 Found 404 hits with Last Name = 'marsiglio' and Initial = 'a'
Found 404 hits with Last Name = 'marsiglio' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7167

(3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)S(N)(=O)=O)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C15H12N6O3S/c16-15(22)13-10-5-6-12-11(7-18-19-12)14(10)21(20-13)8-1-3-9(4-2-8)25(17,23)24/h1-7H,(H2,16,22)(H,18,19)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7177

(3-(4-methanesulfonylphenyl)-3,4,10,11-tetraazatric...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C16H13N5O3S/c1-25(23,24)10-4-2-9(3-5-10)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021624
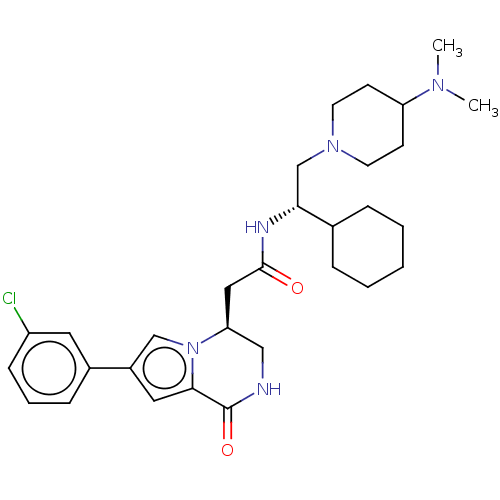
(CHEMBL3297766 | US9145418, 23)Show SMILES CN(C)C1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C30H42ClN5O2/c1-34(2)25-11-13-35(14-12-25)20-27(21-7-4-3-5-8-21)33-29(37)17-26-18-32-30(38)28-16-23(19-36(26)28)22-9-6-10-24(31)15-22/h6,9-10,15-16,19,21,25-27H,3-5,7-8,11-14,17-18,20H2,1-2H3,(H,32,38)(H,33,37)/t26-,27+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021620
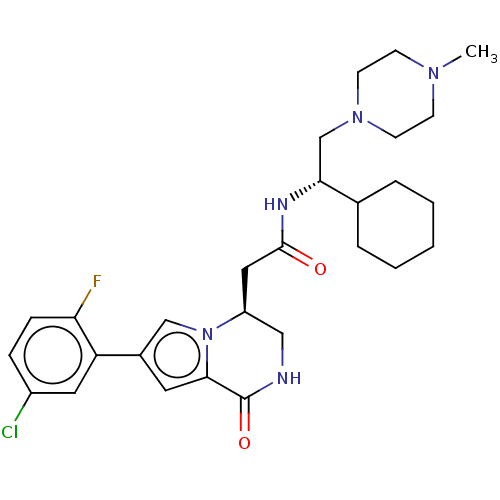
(CHEMBL3297764 | US9145418, 26)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cc(Cl)ccc2F)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H37ClFN5O2/c1-33-9-11-34(12-10-33)18-25(19-5-3-2-4-6-19)32-27(36)15-22-16-31-28(37)26-13-20(17-35(22)26)23-14-21(29)7-8-24(23)30/h7-8,13-14,17,19,22,25H,2-6,9-12,15-16,18H2,1H3,(H,31,37)(H,32,36)/t22-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7107
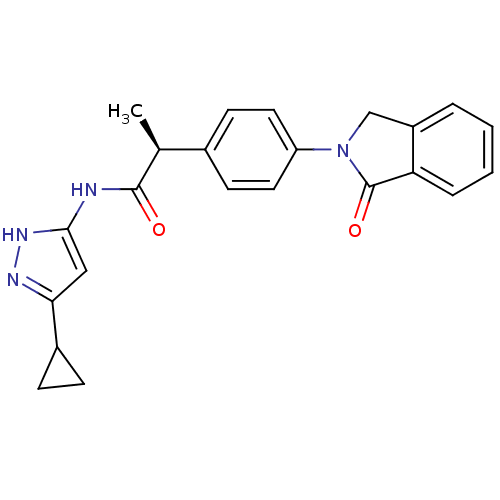
