

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474596 (CHEMBL79332) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474584 (CHEMBL79387) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474594 (CHEMBL86050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474583 (CHEMBL86051) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474587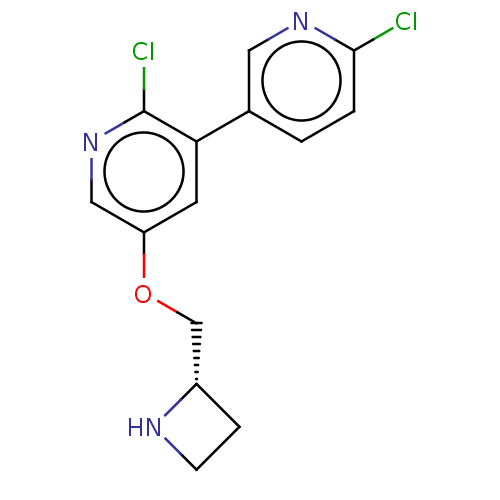 (CHEMBL314718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474595 (CHEMBL79594) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474582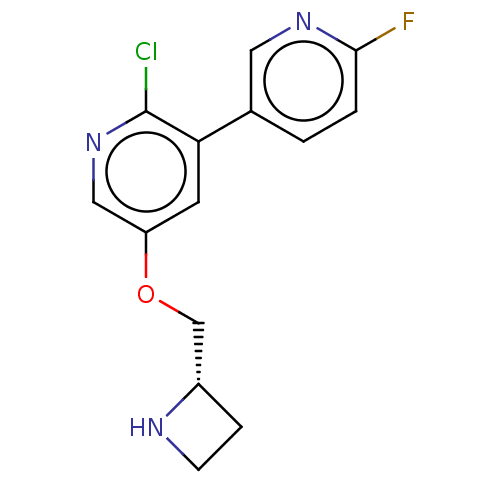 (CHEMBL83444) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474593 (CHEMBL313877) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474591 (CHEMBL79515) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474598 (CHEMBL79702) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474592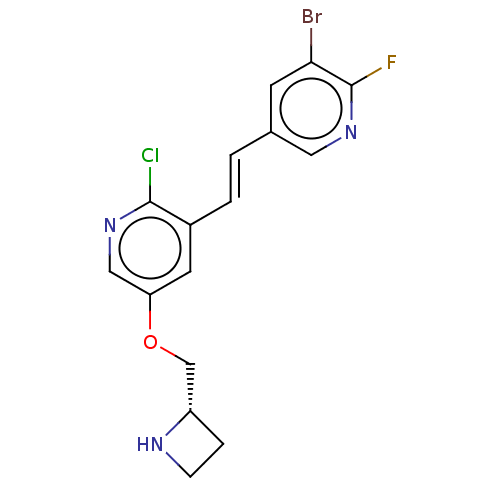 (CHEMBL83738) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474585 (CHEMBL312132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474590 (CHEMBL309292) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474586 (CHEMBL80040) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474588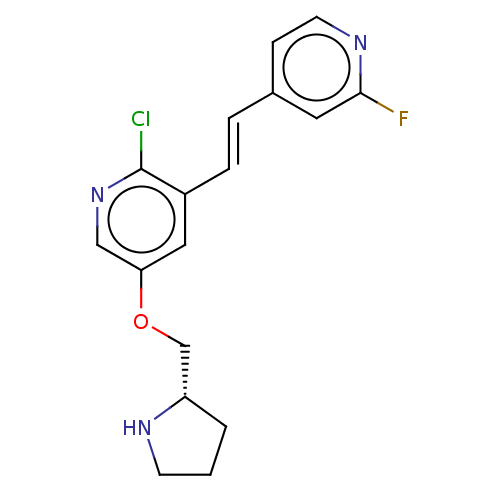 (CHEMBL310197) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.00940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474597 (CHEMBL83608) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474581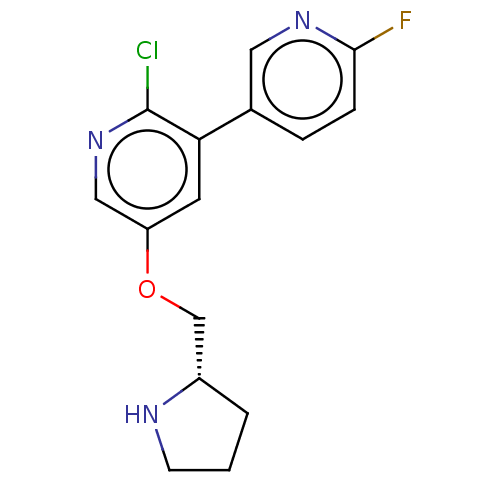 (CHEMBL83986) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474580 (CHEMBL84154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474599 (CHEMBL79737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370083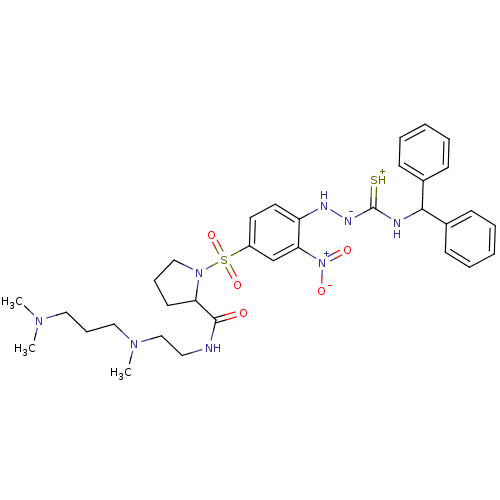 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50062639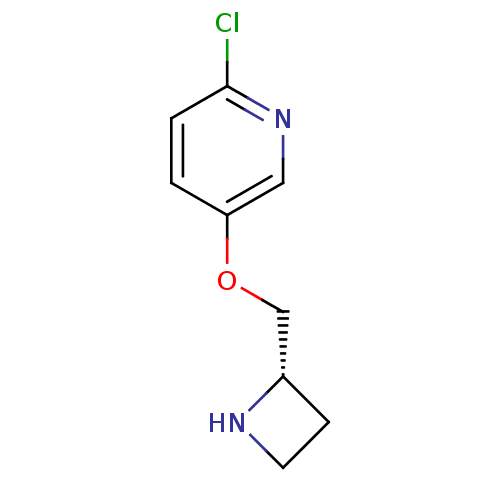 (5-((S)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121507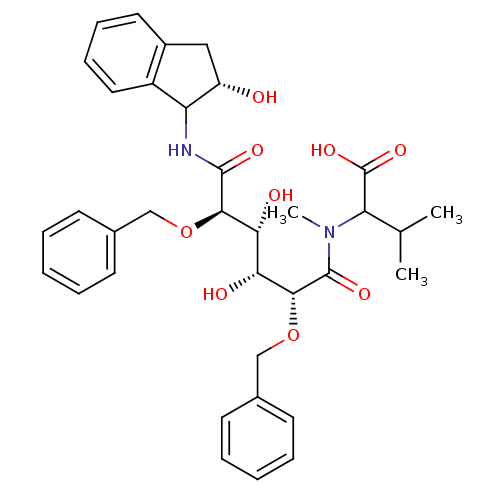 (2-{[2,5-Bis-benzyloxy-3,4-dihydroxy-5-(2-hydroxy-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0540 | n/a | n/a | 4.42 | n/a | n/a | 5.97E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50066788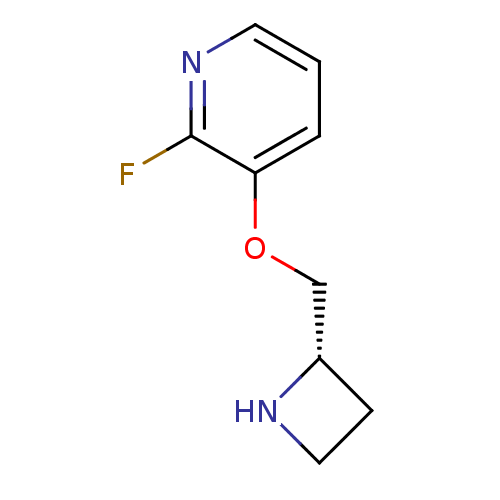 ((S)-3-(azetidin-2-ylmethoxy)-2-fluoropyridine | 3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474589 (CHEMBL79854) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Ability of compound to compete with 50 pM of 5-[125I]iodo-A-85,380 for receptor binding sites in rat brain membranes at room temperature | J Med Chem 47: 2453-65 (2004) Article DOI: 10.1021/jm030432v BindingDB Entry DOI: 10.7270/Q21C20MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM854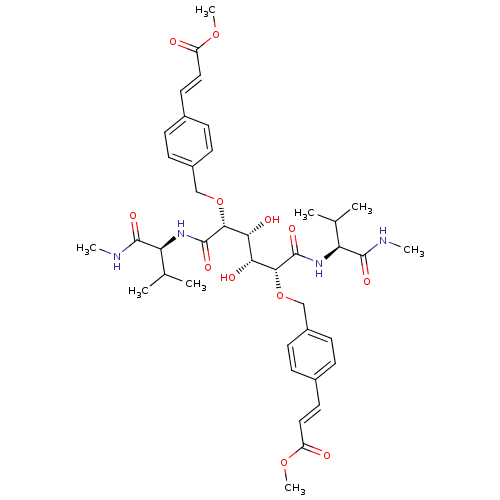 (C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | -58.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description Ki values were determined by using a fluorescent substrate (DABCYL-gamma-Abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS). All incubations were performed a... | J Med Chem 42: 3835-44 (1999) Article DOI: 10.1021/jm9910371 BindingDB Entry DOI: 10.7270/Q2T151VX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM854 (C2-Symmetric inhibitor 12 | CHEMBL127045 | N1,N6-B...