

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM33283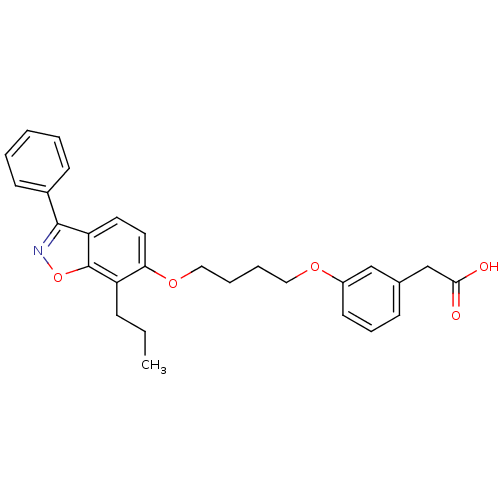 ((3-{4-[(3-phenyl-7-propyl-1,2-benzisoxazol-6-yl)ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR gamma receptor | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50148259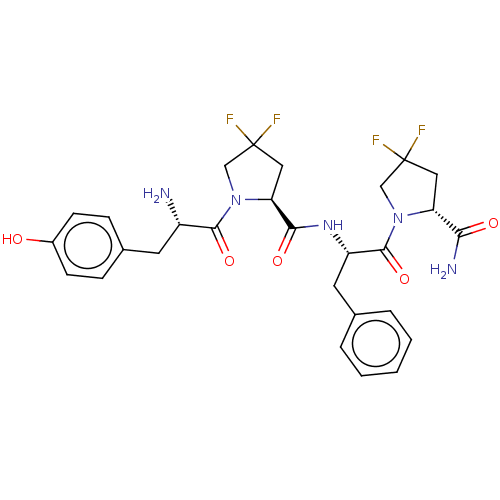 (CHEMBL3765406) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenate by scintillation counting analysis | Bioorg Med Chem 24: 1582-8 (2016) Article DOI: 10.1016/j.bmc.2016.02.034 BindingDB Entry DOI: 10.7270/Q2Z321H7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50256733 (CHEMBL4083221) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation counter an... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50359546 (CHEMBL1927270) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation counter an... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329161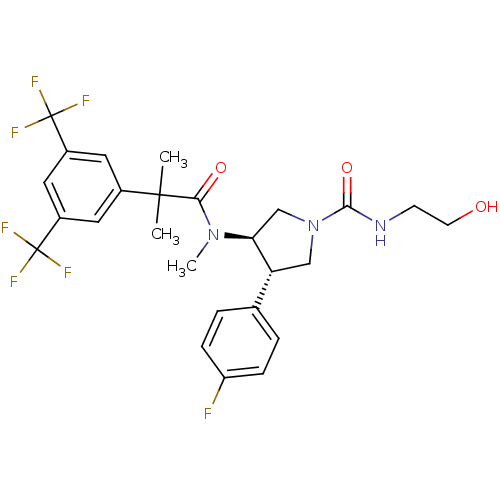 ((3R,4S)-3-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50237596 (CHEMBL4099423) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from MOR in Wistar rat brain homogenates after 180 mins by liquid scintillation counting analysis | Bioorg Med Chem Lett 27: 1644-1648 (2017) Article DOI: 10.1016/j.bmcl.2017.03.016 BindingDB Entry DOI: 10.7270/Q2BG2R9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50256752 (CHEMBL4095366) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation counter an... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329160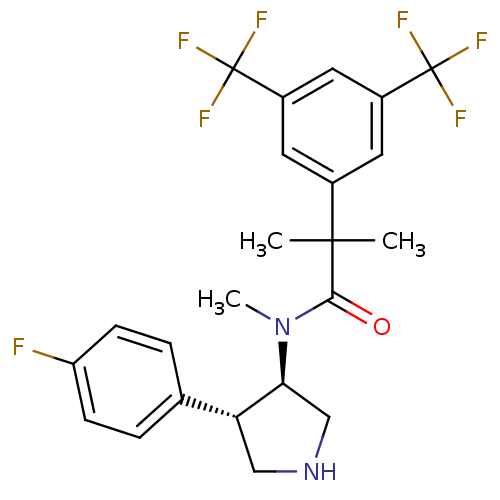 (2-(3,5-bis(trifluoromethyl)phenyl)-N-((3R,4S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329162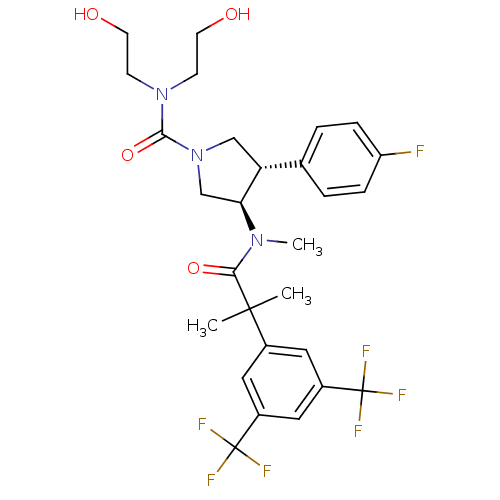 ((3R,4S)-3-(2-(3,5-bis(trifluoromethyl)phenyl)-N,2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50256743 (CHEMBL4085033) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation counter an... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM360648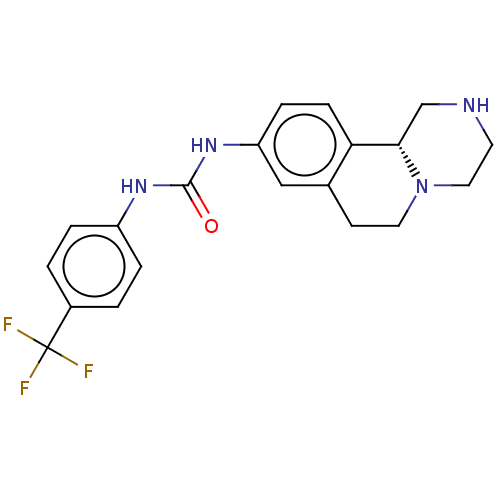 ((R)-1-(2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-a]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37° C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329163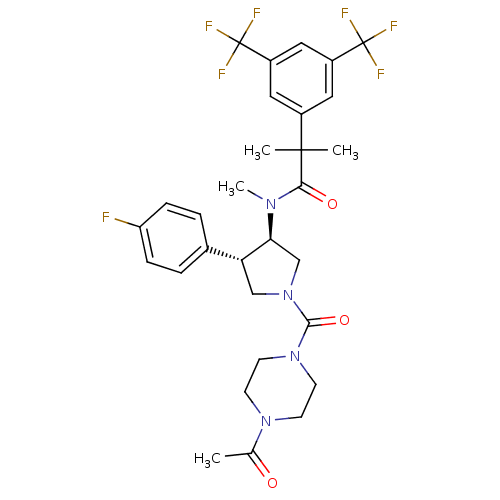 (CHEMBL1269639 | N-((3R,4S)-1-(4-acetylpiperazine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178953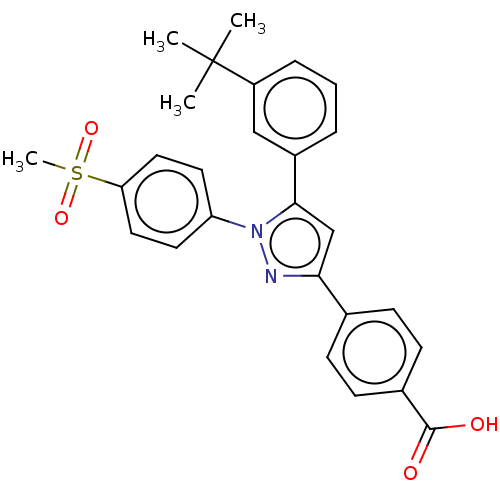 (CHEMBL3815166) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124377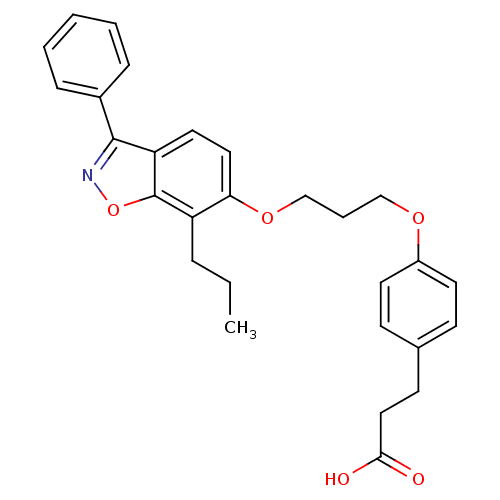 (3-{4-[3-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor delta (PPAR delta) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50329164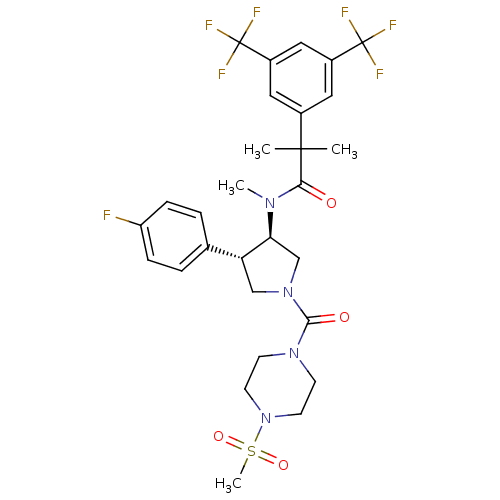 (2-(3,5-bis(trifluoromethyl)phenyl)-N-((3R,4S)-4-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]substance P from human neurokinin NK1 receptor expressed in CHO cell | Bioorg