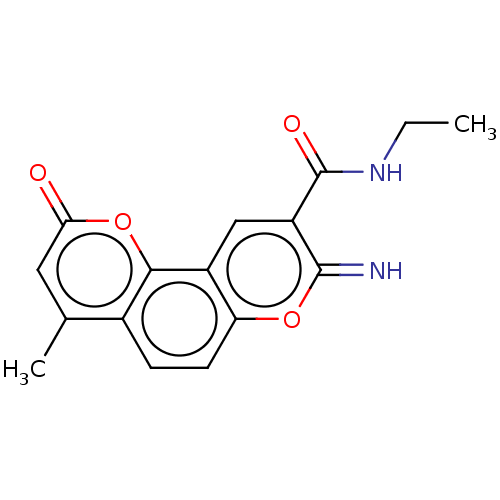 (CHEMBL3746173) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 294 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500459 (CHEMBL3747030) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 372 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 665 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500452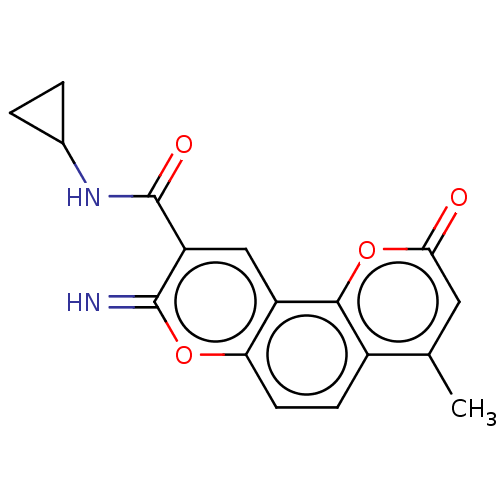 (CHEMBL3746215) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500463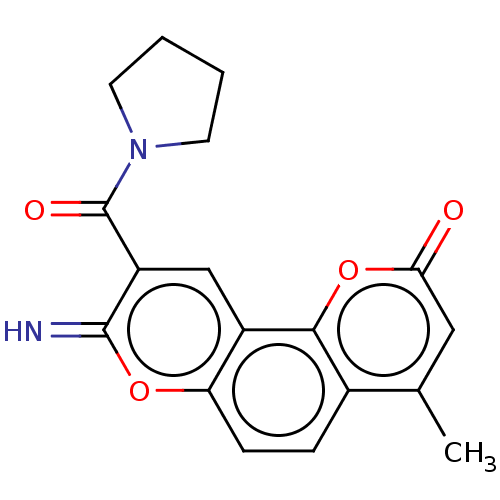 (CHEMBL3746086) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 984 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500454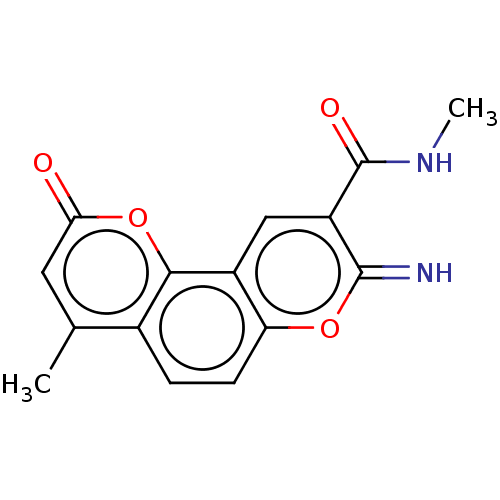 (CHEMBL3746327) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500461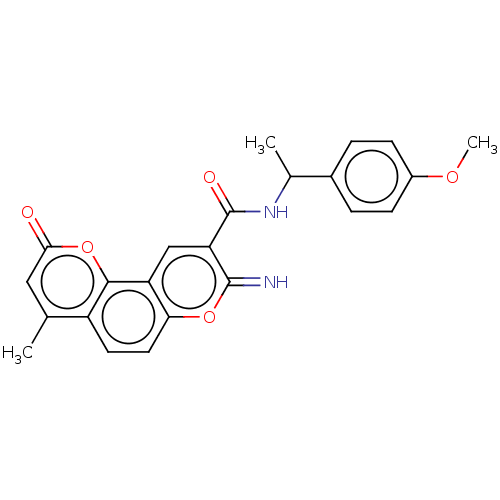 (CHEMBL3746509) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500465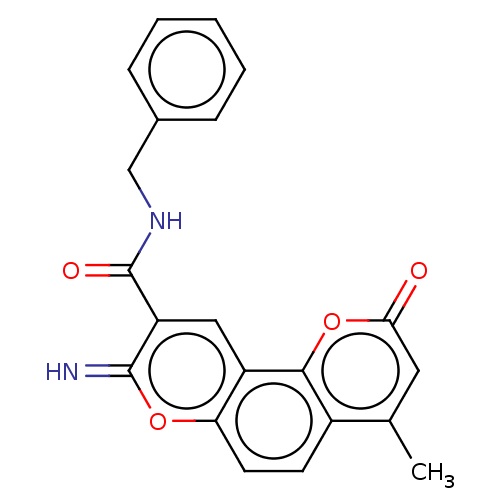 (CHEMBL3747234) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50500453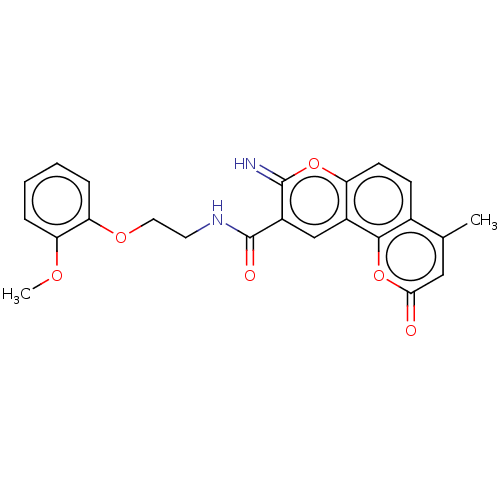 (CHEMBL3746191) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured after 5 m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM11682 (2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500460 (CHEMBL3746173) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500453 (CHEMBL3746191) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500468 (CHEMBL3745771) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500452 (CHEMBL3746215) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500464 (CHEMBL3745983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM189377 (N-(4-(tert-butyl)benzyl)-8-imino-4-methyl-2-oxo-2H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500463 (CHEMBL3746086) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500456 (CHEMBL3747774) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500465 (CHEMBL3747234) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500466 (CHEMBL3747027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500455 (CHEMBL3746856) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500454 (CHEMBL3746327) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500467 (CHEMBL3745757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500459 (CHEMBL3747030) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500457 (CHEMBL3746815) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50500458 (CHEMBL3746565) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Yogi Vemana University Curated by ChEMBL | Assay Description Inhibition of horse serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition m... | Eur J Med Chem 107: 219-32 (2016) Article DOI: 10.1016/j.ejmech.2015.10.046 BindingDB Entry DOI: 10.7270/Q2JW8HWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||