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0900 | n/a | n/a | 1.95 | n/a | n/a | 2.04E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121505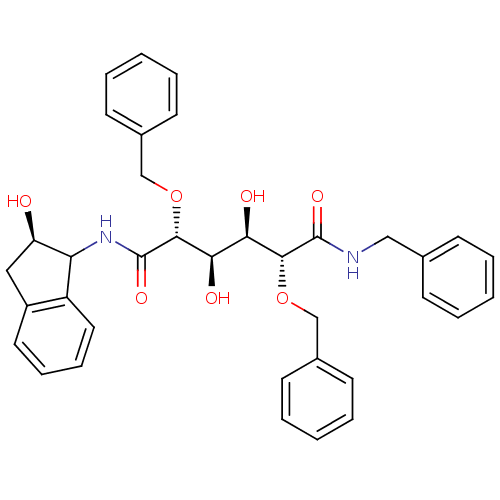 (2,5-Bis-benzyloxy-3,4-dihydroxy-hexanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | 70 | n/a | n/a | 1.01E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM851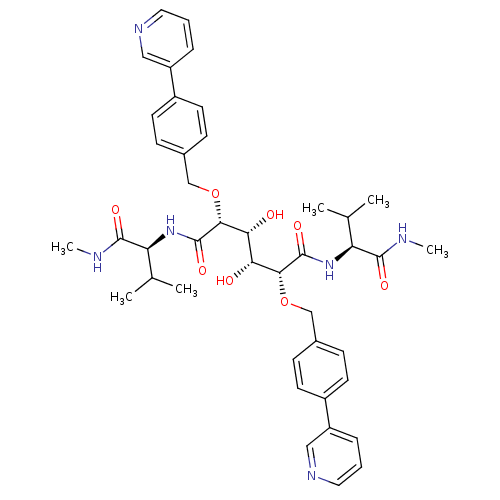 ((2R,3R,4R,5R)-3,4-dihydroxy-N,N'-bis[(1S)-2-methyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | Eur J Biochem 270: 1746-58 (2003) Article DOI: 10.1046/j.1432-1033.2003.03533.x BindingDB Entry DOI: 10.7270/Q2NZ85WX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12218 ((2R,3R,4R,5R)-N-benzyl-2,5-bis(benzyloxy)-3,4-dihy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | Eur J Biochem 270: 1746-58 (2003) Article DOI: 10.1046/j.1432-1033.2003.03533.x BindingDB Entry DOI: 10.7270/Q2NZ85WX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50030815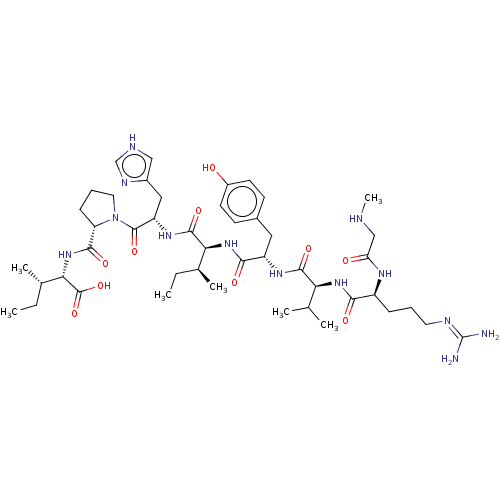 (CHEMBL404594) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity to type-2 angiotensin-2 receptor (unknown origin) | Bioorg Med Chem Lett 26: 1355-9 (2016) Article DOI: 10.1016/j.bmcl.2015.10.084 BindingDB Entry DOI: 10.7270/Q2SN0BT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50370684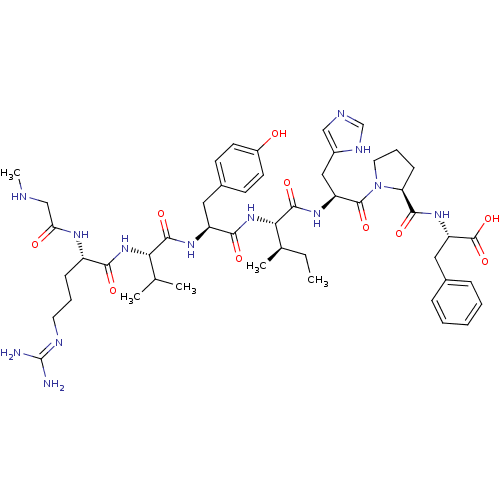 (CHEMBL1791349) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity for angiotensin II receptor, type 1 in rat liver membrane using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 2 hr at ... | J Med Chem 48: 6620-31 (2005) Article DOI: 10.1021/jm050280z BindingDB Entry DOI: 10.7270/Q2HX1DG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50370684 (CHEMBL1791349) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Uppsala University Curated by ChEMBL | Assay Description Binding affinity for angiotensin II receptor, type 2 in pig uterus myometrium using [125I]-Ang II as radioligand, in pH 7.4 Tris-HCl buffer for 1.5 h... | J Med Chem 48: 6620-31 (2005) Article DOI: 10.1021/jm050280z BindingDB Entry DOI: 10.7270/Q2HX1DG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A/B (RAT) | BDBM50472361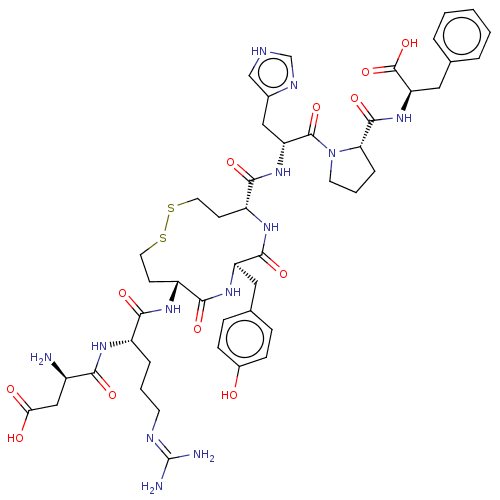 (CHEMBL406349) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro binding affinity against Angiotensin II receptor, type 1 from rat liver membranes | J Med Chem 42: 4524-37 (1999) Article DOI: 10.1021/jm991089q BindingDB Entry DOI: 10.7270/Q2PN98C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... | Bioorg Med Chem Lett 26: 1355-9 (2016) Article DOI: 10.1016/j.bmcl.2015.10.084 BindingDB Entry DOI: 10.7270/Q2SN0BT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50066918 ((2R,3R,4R,5R)-2,5-Bis-benzyloxy-3,4-dihydroxy-hexa...