Med Chem Lett 20: 6735-8 (2010) Article DOI: 10.1016/j.bmcl.2010.08.138 BindingDB Entry DOI: 10.7270/Q22Z15SN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50359546 (CHEMBL1927270) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from recombinant human kappa opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation count... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50124394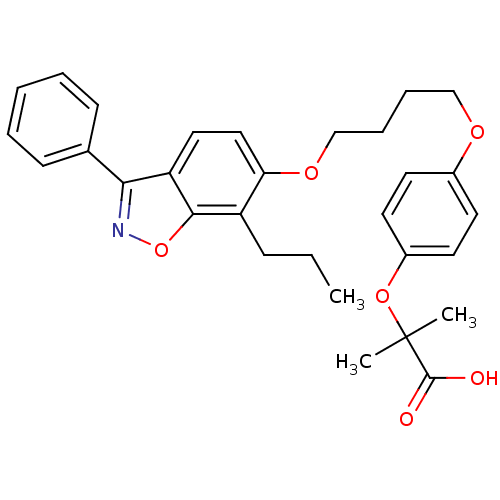 (2-Methyl-2-{4-[4-(3-phenyl-7-propyl-benzo[d]isoxaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360648 ((R)-1-(2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-a]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178952 (CHEMBL3814574) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124379 (2-(4-(3-(3-phenyl-7-propylbenzo[d]isoxazol-6-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM33283 ((3-{4-[(3-phenyl-7-propyl-1,2-benzisoxazol-6-yl)ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50256756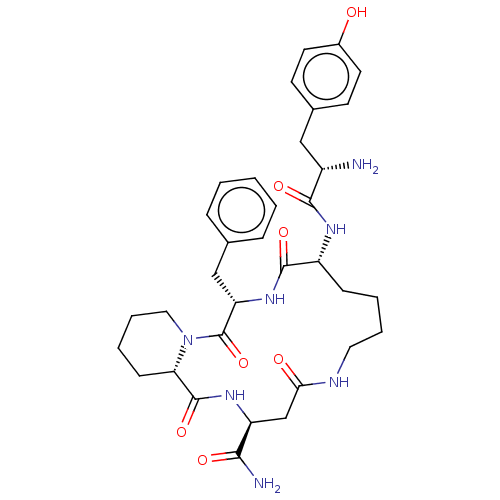 (CHEMBL4074931) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Biomolecular Chemistry, Faculty of Medicine, Medical University of Lodz, Mazowiecka 6/8, 92-215 Lodz, Poland. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from recombinant human mu opioid receptor expressed in CHO cell membranes after 120 mins by liquid scintillation counter an... | Bioorg Med Chem 25: 2399-2405 (2017) Article DOI: 