

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231998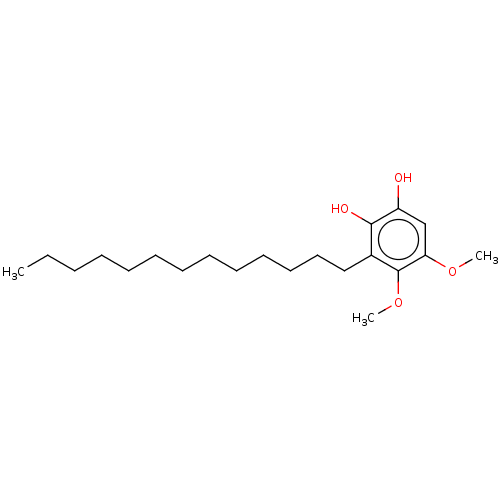 (CHEMBL4085835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231991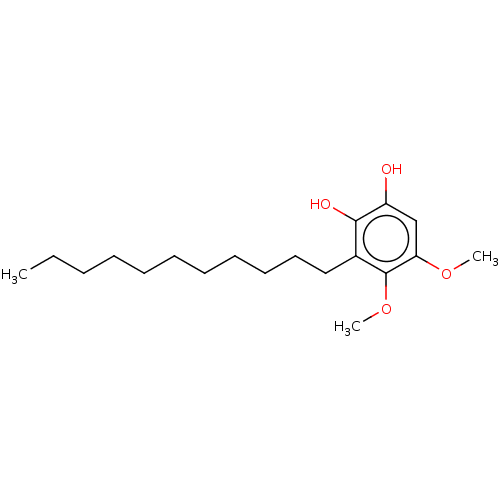 (CHEMBL4093584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231998 (CHEMBL4085835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231999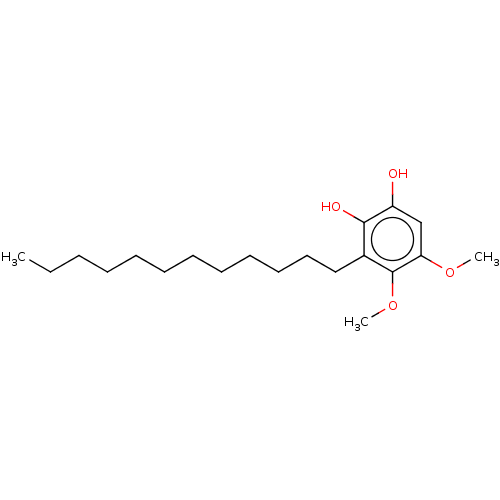 (CHEMBL4103758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231991 (CHEMBL4093584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232002 (CHEMBL4095644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231996 (CHEMBL4069339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231993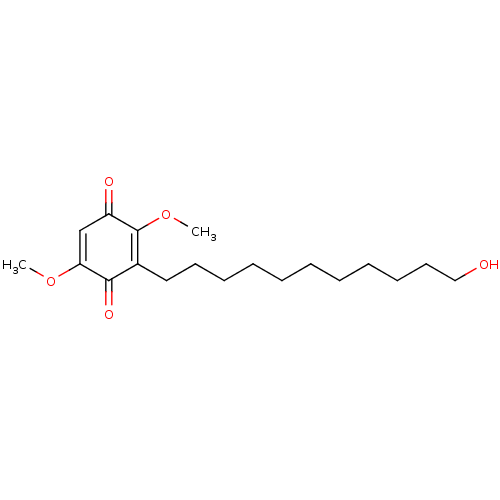 (CHEMBL4088278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231996 (CHEMBL4069339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231999 (CHEMBL4103758) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231997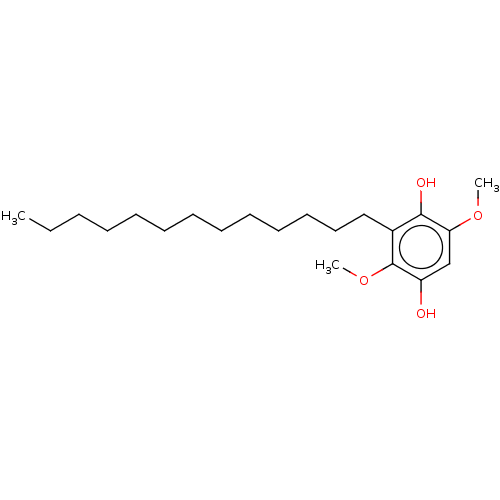 (CHEMBL4089216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232003 (CHEMBL4061044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231997 (CHEMBL4089216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232001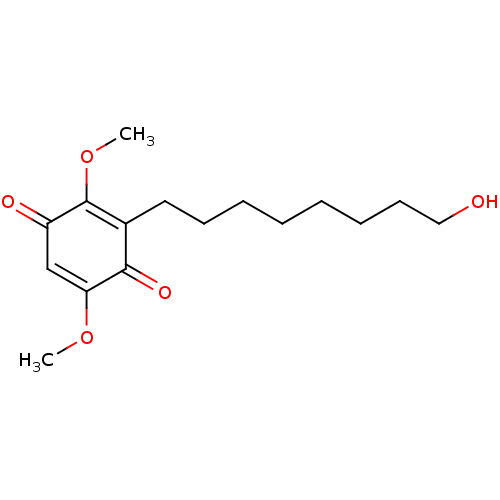 (CHEMBL4096012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231995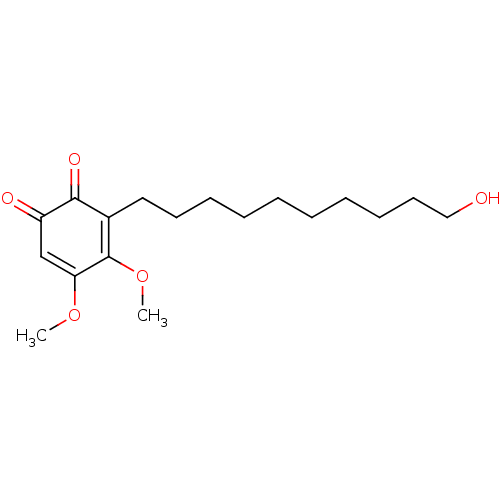 (CHEMBL4075247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232000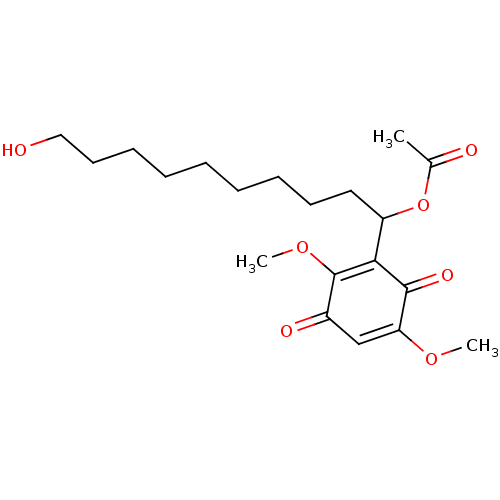 (CHEMBL4065980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231994 (CHEMBL4077966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231992 (CHEMBL4067007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231995 (CHEMBL4075247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231992 (CHEMBL4067007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231993 (CHEMBL4088278) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232003 (CHEMBL4061044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232000 (CHEMBL4065980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of 5-lipoxygenase in human neutrophils assessed as inhibition of A23187-induced product formation using arachidonic acid as substrate prei... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50231994 (CHEMBL4077966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232001 (CHEMBL4096012) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50232002 (CHEMBL4095644) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Second University of Naples Curated by ChEMBL | Assay Description Inhibition of recombinant human 5-lipoxygenase expressed in Escherichia coli BL21 using arachidonic acid as substrate preincubated for 15 mins follow... | Eur J Med Chem 127: 715-726 (2017) Article DOI: 10.1016/j.ejmech.2016.10.046 BindingDB Entry DOI: 10.7270/Q2DR2XQ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||