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV-1 protease expressed in E. coli in sensitive fluorometric assay | J Med Chem 41: 3782-92 (1998) Article DOI: 10.1021/jm970777b BindingDB Entry DOI: 10.7270/Q2PK0F93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50213021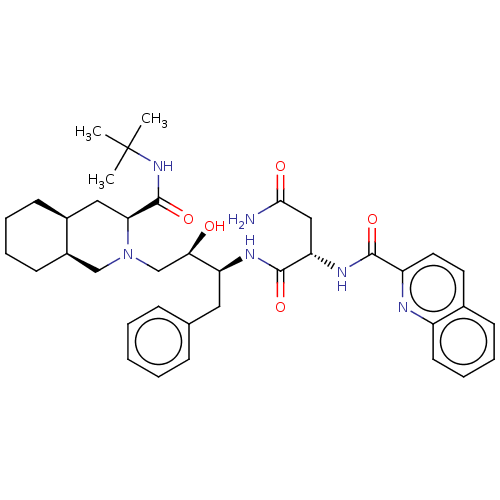 (CHEBI:63621 | Fortovase | Invirase | Ro-31-8959 | ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli by fluorometric assay | J Med Chem 53: 607-15 (2010) Article DOI: 10.1021/jm901165g BindingDB Entry DOI: 10.7270/Q29Z97R7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12216 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N-[...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant protease by fluorometric assay | Eur J Med Chem 45: 160-70 (2010) Article DOI: 10.1016/j.ejmech.2009.09.038 BindingDB Entry DOI: 10.7270/Q2KD21QM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM12216 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N-[...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stockholm University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | Eur J Med Chem 45: 160-70 (2010) Article DOI: 10.1016/j.ejmech.2009.09.038 BindingDB Entry DOI: 10.7270/Q2KD21QM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM358 ((2R,3R,4R,5R)-2,5-bis(benzyloxy)-3,4-dihydroxy-N,N...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 5.0 | 30 |