10.1016/j.bmc.2017.02.057 BindingDB Entry DOI: 10.7270/Q2ZP48J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124384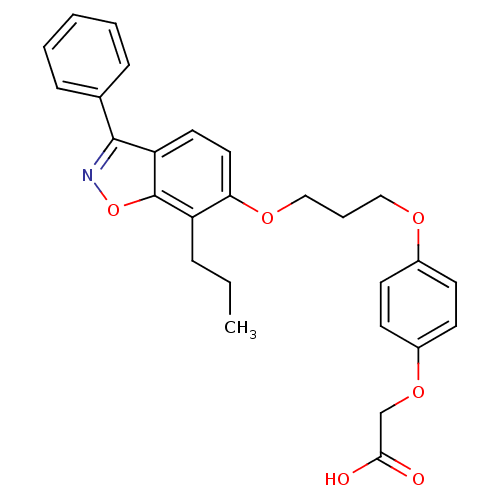 (CHEMBL173494 | {4-[3-(3-Phenyl-7-propyl-benzo[d]is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR alpha receptor | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124369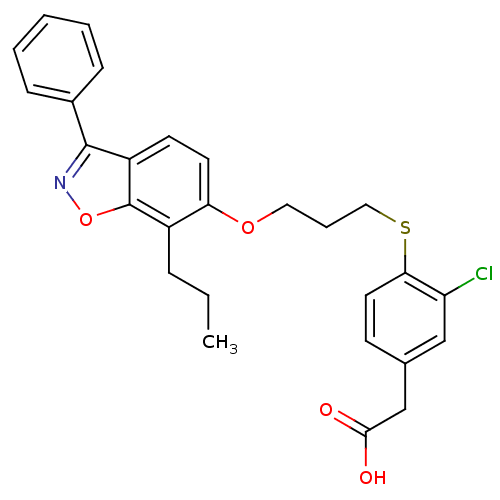 (CHEMBL367019 | {3-Chloro-4-[3-(3-phenyl-7-propyl-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor delta (PPAR delta) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124378 (2-{4-[3-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR gamma receptor | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50200369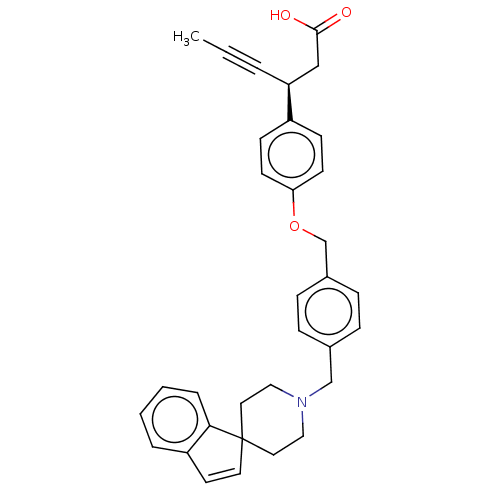 (CHEMBL3915620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... | J Med Chem 61: 934-945 (2018) Article DOI: 10.1021/acs.jmedchem.7b01411 BindingDB Entry DOI: 10.7270/Q2CZ39K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124379 (2-(4-(3-(3-phenyl-7-propylbenzo[d]isoxazol-6-yloxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124385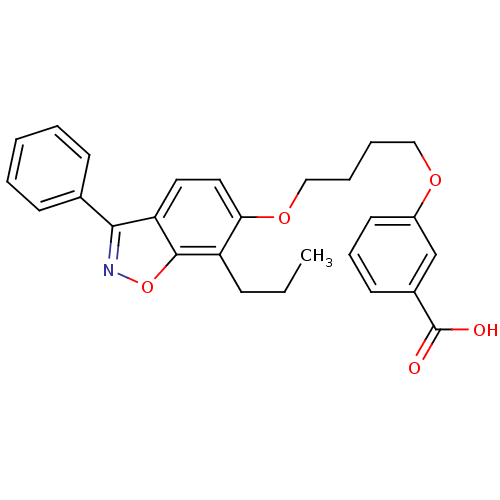 (3-[4-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-yloxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR delta receptor | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178954 (CHEMBL3813779) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM360621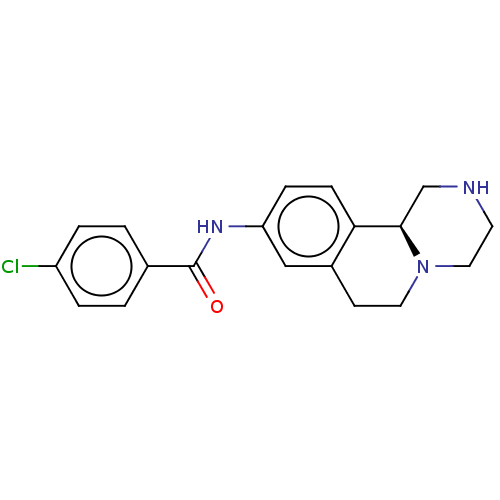 (N-[(11bS)-2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37° C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360627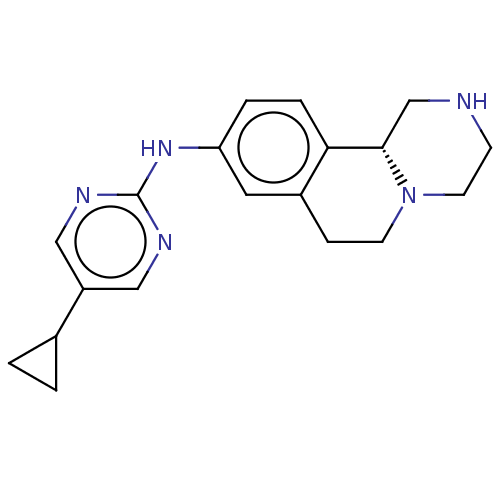 ((11bR)-N-(5-cyclopropylpyrimidin-2-yl)-2,3,4,6,7,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124386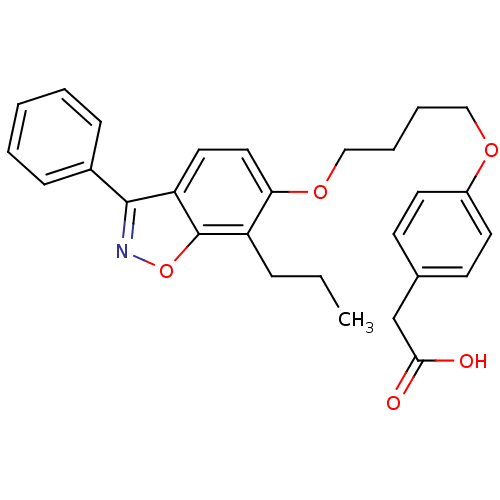 (CHEMBL367311 | {4-[4-(3-Phenyl-7-propyl-benzo[d]is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonistic activity was determined in COS1 cells transfected with GAL 4-PPAR alpha receptor | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM33283 ((3-{4-[(3-phenyl-7-propyl-1,2-benzisoxazol-6-yl)ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124377 (3-{4-[3-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360621 (N-[(11bS)-2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM360632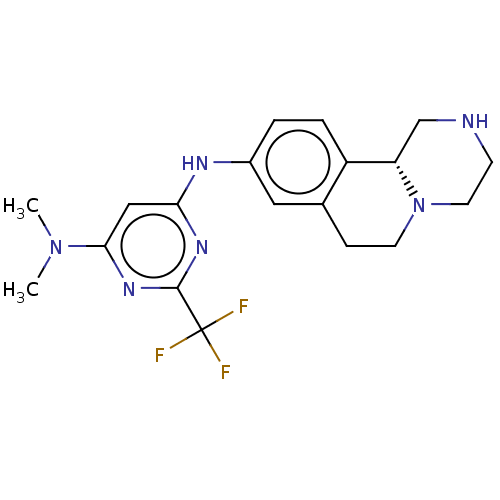 (N4-[(11bR)-2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37° C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124374 (3-{4-[4-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha/alpha (Homo sapiens (Human)) | BDBM50178961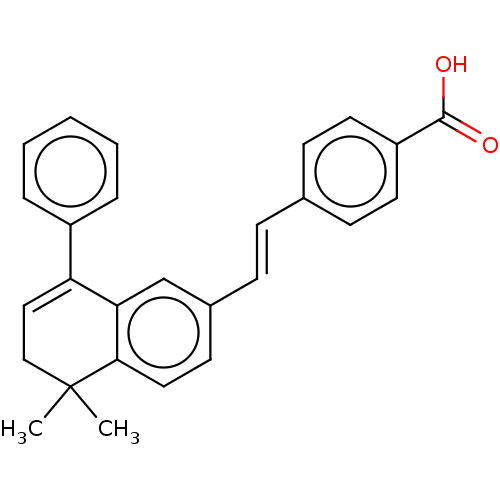 (CHEMBL2385268) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by ChEMBL | Assay Description Displacement of [3H]-TTNPB from RARalpha/RXRalpha (unknown origin) expressed in baculovirus expression system by scintillation proximity assay | Bioorg Med