((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(1-oxo...)Show SMILES C[C@H](C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)N1Cc2ccccc2C1=O |r| Show InChI InChI=1S/C23H22N4O2/c1-14(22(28)24-21-12-20(25-26-21)16-6-7-16)15-8-10-18(11-9-15)27-13-17-4-2-3-5-19(17)23(27)29/h2-5,8-12,14,16H,6-7,13H2,1H3,(H2,24,25,26,28)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 2944-56 (2005)
Article DOI: 10.1021/jm0408870
BindingDB Entry DOI: 10.7270/Q2WQ020R |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7214

(3-(2,2,2-trifluoroethyl)-3,4,10,11-tetraazatricycl...)Show InChI InChI=1S/C11H8F3N5O/c12-11(13,14)4-19-9-5(8(18-19)10(15)20)1-2-7-6(9)3-16-17-7/h1-3H,4H2,(H2,15,20)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7169
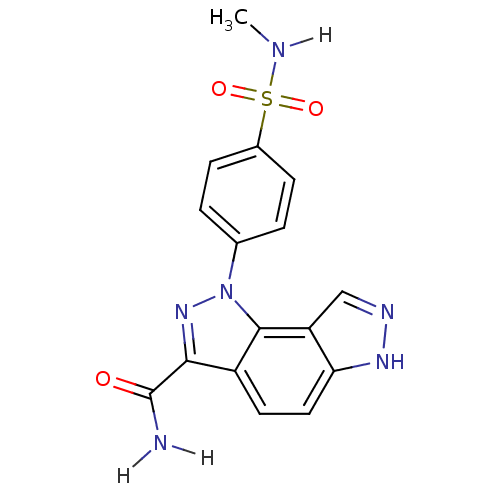
(3-[4-(methylsulfamoyl)phenyl]-3,4,10,11-tetraazatr...)Show SMILES CNS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C16H14N6O3S/c1-18-26(24,25)10-4-2-9(3-5-10)22-15-11(14(21-22)16(17)23)6-7-13-12(15)8-19-20-13/h2-8,18H,1H3,(H2,17,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7173

(3-[4-(butylsulfamoyl)phenyl]-3,4,10,11-tetraazatri...)Show SMILES CCCCNS(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C19H20N6O3S/c1-2-3-10-22-29(27,28)13-6-4-12(5-7-13)25-18-14(17(24-25)19(20)26)8-9-16-15(18)11-21-23-16/h4-9,11,22H,2-3,10H2,1H3,(H2,20,26)(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7111
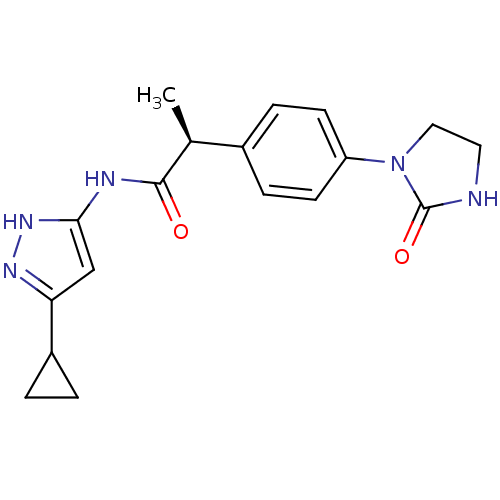
