 Found 33 hits in this display
Found 33 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501641

(CHEMBL4081024)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-c3ccccc3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C16H21N3O11P2.3Na/c17-11-6-7-19(16(22)18-11)14-13(21)12(20)10(29-14)8-28-32(26,27)30-15(31(23,24)25)9-4-2-1-3-5-9;;;/h1-7,10,12-15,20-21H,8H2,(H,26,27)(H2,17,18,22)(H2,23,24,25);;;/q;3*+1/p-3/t10-,12-,13-,14-,15?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50501641

(CHEMBL4081024)Show SMILES [Na;v0+].[Na;v0+].[Na;v0+].[#7]-c1ccn(-[#6@@H]-2-[#8]-[#6@H](-[#6]-[#8]P([#8-])(=O)[#8]-[#6](-c3ccccc3)P([#8-])([#8-])=O)-[#6@@H](-[#8])-[#6@H]-2-[#8])c(=O)n1 |r| Show InChI InChI=1S/C16H21N3O11P2.3Na/c17-11-6-7-19(16(22)18-11)14-13(21)12(20)10(29-14)8-28-32(26,27)30-15(31(23,24)25)9-4-2-1-3-5-9;;;/h1-7,10,12-15,20-21H,8H2,(H,26,27)(H2,17,18,22)(H2,23,24,25);;;/q;3*+1/p-3/t10-,12-,13-,14-,15?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127709
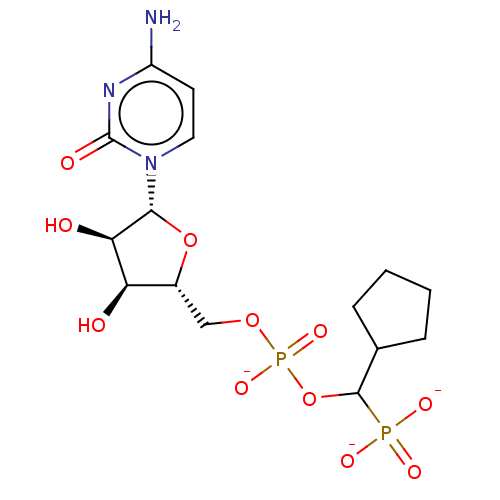
(CHEMBL3629689)Show SMILES [Na+].[Na+].[Na+].Nc1ccn([C@@H]2O[C@H](COP([O-])(=O)OC(C3CCCC3)P([O-])([O-])=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H25N3O11P2.3Na/c16-10-5-6-18(15(21)17-10)13-12(20)11(19)9(28-13)7-27-31(25,26)29-14(30(22,23)24)8-3-1-2-4-8;;;/h5-6,8-9,11-14,19-20H,1-4,7H2,(H,25,26)(H2,16,17,21)(H2,22,23,24);;;/q;3*+1/p-3/t9-,11-,12-,13-,14?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant ST6Gal-1 using CMP-Neu5Ac and p-nitrophenyl LacNAc as donar and acceptor by Lineweaver-Burk double recipr... |
J Med Chem 58: 7972-90 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01181
BindingDB Entry DOI: 10.7270/Q20V8FMQ |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50127709

(CHEMBL3629689)Show SMILES [Na+].[Na+].[Na+].Nc1ccn([C@@H]2O[C@H](COP([O-])(=O)OC(C3CCCC3)P([O-])([O-])=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C15H25N3O11P2.3Na/c16-10-5-6-18(15(21)17-10)13-12(20)11(19)9(28-13)7-27-31(25,26)29-14(30(22,23)24)8-3-1-2-4-8;;;/h5-6,8-9,11-14,19-20H,1-4,7H2,(H,25,26)(H2,16,17,21)(H2,22,23,24);;;/q;3*+1/p-3/t9-,11-,12-,13-,14?;;;/m1.../s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged ST6Gal-1 (44 to 406 residues) using CMP-Neu5Ac as substrate after 20 mins by UV/RP-HPLC method |
J Med Chem 60: 2135-2141 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01644
BindingDB Entry DOI: 10.7270/Q2XP77Z0 |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50559954