Chem Lett 26: 3274-3277 (2016) Article DOI: 10.1016/j.bmcl.2016.05.056 BindingDB Entry DOI: 10.7270/Q2FF3V9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360626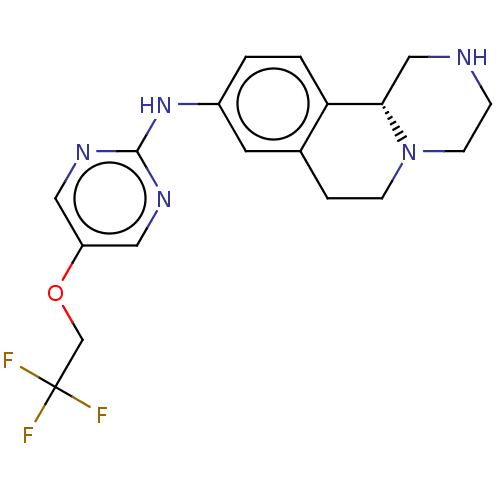 ((R)-N-(5-(2,2,2-trifluoroethoxy)pyrimidin-2-yl)-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124384 (CHEMBL173494 | {4-[3-(3-Phenyl-7-propyl-benzo[d]is...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50247164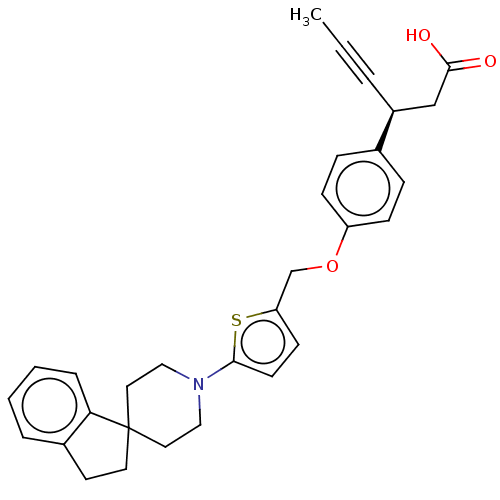 (CHEMBL4101901) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Competitive displacement of [3H]-TAK-875 from full length human recombinant GPR40 expressed in HEK293 cell membranes after 2 hrs by scintillation cou... | J Med Chem 61: 934-945 (2018) Article DOI: 10.1021/acs.jmedchem.7b01411 BindingDB Entry DOI: 10.7270/Q2CZ39K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50124374 (3-{4-[4-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM360631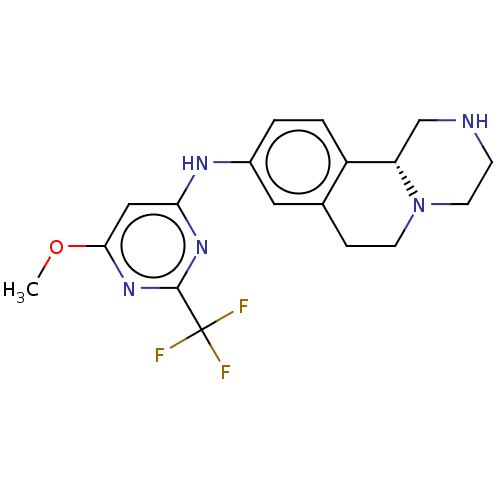 ((11bR)-N-[6-methoxy-2-(trifluoromethyl)pyrimidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37° C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124378 (2-{4-[3-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360616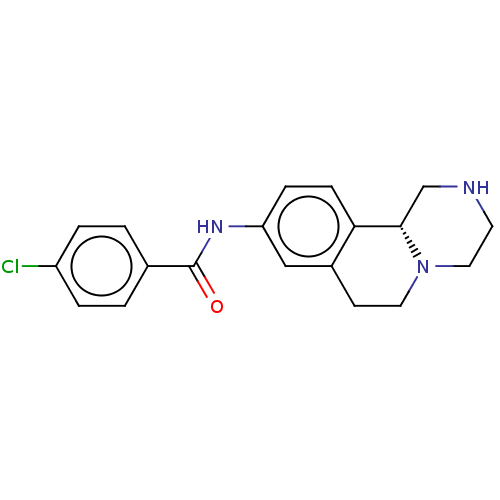 (N-[(11bR)-2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Rattus norvegicus (Rat)) | BDBM360649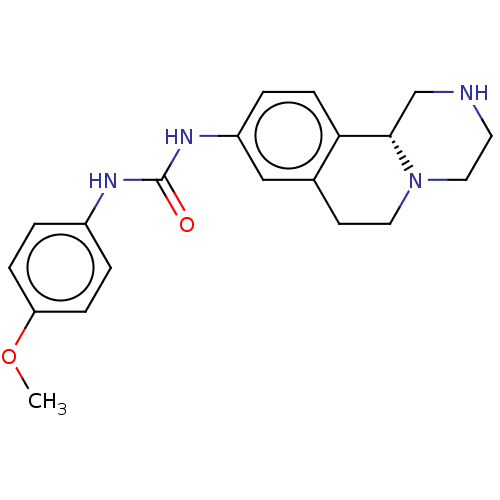 ((R)-1-(2,3,4,6,7,11b-hexahydro-1H-pyrazino[2,1-a]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 10.