Uppsala University | Assay Description A fluorimetric assay was used to determine the effects of the inhibitors on HIV-1 protease. This assay used an internally quenched fluorescent peptid... | Eur J Biochem 270: 1746-58 (2003) Article DOI: 10.1046/j.1432-1033.2003.03533.x BindingDB Entry DOI: 10.7270/Q2NZ85WX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50121499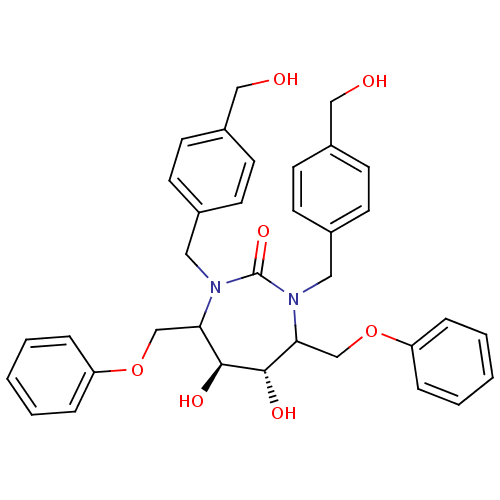 (5,6-Dihydroxy-1,3-bis-(4-hydroxymethyl-benzyl)-4,7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | 7 | n/a | n/a | 7.06E+9 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50015662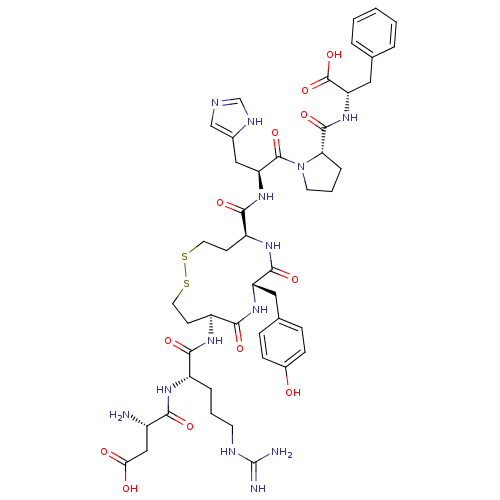 (3-Amino-N-{1-[5-[2-[2-(1-carboxy-2-phenyl-ethylcar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description In vitro binding affinity at rat liver Angiotensin II receptor, type 1 was determined based on displacement of [125I]-Ang II | J Med Chem 45: 1767-77 (2002) BindingDB Entry DOI: 10.7270/Q2QV3N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.230 | n/a | n/a | 0.315 | n/a | n/a | 8.17E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM18137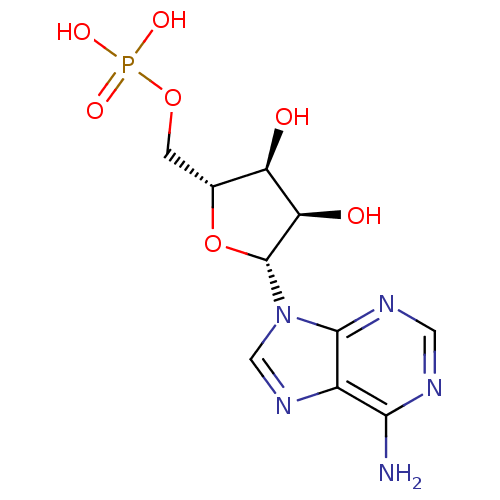 (AMP | CHEMBL752 | US11185100, TABLE 7.3 | [(2R,3S,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | 1.13 | n/a | n/a | 4.43E+6 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150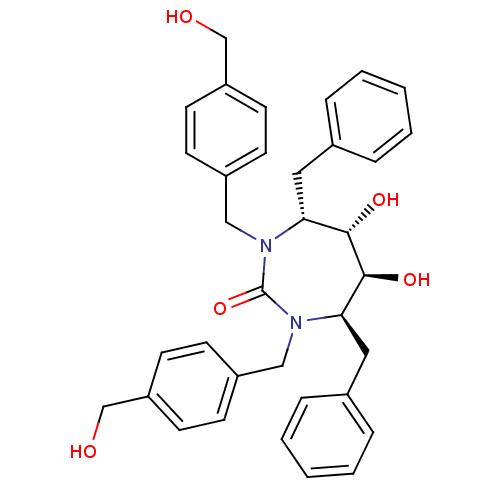 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.270 | n/a | n/a | 3.83 | n/a | n/a | 2.52E+10 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50160917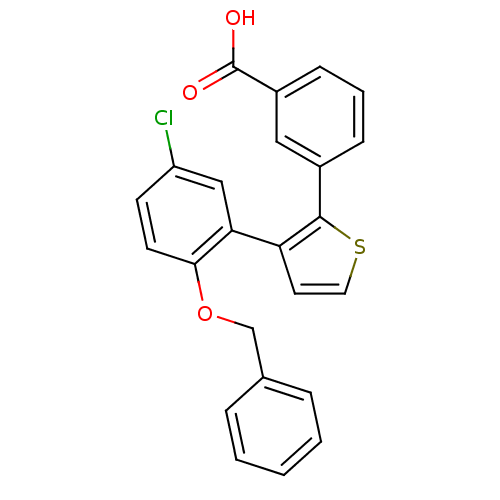 (3-(3-(2-(benzyloxy)-5-chlorophenyl)thiophen-2-yl)b...