((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(2-oxo...)Show SMILES C[C@H](C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)N1CCNC1=O |r| Show InChI InChI=1S/C18H21N5O2/c1-11(17(24)20-16-10-15(21-22-16)13-2-3-13)12-4-6-14(7-5-12)23-9-8-19-18(23)25/h4-7,10-11,13H,2-3,8-9H2,1H3,(H,19,25)(H2,20,21,22,24)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 2944-56 (2005)
Article DOI: 10.1021/jm0408870
BindingDB Entry DOI: 10.7270/Q2WQ020R |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7163

(3-Phenylacetamidoaminopyrazole deriv. 40 | CS10 | ...)Show InChI InChI=1S/C18H17N3OS/c22-18(19-17-11-15(20-21-17)13-7-8-13)10-12-3-5-14(6-4-12)16-2-1-9-23-16/h1-6,9,11,13H,7-8,10H2,(H2,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7159
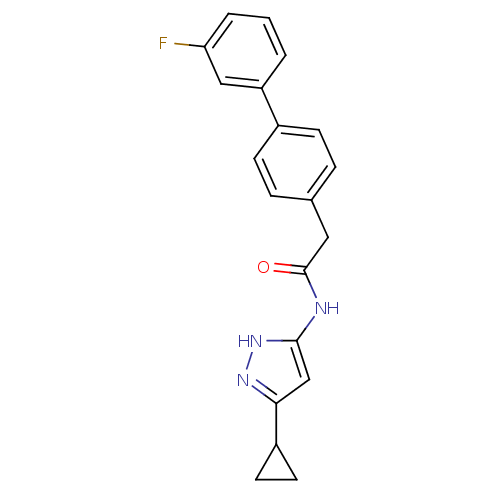
(3-Phenylacetamidoaminopyrazole deriv. 36 | N-(5-Cy...)Show SMILES Fc1cccc(c1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C20H18FN3O/c21-17-3-1-2-16(11-17)14-6-4-13(5-7-14)10-20(25)22-19-12-18(23-24-19)15-8-9-15/h1-7,11-12,15H,8-10H2,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7166
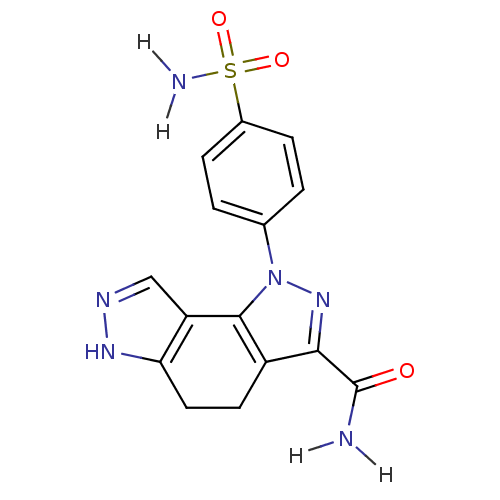
(3-(4-sulfamoylphenyl)-3,4,10,11-tetraazatricyclo[7...)Show SMILES NC(=O)c1nn(c-2c1CCc1[nH]ncc-21)-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C15H14N6O3S/c16-15(22)13-10-5-6-12-11(7-18-19-12)14(10)21(20-13)8-1-3-9(4-2-8)25(17,23)24/h1-4,7H,5-6H2,(H2,16,22)(H,18,19)(H2,17,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7162

(3-Phenylacetamidoaminopyrazole deriv. 39 | 4 -{2-[...)Show SMILES NC(=O)c1ccc(cc1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H20N4O2/c22-21(27)17-9-5-15(6-10-17)14-3-1-13(2-4-14)11-20(26)23-19-12-18(24-25-19)16-7-8-16/h1-6,9-10,12,16H,7-8,11H2,(H2,22,27)(H2,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021622