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description The TAAR1 radioligand 3[H]-(S)-4-[(ethyl-phenyl-amino)-methyl]-4,5-dihydro-oxazol-2-ylamine (described in WO 2008/098857) was used at a concentration... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50124383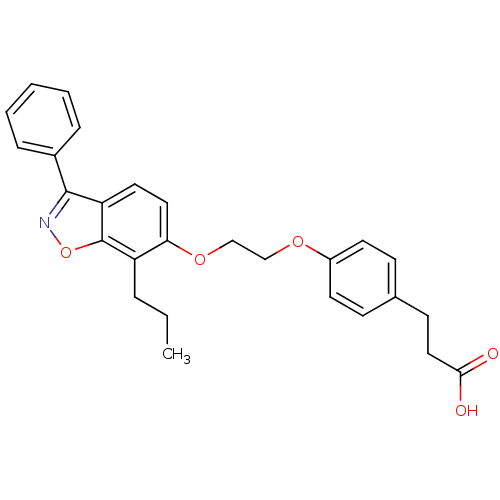 (3-{4-[2-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor delta (PPAR delta) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50124383 (3-{4-[2-(3-Phenyl-7-propyl-benzo[d]isoxazol-6-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor alpha (PPAR alpha) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50124369 (CHEMBL367019 | {3-Chloro-4-[3-(3-phenyl-7-propyl-b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro binding affinity towards human peroxisome proliferator activated receptor gamma (PPAR gamma) | Bioorg Med Chem Lett 13: 931-5 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM360633 ((11bR)-N-[6-methyl-2-(trifluoromethyl)pyrimidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. US Patent | Assay Description HEK-293 cells stably expressing mouse TAAR1 were maintained at 37° C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ... | US Patent US9828374 (2017) BindingDB Entry DOI: 10.7270/Q28W3GM2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2204 total ) | Next | Last >> |