(CHEMBL4755740)Show SMILES O[C@@H]1[C@@H](CNC(=O)OC(c2cc3ccccc3s2)P([O-])([O-])=O)O[C@H]([C@@H]1O)n1ccc(=O)[nH]c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115561
BindingDB Entry DOI: 10.7270/Q25M69DR |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50559951
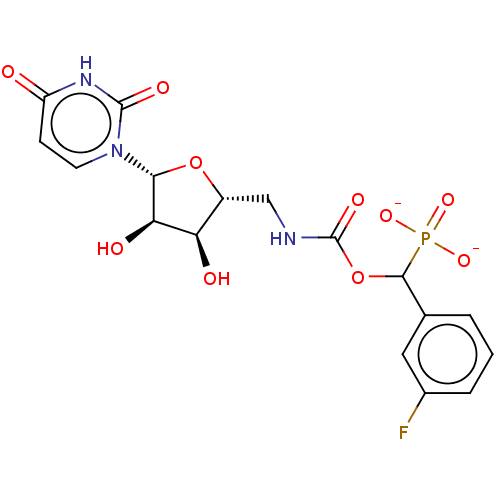
(CHEMBL4751247)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1CNC(=O)OC(c1cccc(F)c1)P([O-])([O-])=O)n1ccc(=O)[nH]c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115561
BindingDB Entry DOI: 10.7270/Q25M69DR |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50559952
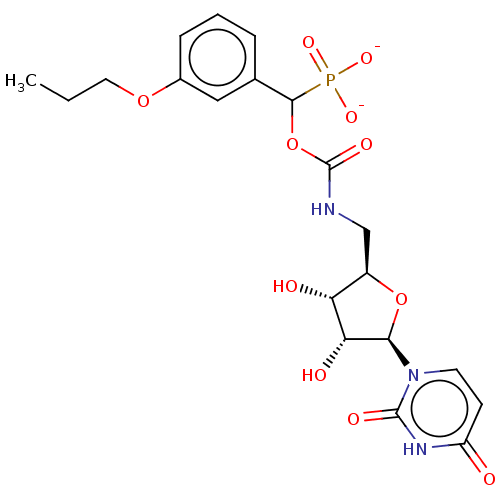
(CHEMBL4743892)Show SMILES CCCOc1cccc(c1)C(OC(=O)NC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1ccc(=O)[nH]c1=O)P([O-])([O-])=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115561
BindingDB Entry DOI: 10.7270/Q25M69DR |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50310540
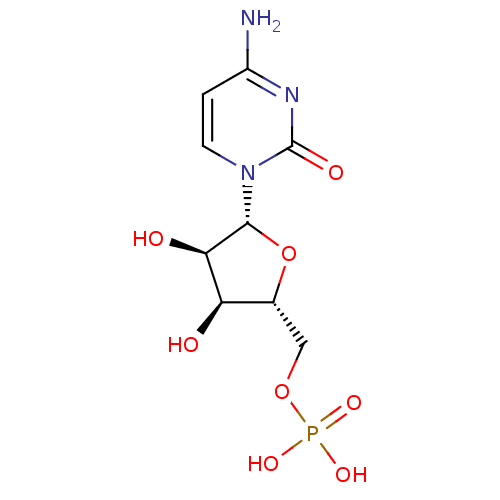
(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366833
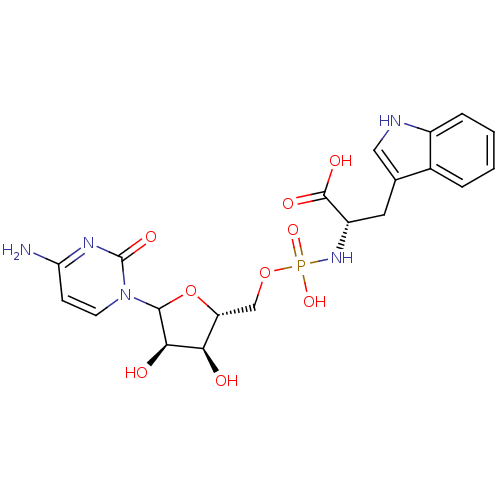
(CHEMBL608928)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C20H24N5O9P/c21-15-5-6-25(20(30)23-15)18-17(27)16(26)14(34-18)9-33-35(31,32)24-13(19(28)29)7-10-8-22-12-4-2-1-3-11(10)12/h1-6,8,13-14,16-18,22,26-27H,7,9H2,(H,28,29)(H2,21,23,30)(H2,24,31,32)/t13-,14+,16+,17+,18?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50559950

(CHEMBL4745184)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1CNC(=O)OC(c1ccc(F)cc1)P([O-])([O-])=O)n1ccc(=O)[nH]c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115561
BindingDB Entry DOI: 10.7270/Q25M69DR |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50559950