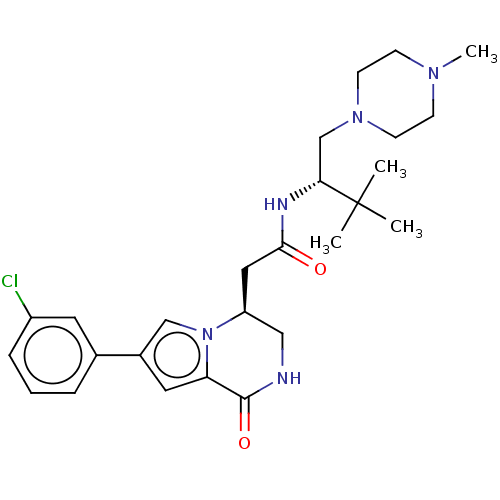
(CHEMBL3297767)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C(C)(C)C)CC1 |r| Show InChI InChI=1S/C26H36ClN5O2/c1-26(2,3)23(17-31-10-8-30(4)9-11-31)29-24(33)14-21-15-28-25(34)22-13-19(16-32(21)22)18-6-5-7-20(27)12-18/h5-7,12-13,16,21,23H,8-11,14-15,17H2,1-4H3,(H,28,34)(H,29,33)/t21-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12103
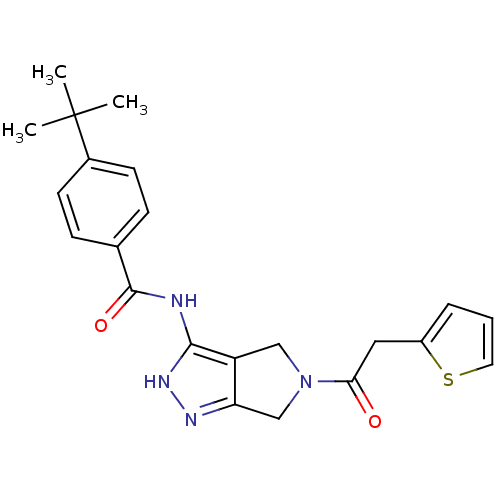
(1,4,5,6-Tetrahydropyrrolo[3,4-c]pyrazole 11 | 4-te...)Show SMILES CC(C)(C)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)Cc1cccs1 Show InChI InChI=1S/C22H24N4O2S/c1-22(2,3)15-8-6-14(7-9-15)21(28)23-20-17-12-26(13-18(17)24-25-20)19(27)11-16-5-4-10-29-16/h4-10H,11-13H2,1-3H3,(H2,23,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 3080-4 (2005)
Article DOI: 10.1021/jm049076m
BindingDB Entry DOI: 10.7270/Q2FF3QKK |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7195

(3-(4-cyanophenyl)-3,4,10,11-tetraazatricyclo[7.3.0...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)C#N)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C16H10N6O/c17-7-9-1-3-10(4-2-9)22-15-11(14(21-22)16(18)23)5-6-13-12(15)8-19-20-13/h1-6,8H,(H2,18,23)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327930

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C25H25N5O3S/c1-16(17-5-3-2-4-6-17)26-25(32)21-15-20-22(34-21)23(29-28-20)27-24(31)18-7-9-19(10-8-18)30-11-13-33-14-12-30/h2-10,15-16H,11-14H2,1H3,(H,26,32)(H2,27,28,29,31)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327929
