

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151154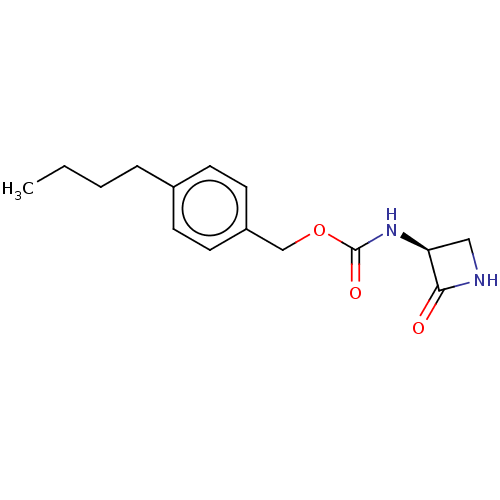 (CHEMBL3770896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151242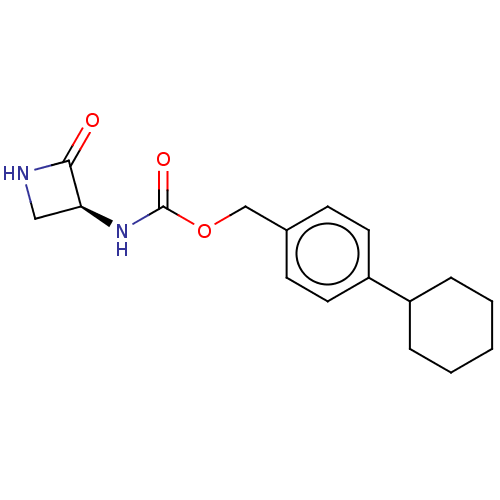 (CHEMBL3770525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151055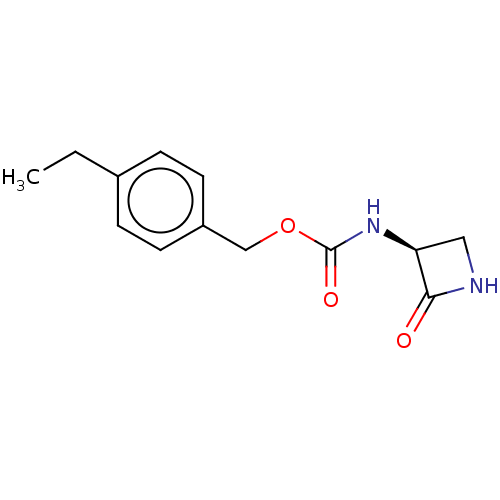 (CHEMBL3771111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151154 (CHEMBL3770896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151057 (CHEMBL3770726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151155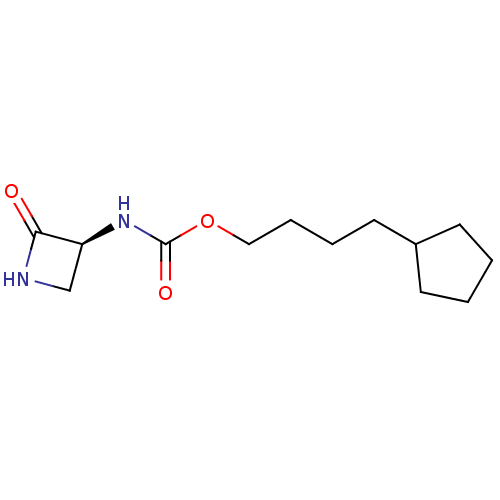 (CHEMBL3769844) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151240 (CHEMBL3770558) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151242 (CHEMBL3770525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151239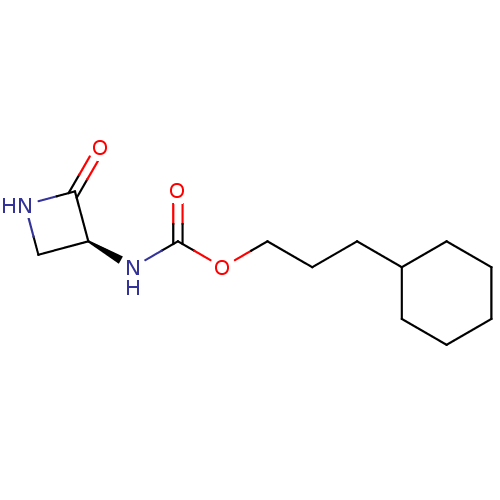 (CHEMBL3769455) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151096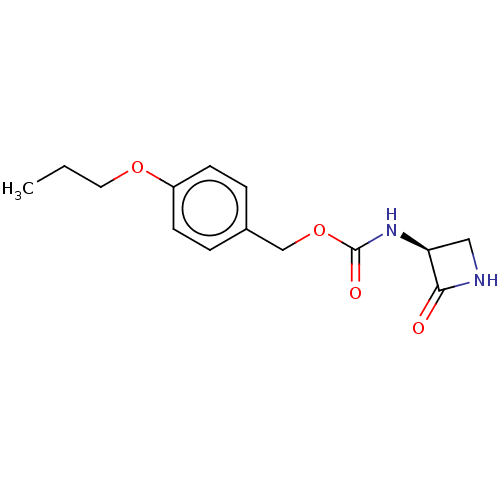 (CHEMBL3769689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151234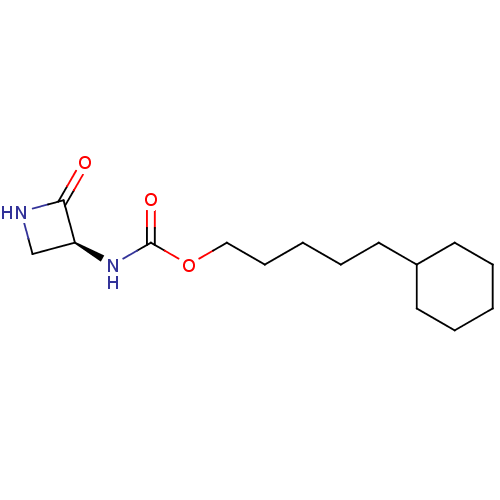 (CHEMBL3770490) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151237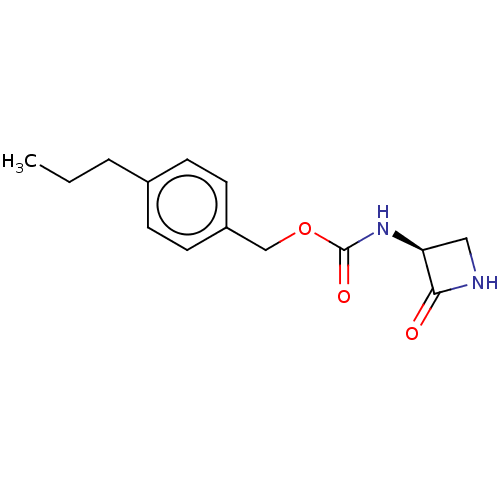 (CHEMBL3770240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151232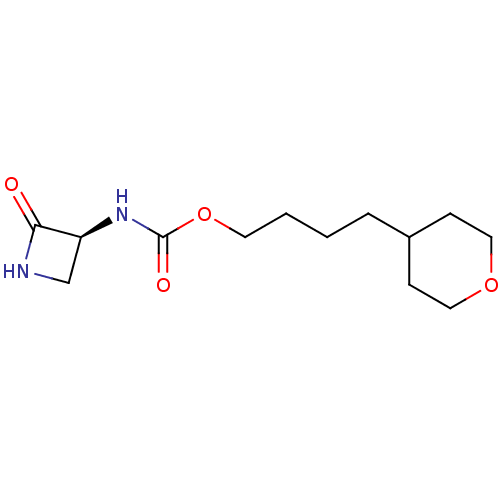 (CHEMBL3769763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151057 (CHEMBL3770726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged human BRD4 bromodomain1 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151149 (CHEMBL3769639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151220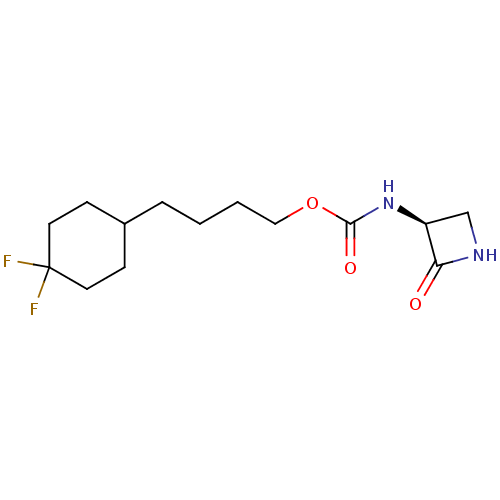 (CHEMBL3769678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151055 (CHEMBL3771111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Binding affinity to his-tagged human BRD4 bromodomain1 by isothermal titration calorimetric analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151127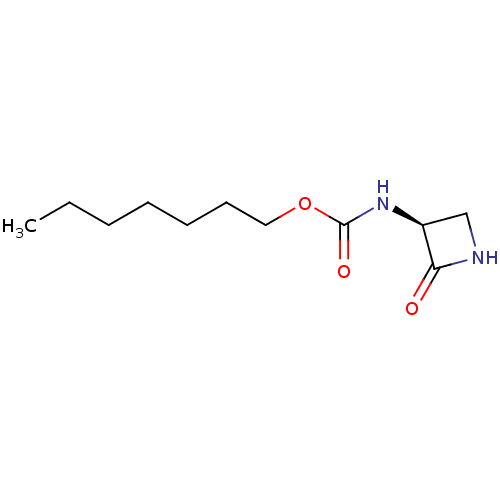 (CHEMBL3770640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151148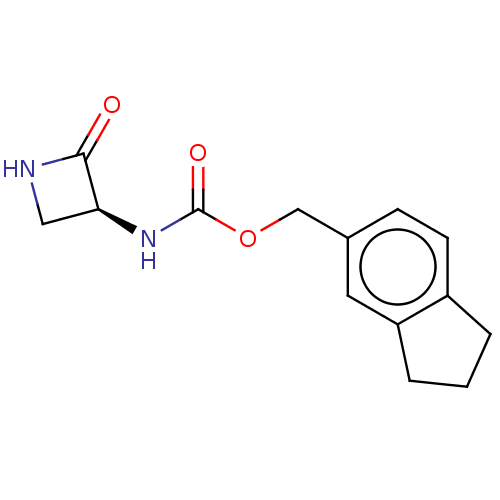 (CHEMBL3769700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151152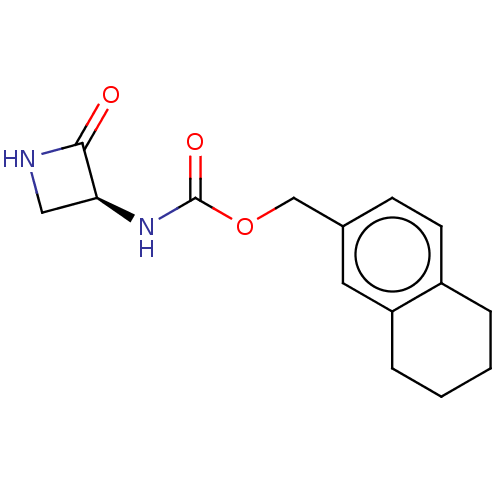 (CHEMBL3771196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151055 (CHEMBL3771111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151151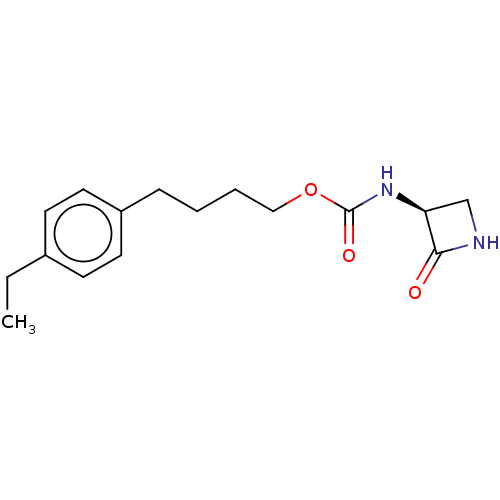 (CHEMBL3770354) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151236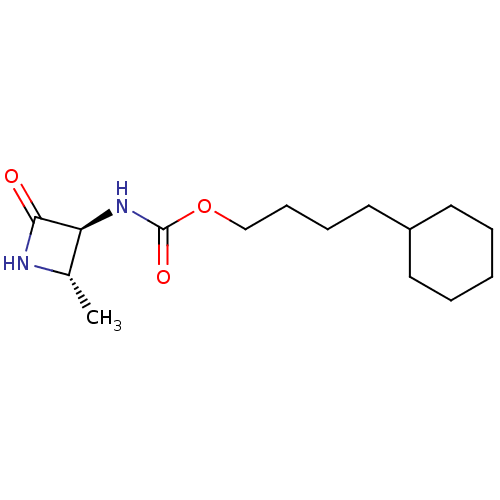 (CHEMBL3770695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151096 (CHEMBL3769689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged human BRD4 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151241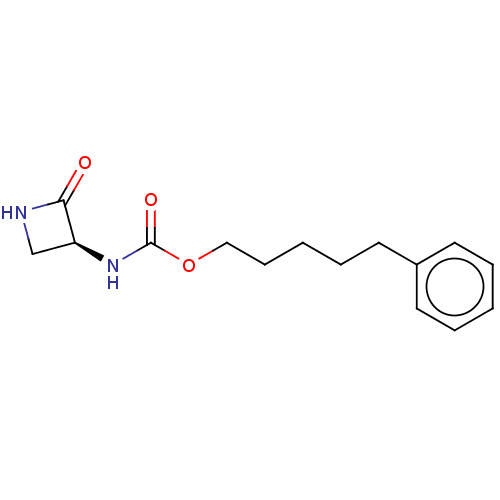 (CHEMBL3770549) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 298 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151153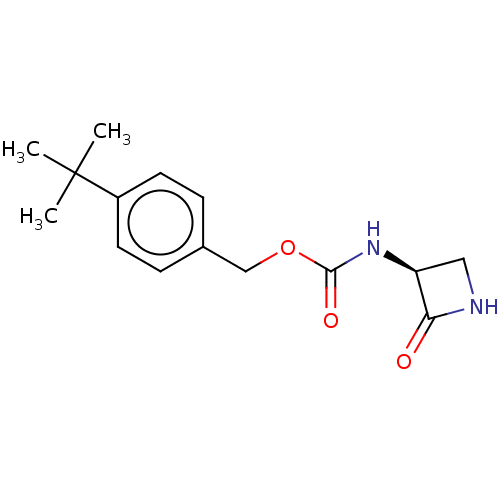 (CHEMBL3769597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151222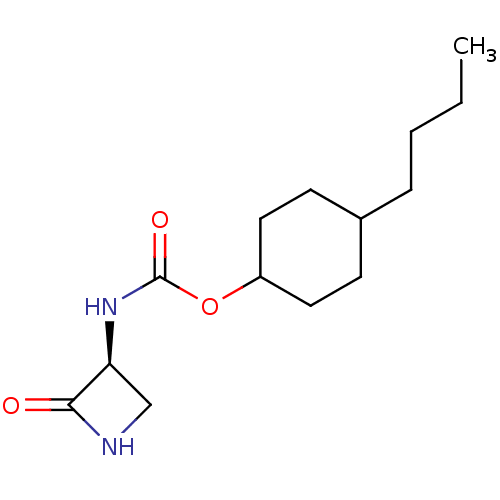 (CHEMBL3770519) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151220 (CHEMBL3769678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human BRD3 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151053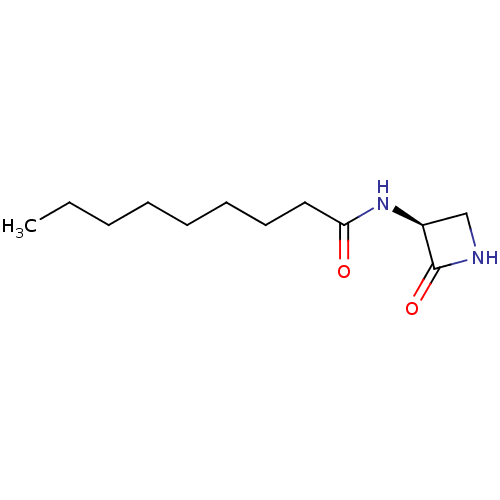 (CHEMBL3770509) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151154 (CHEMBL3770896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151238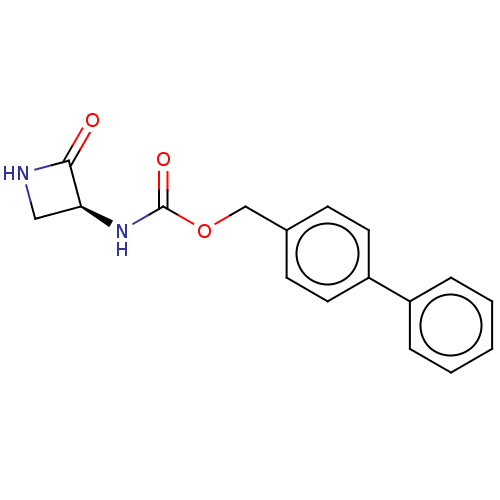 (CHEMBL3770031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 513 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151147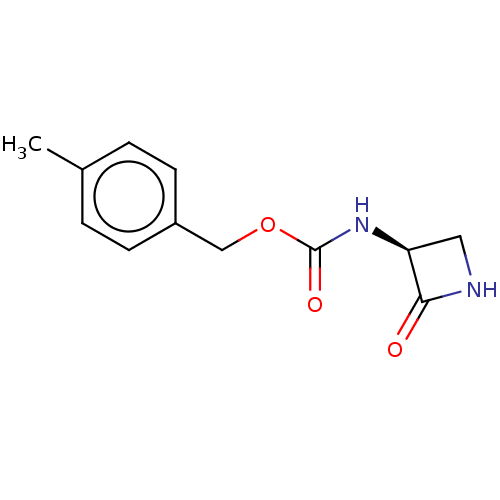 (CHEMBL3770722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151147 (CHEMBL3770722) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151150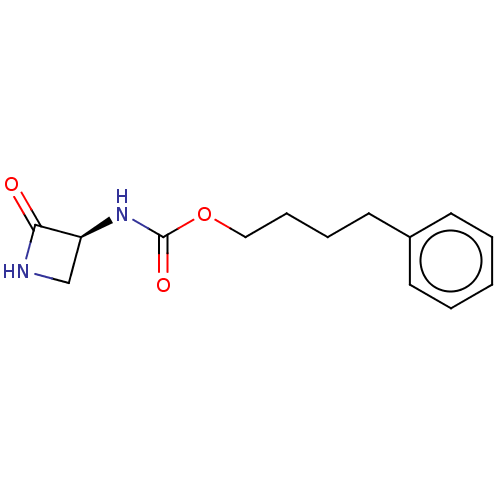 (CHEMBL3769700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151235 (CHEMBL3770450) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged human BRD2 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151039 (CHEMBL3770901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151039 (CHEMBL3770901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged human BRD2 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151242 (CHEMBL3770525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151130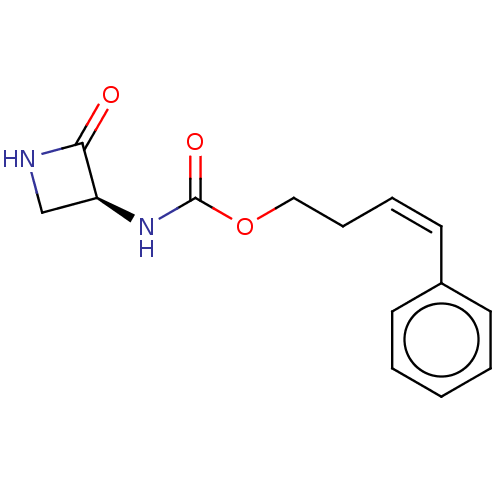 (CHEMBL3771259) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151144 (CHEMBL3771368) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151129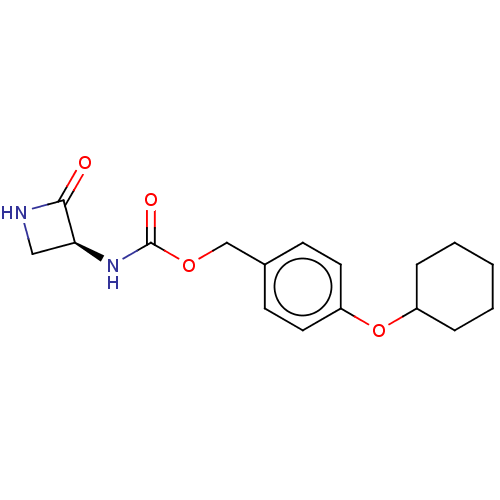 (CHEMBL3769503) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151099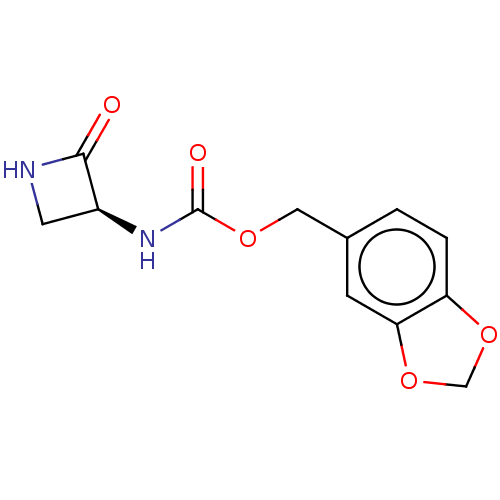 (CHEMBL3770849) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151096 (CHEMBL3769689) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151224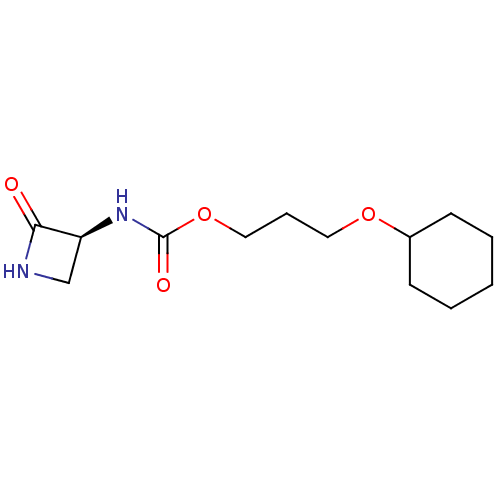 (CHEMBL3770547) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151146 (CHEMBL3770146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151054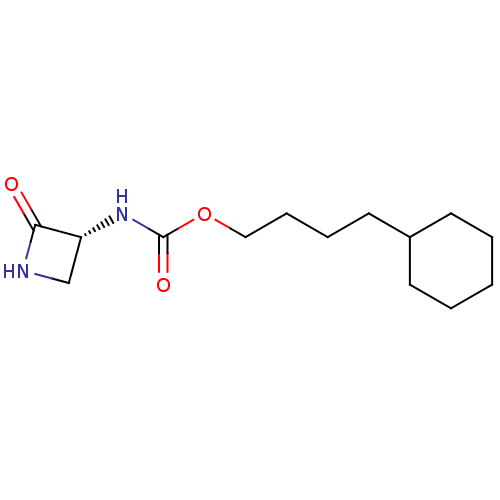 (CHEMBL3770250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151126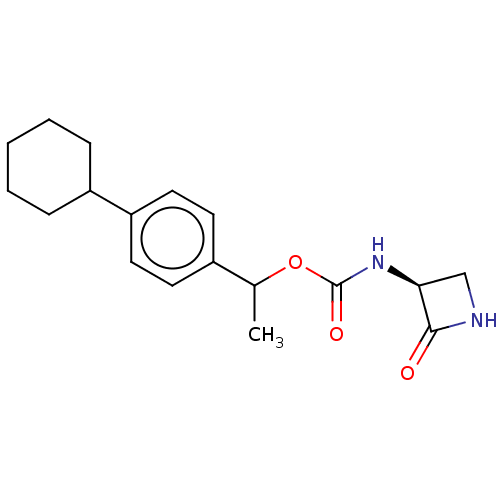 (CHEMBL3770640) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151057 (CHEMBL3770726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151055 (CHEMBL3771111) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151056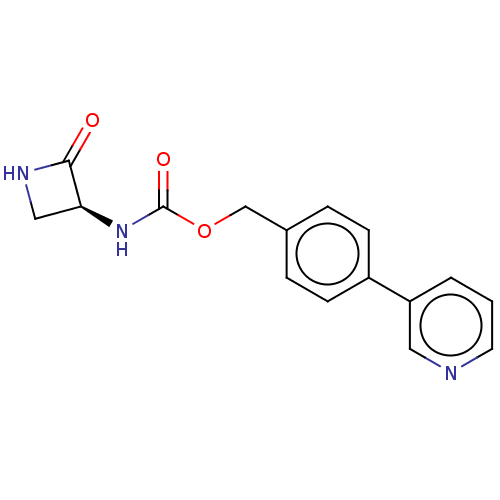 (CHEMBL3769770) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151232 (CHEMBL3769763) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acid ceramidase (Homo sapiens (Human)) | BDBM50151220 (CHEMBL3769678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human acid ceramidase by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151103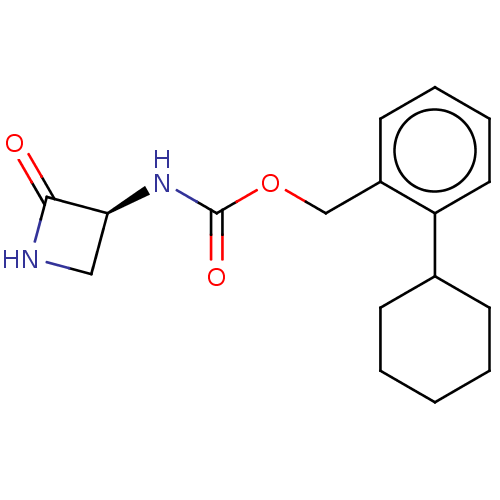 (CHEMBL3769976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151221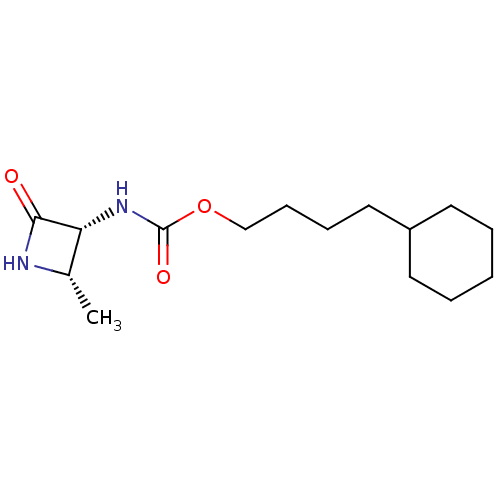 (CHEMBL3771160) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human BRD3 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151156 (CHEMBL3769863) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of GST-tagged human BRD4 bromodomain2 using H4KAc 5/8/12/16 peptide as substrate incubated overnight by HTRF assay | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151223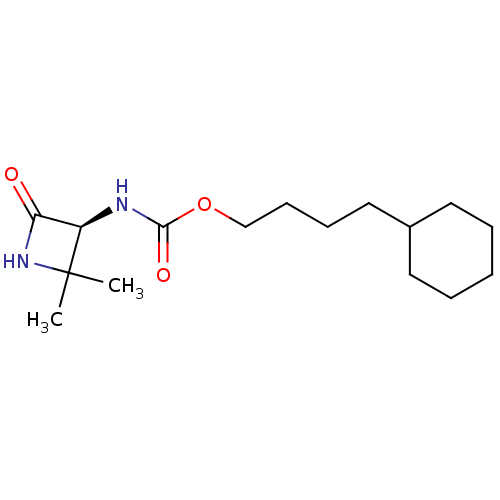 (CHEMBL3771292) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151225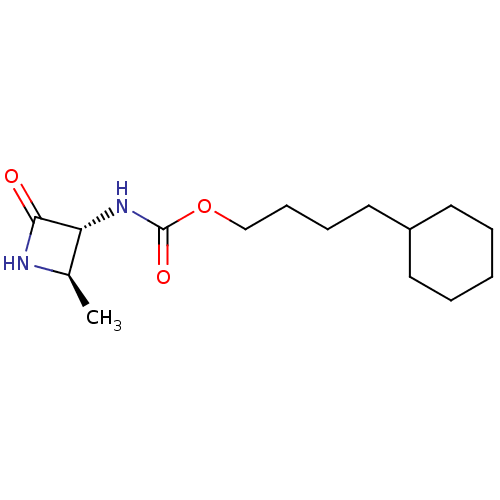 (CHEMBL3771358) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151145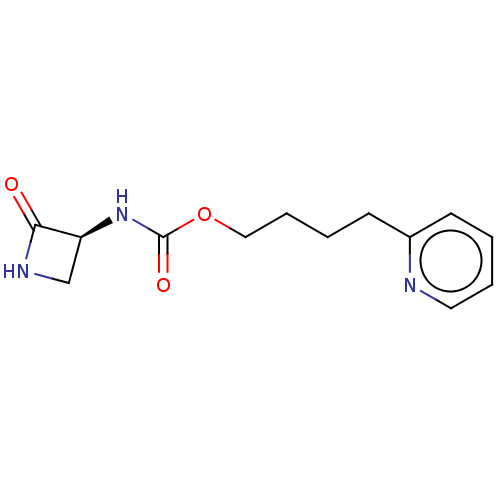 (CHEMBL3771150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151233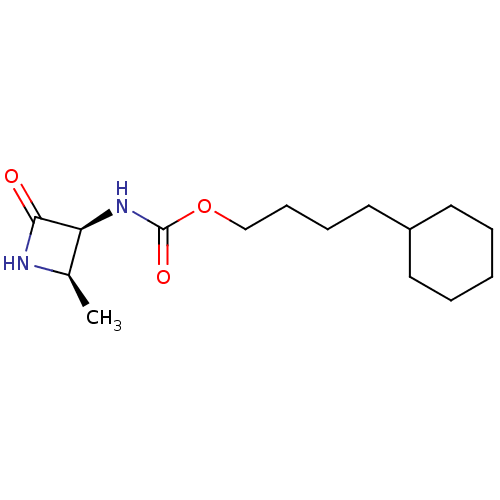 (CHEMBL3770388) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human NAAA expressed in HEK293 cells preincubated for 10 mins followed by N-(4-methyl-2-oxo-chromen-7-yl)-hexadecanamide substrate addi... | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||