(CHEMBL4745184)Show SMILES O[C@H]1[C@@H](O)[C@@H](O[C@@H]1CNC(=O)OC(c1ccc(F)cc1)P([O-])([O-])=O)n1ccc(=O)[nH]c1=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human N-terminal 6His-tagged ST6Gal-1 (Glu44 to Cys406 residues) |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115561
BindingDB Entry DOI: 10.7270/Q25M69DR |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366837

(CHEMBL608619)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)OCC(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C11H16N3O10P/c12-6-1-2-14(11(19)13-6)10-9(18)8(17)5(24-10)3-22-25(20,21)23-4-7(15)16/h1-2,5,8-10,17-18H,3-4H2,(H,15,16)(H,20,21)(H2,12,13,19)/t5-,8-,9-,10?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366837

(CHEMBL608619)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)OCC(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C11H16N3O10P/c12-6-1-2-14(11(19)13-6)10-9(18)8(17)5(24-10)3-22-25(20,21)23-4-7(15)16/h1-2,5,8-10,17-18H,3-4H2,(H,15,16)(H,20,21)(H2,12,13,19)/t5-,8-,9-,10?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366833

(CHEMBL608928)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](Cc3c[nH]c4ccccc34)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C20H24N5O9P/c21-15-5-6-25(20(30)23-15)18-17(27)16(26)14(34-18)9-33-35(31,32)24-13(19(28)29)7-10-8-22-12-4-2-1-3-11(10)12/h1-6,8,13-14,16-18,22,26-27H,7,9H2,(H,28,29)(H2,21,23,30)(H2,24,31,32)/t13-,14+,16+,17+,18?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Ribonuclease pancreatic
(Bison bison (American bison)) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of bovine pancreatic ribonuclease A assessed as enzyme activity by spectrophotometric method pH 6 |
Eur J Med Chem 44: 4496-508 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.039
BindingDB Entry DOI: 10.7270/Q26H4HJJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366832

(CHEMBL609516)Show SMILES CC(C)C[C@H](NP(O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1ccc(N)nc1=O)C(O)=O |r| Show InChI InChI=1S/C15H25N4O9P/c1-7(2)5-8(14(22)23)18-29(25,26)27-6-9-11(20)12(21)13(28-9)19-4-3-10(16)17-15(19)24/h3-4,7-9,11-13,20-21H,5-6H2,1-2H3,(H,22,23)(H2,16,17,24)(H2,18,25,26)/t8-,9+,11+,12+,13?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Orotidine 5'-phosphate decarboxylase
(Methanobacterium thermoautotrophicum) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network
Curated by ChEMBL
| Assay Description
Inhibition of Methanobacterium thermoautotrophicum ODCase by isothermal titration calorimetry |
J Med Chem 55: 9988-97 (2012)
Article DOI: 10.1021/jm301176r
BindingDB Entry DOI: 10.7270/Q2DR2WPD |
More data for this
Ligand-Target Pair | |
Uridine 5'-monophosphate synthase
(Homo sapiens (Human)) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network
Curated by ChEMBL
| Assay Description
Inhibition of human ODCase by isothermal titration calorimetry |
J Med Chem 55: 9988-97 (2012)
Article DOI: 10.1021/jm301176r
BindingDB Entry DOI: 10.7270/Q2DR2WPD |
More data for this
Ligand-Target Pair | |
Seminal ribonuclease
(Bos taurus) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.56E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
National Hellenic Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of bovine seminal ribonuclease assessed as enzyme activity by spectrophotometric method at pH 6 |
Eur J Med Chem 44: 4496-508 (2009)
Article DOI: 10.1016/j.ejmech.2009.06.039
BindingDB Entry DOI: 10.7270/Q26H4HJJ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366836
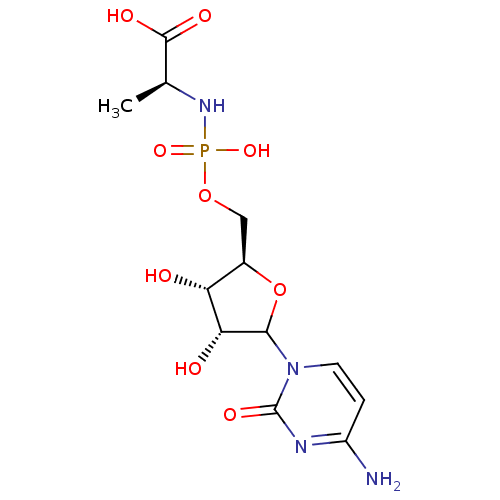
(CHEMBL610554)Show SMILES C[C@H](NP(O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1ccc(N)nc1=O)C(O)=O |r| Show InChI InChI=1S/C12H19N4O9P/c1-5(11(19)20)15-26(22,23)24-4-6-8(17)9(18)10(25-6)16-3-2-7(13)14-12(16)21/h2-3,5-6,8-10,17-18H,4H2,1H3,(H,19,20)(H2,13,14,21)(H2,15,22,23)/t5-,6+,8+,9+,10?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366835
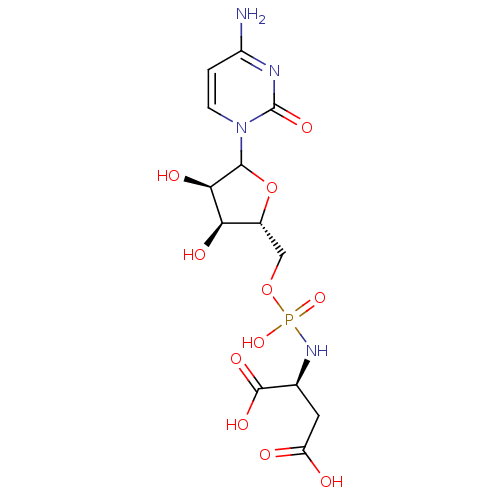
(CHEMBL609514)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C13H19N4O11P/c14-7-1-2-17(13(24)15-7)11-10(21)9(20)6(28-11)4-27-29(25,26)16-5(12(22)23)3-8(18)19/h1-2,5-6,9-11,20-21H,3-4H2,(H,18,19)(H,22,23)(H2,14,15,24)(H2,16,25,26)/t5-,6+,9+,10+,11?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366836