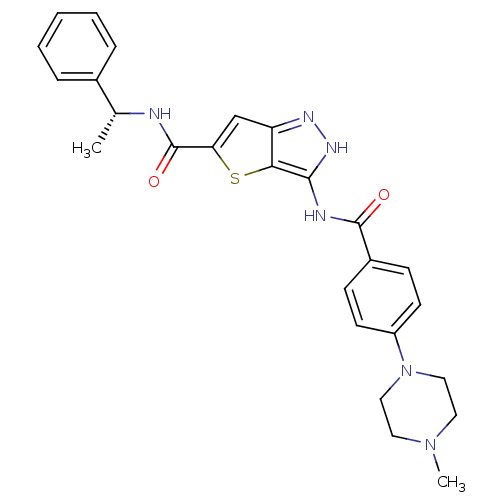
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12983
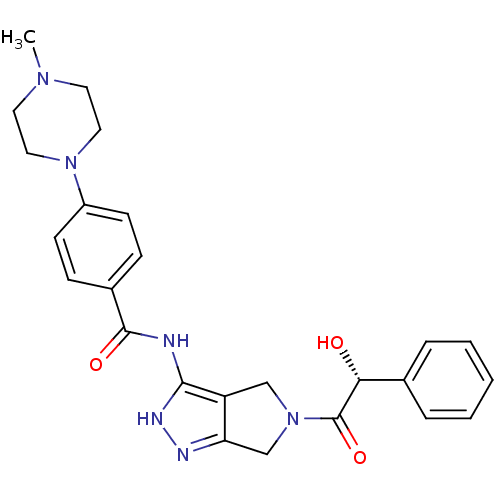
(5-Amido-pyrrolopyrazole 9b | CHEMBL385872 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](O)c1ccccc1 |r| Show InChI InChI=1S/C25H28N6O3/c1-29-11-13-30(14-12-29)19-9-7-18(8-10-19)24(33)26-23-20-15-31(16-21(20)27-28-23)25(34)22(32)17-5-3-2-4-6-17/h2-10,22,32H,11-16H2,1H3,(H2,26,27,28,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7206

(3-(pyridin-2-yl)-3,4,10,11-tetraazatricyclo[7.3.0....)Show InChI InChI=1S/C14H10N6O/c15-14(21)12-8-4-5-10-9(7-17-18-10)13(8)20(19-12)11-3-1-2-6-16-11/h1-7H,(H2,15,21)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7187
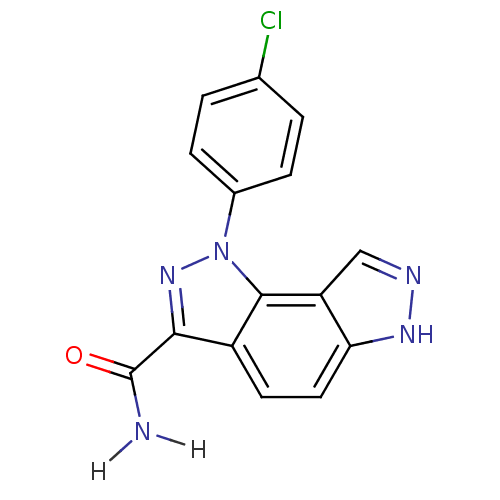
(3-(4-chlorophenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C15H10ClN5O/c16-8-1-3-9(4-2-8)21-14-10(13(20-21)15(17)22)5-6-12-11(14)7-18-19-12/h1-7H,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12982
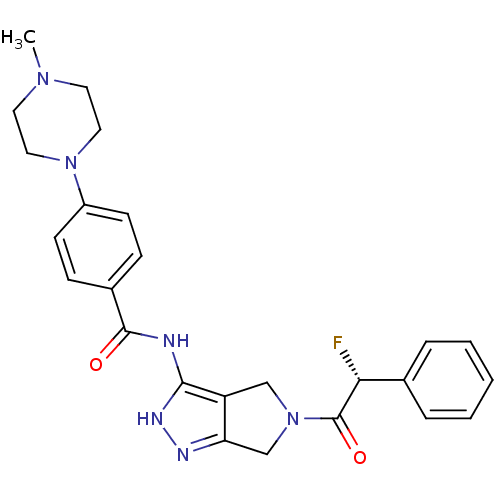
(5-Amido-pyrrolopyrazole 9a | CHEMBL385266 | N-{5-[...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)Nc1[nH]nc2CN(Cc12)C(=O)[C@H](F)c1ccccc1 |r| Show InChI InChI=1S/C25H27FN6O2/c1-30-11-13-31(14-12-30)19-9-7-18(8-10-19)24(33)27-23-20-15-32(16-21(20)28-29-23)25(34)22(26)17-5-3-2-4-6-17/h2-10,22H,11-16H2,1H3,(H2,27,28,29,33)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021617
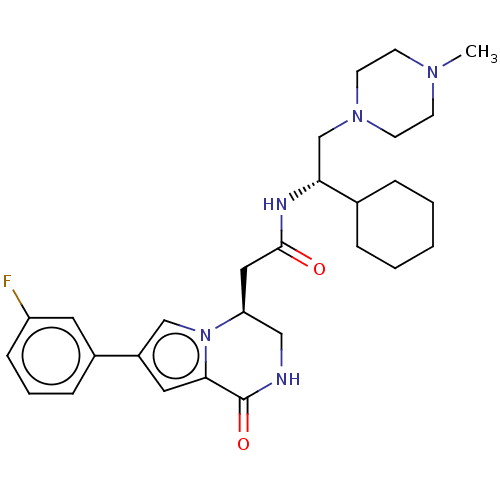