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity against PGE2 activated EP1 receptor assessed as ability to inhibit intracellular calcium mobilisation by FLIPR | Bioorg Med Chem Lett 16: 2666-71 (2006) Article DOI: 10.1016/j.bmcl.2006.02.014 BindingDB Entry DOI: 10.7270/Q2J102RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169098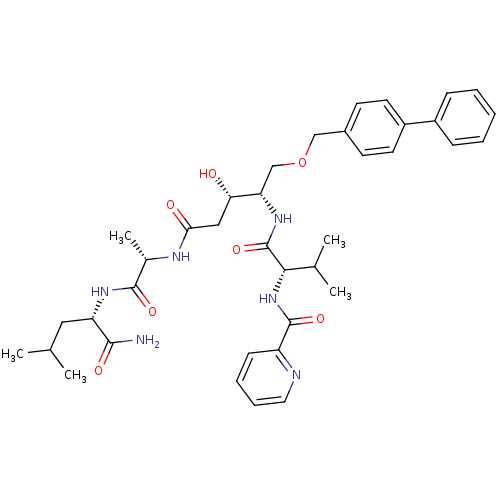 (CHEMBL264770 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50169100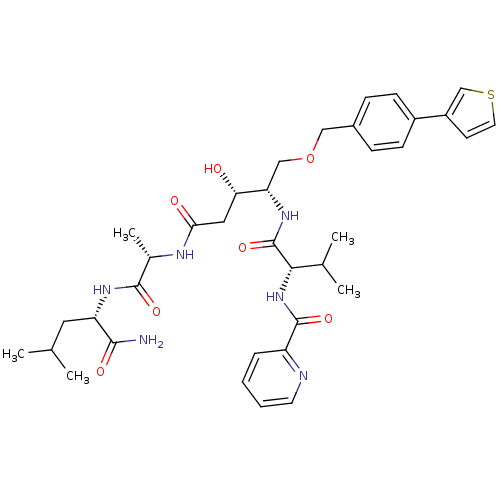 (CHEMBL191260 | Pyridine-2-carboxylic acid {(S)-1-[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Linköping University Curated by ChEMBL | Assay Description Inhibitory concentration against the Plasmepsin I of Plasmodium falciparum | J Med Chem 48: 4400-9 (2005) Article DOI: 10.1021/jm040884n BindingDB Entry DOI: 10.7270/Q2R78DRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM845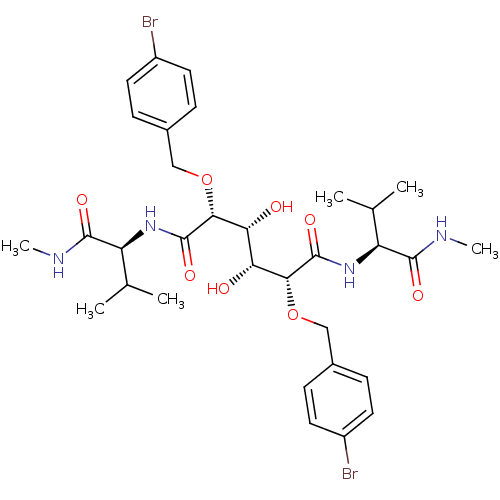 ((2R,3R,4R,5R)-2,5-bis[(4-bromophenyl)methoxy]-3,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | 21 | n/a | n/a | 8.89E+5 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM855 ((2R,3R,4R,5R)-3,4-dihydroxy-2,5-bis({[4-(2-hydroxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.300 | n/a | n/a | 18 | n/a | n/a | 2.93E+4 | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Association rate constant for the interaction between inhibitor and HIV-1 protease | J Med Chem 45: 5430-9 (2002) BindingDB Entry DOI: 10.7270/Q2GH9JP4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5974 total ) | Next | Last >> |