(CHEMBL610554)Show SMILES C[C@H](NP(O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1ccc(N)nc1=O)C(O)=O |r| Show InChI InChI=1S/C12H19N4O9P/c1-5(11(19)20)15-26(22,23)24-4-6-8(17)9(18)10(25-6)16-3-2-7(13)14-12(16)21/h2-3,5-6,8-10,17-18H,4H2,1H3,(H,19,20)(H2,13,14,21)(H2,15,22,23)/t5-,6+,8+,9+,10?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366834

(CHEMBL609800)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](Cc3ccccc3)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C18H23N4O9P/c19-13-6-7-22(18(27)20-13)16-15(24)14(23)12(31-16)9-30-32(28,29)21-11(17(25)26)8-10-4-2-1-3-5-10/h1-7,11-12,14-16,23-24H,8-9H2,(H,25,26)(H2,19,20,27)(H2,21,28,29)/t11-,12+,14+,15+,16?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366838
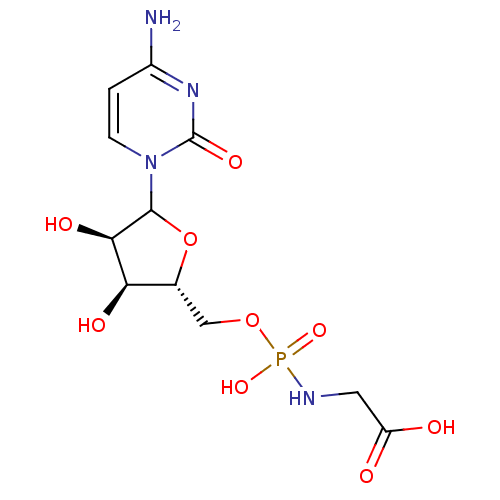
(CHEMBL609215)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)NCC(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C11H17N4O9P/c12-6-1-2-15(11(20)14-6)10-9(19)8(18)5(24-10)4-23-25(21,22)13-3-7(16)17/h1-2,5,8-10,18-19H,3-4H2,(H,16,17)(H2,12,14,20)(H2,13,21,22)/t5-,8-,9-,10?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366832

(CHEMBL609516)Show SMILES CC(C)C[C@H](NP(O)(=O)OC[C@H]1OC([C@H](O)[C@@H]1O)n1ccc(N)nc1=O)C(O)=O |r| Show InChI InChI=1S/C15H25N4O9P/c1-7(2)5-8(14(22)23)18-29(25,26)27-6-9-11(20)12(21)13(28-9)19-4-3-10(16)17-15(19)24/h3-4,7-9,11-13,20-21H,5-6H2,1-2H3,(H,22,23)(H2,16,17,24)(H2,18,25,26)/t8-,9+,11+,12+,13?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Rattus norvegicus) | BDBM50366834