(CHEMBL3297761)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(F)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H38FN5O2/c1-32-10-12-33(13-11-32)19-25(20-6-3-2-4-7-20)31-27(35)16-24-17-30-28(36)26-15-22(18-34(24)26)21-8-5-9-23(29)14-21/h5,8-9,14-15,18,20,24-25H,2-4,6-7,10-13,16-17,19H2,1H3,(H,30,36)(H,31,35)/t24-,25+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7200

(3-(3-methylphenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C16H13N5O/c1-9-3-2-4-10(7-9)21-15-11(14(20-21)16(17)22)5-6-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7160
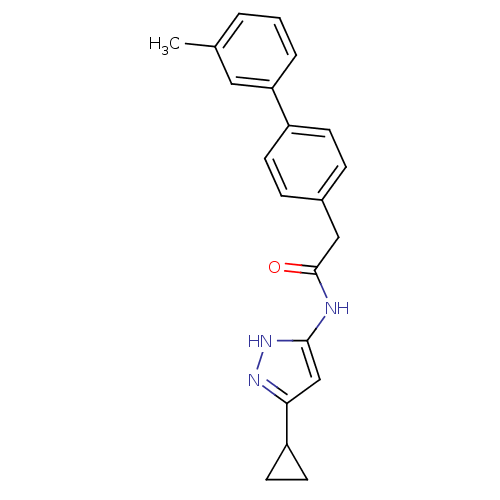
(3-Phenylacetamidoaminopyrazole deriv. 37 | N-(5-Cy...)Show SMILES Cc1cccc(c1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H21N3O/c1-14-3-2-4-18(11-14)16-7-5-15(6-8-16)12-21(25)22-20-13-19(23-24-20)17-9-10-17/h2-8,11,13,17H,9-10,12H2,1H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7185

(3-(4-methoxyphenyl)-3,4,10,11-tetraazatricyclo[7.3...)Show InChI InChI=1S/C16H13N5O2/c1-23-10-4-2-9(3-5-10)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327928
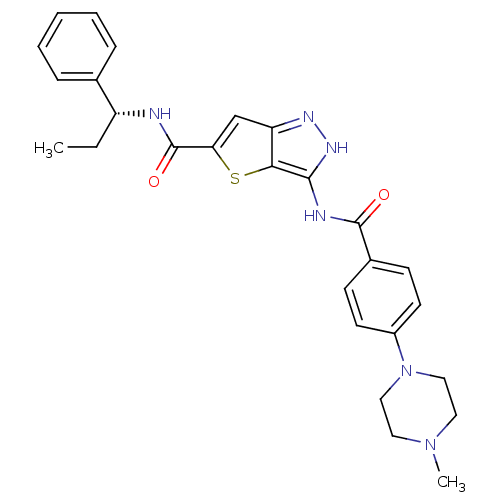
(3-({[4-(4-METHYLPIPERAZIN-1-YL)PHENYL]CARBONYL}AMI...)Show SMILES CC[C@@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021649
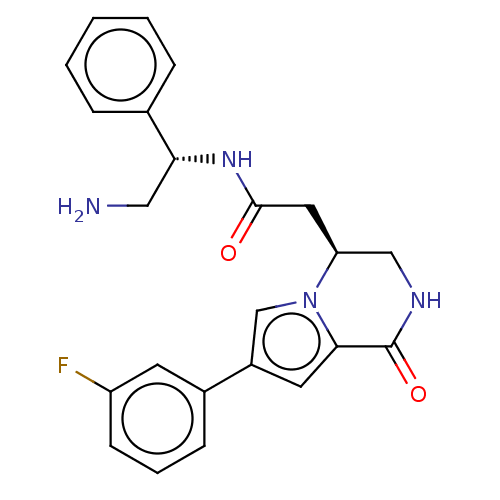
(CHEMBL3298891)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C23H23FN4O2/c24-18-8-4-7-16(9-18)17-10-21-23(30)26-13-19(28(21)14-17)11-22(29)27-20(12-25)15-5-2-1-3-6-15/h1-10,14,19-20H,11-13,25H2,(H,26,30)(H,27,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7171
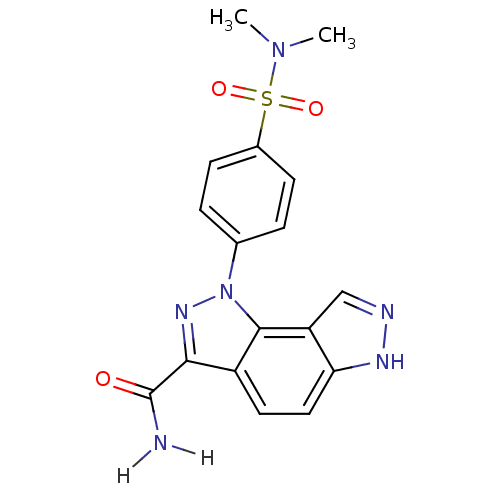