(CHEMBL609800)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](Cc3ccccc3)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C18H23N4O9P/c19-13-6-7-22(18(27)20-13)16-15(24)14(23)12(31-16)9-30-32(28,29)21-11(17(25)26)8-10-4-2-1-3-5-10/h1-7,11-12,14-16,23-24H,8-9H2,(H,25,26)(H2,19,20,27)(H2,21,28,29)/t11-,12+,14+,15+,16?/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,6-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366835

(CHEMBL609514)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)N[C@@H](CC(O)=O)C(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C13H19N4O11P/c14-7-1-2-17(13(24)15-7)11-10(21)9(20)6(28-11)4-27-29(25,26)16-5(12(22)23)3-8(18)19/h1-2,5-6,9-11,20-21H,3-4H2,(H,18,19)(H,22,23)(H2,14,15,24)(H2,16,25,26)/t5-,6+,9+,10+,11?/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50366838

(CHEMBL609215)Show SMILES Nc1ccn(C2O[C@H](COP(O)(=O)NCC(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C11H17N4O9P/c12-6-1-2-15(11(20)14-6)10-9(19)8(18)5(24-10)4-23-25(21,22)13-3-7(16)17/h1-2,5,8-10,18-19H,3-4H2,(H,16,17)(H2,12,14,20)(H2,13,21,22)/t5-,8-,9-,10?/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Colorado
Curated by ChEMBL
| Assay Description
Inhibitory activity evaluated against alpha-2,3-sialyltransferase from rat liver |
Bioorg Med Chem Lett 13: 301-4 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2HBM |
More data for this
Ligand-Target Pair | |
Orotidine-5'-phosphate decarboxylase
(Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM50310540

(((2R,3S,4R,5R)-5-(4-amino-2-oxopyrimidin-1(2H)-yl)...)Show SMILES Nc1ccn([C@@H]2O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]2O)c(=O)n1 |r| Show InChI InChI=1S/C9H14N3O8P/c10-5-1-2-12(9(15)11-5)8-7(14)6(13)4(20-8)3-19-21(16,17)18/h1-2,4,6-8,13-14H,3H2,(H2,10,11,15)(H2,16,17,18)/t4-,6-,7-,8-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network
Curated by ChEMBL
| Assay Description
Inhibition of Plasmodium falciparum ODCase by isothermal titration calorimetry |
J Med Chem 55: 9988-97 (2012)
Article DOI: 10.1021/jm301176r
BindingDB Entry DOI: 10.7270/Q2DR2WPD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
CMP-N-acetylneuraminate-beta-1,4-galactoside alpha-2,3-sialyltransferase
(Rattus norvegicus) | BDBM50565993
(CHEMBL4797481)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)[C@@H](N)CC(O)=O)[C@H](C)CCC(O)=O |r| | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha-2,3-N-ST3GALIII assessed as reduction in sialylated-product formation using Gal-beta1-4Glc and CMP-NeuSAc incubated for 1.5 h... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01477
BindingDB Entry DOI: 10.7270/Q2T43XVZ |
More data for this
Ligand-Target Pair | |
Beta-galactoside alpha-2,6-sialyltransferase 1
(Homo sapiens (Human)) | BDBM50565993

(CHEMBL4797481)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)[C@@H](N)CC(O)=O)[C@H](C)CCC(O)=O |r| | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human alpha-2,6-ST6GAL1 assessed as reduction in sialylated-product formation using Gal-beta1-4GlcNac and CMP-NeuSAc incubated for 15 m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01477
BindingDB Entry DOI: 10.7270/Q2T43XVZ |
More data for this
Ligand-Target Pair | |
Sialyltransferase ST3Gal-I
(Rattus norvegicus) | BDBM50565993

(CHEMBL4797481)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)[C@@H](N)CC(O)=O)[C@H](C)CCC(O)=O |r| | KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat alpha-2,3-O-ST3GALI assessed as reduction in sialylated-product formation using p-nitrophenyl T-antigen and CMP-NeuSAc incubated fo... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01477
BindingDB Entry DOI: 10.7270/Q2T43XVZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase












 Purchase
Purchase