(3-[4-(dimethylsulfamoyl)phenyl]-3,4,10,11-tetraaza...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(C(N)=O)c2ccc3[nH]ncc3c12 Show InChI InChI=1S/C17H16N6O3S/c1-22(2)27(25,26)11-5-3-10(4-6-11)23-16-12(15(21-23)17(18)24)7-8-14-13(16)9-19-20-14/h3-9H,1-2H3,(H2,18,24)(H,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327923
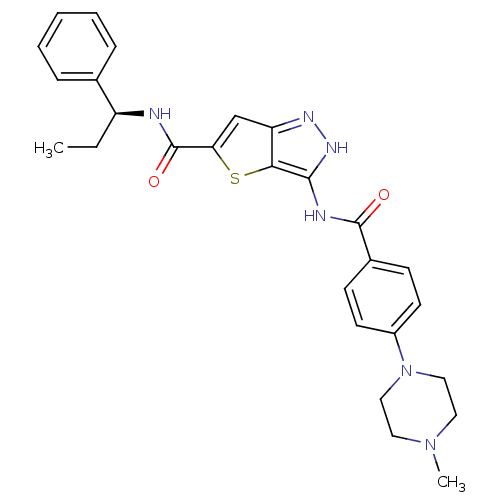
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES CC[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C27H30N6O2S/c1-3-21(18-7-5-4-6-8-18)28-27(35)23-17-22-24(36-23)25(31-30-22)29-26(34)19-9-11-20(12-10-19)33-15-13-32(2)14-16-33/h4-12,17,21H,3,13-16H2,1-2H3,(H,28,35)(H2,29,30,31,34)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327927
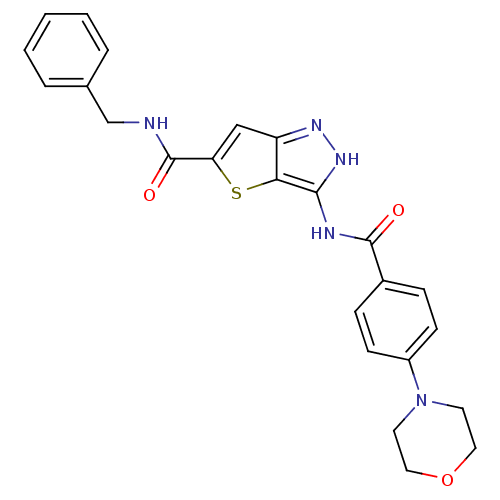
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(NCc1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 Show InChI InChI=1S/C24H23N5O3S/c30-23(17-6-8-18(9-7-17)29-10-12-32-13-11-29)26-22-21-19(27-28-22)14-20(33-21)24(31)25-15-16-4-2-1-3-5-16/h1-9,14H,10-13,15H2,(H,25,31)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7161
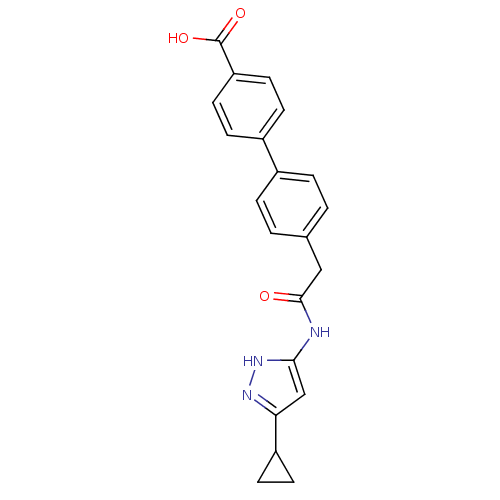
(3-Phenylacetamidoaminopyrazole deriv. 38 | 4 -{2-[...)Show SMILES OC(=O)c1ccc(cc1)-c1ccc(CC(=O)Nc2cc(n[nH]2)C2CC2)cc1 Show InChI InChI=1S/C21H19N3O3/c25-20(22-19-12-18(23-24-19)16-7-8-16)11-13-1-3-14(4-2-13)15-5-9-17(10-6-15)21(26)27/h1-6,9-10,12,16H,7-8,11H2,(H,26,27)(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7114

(3-Aminopyrazole deriv. 27 | N-(5-Cyclopropyl-1H-py...)Show SMILES CC(C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)-n1cc[nH]c1=O Show InChI InChI=1S/C18H19N5O2/c1-11(17(24)20-16-10-15(21-22-16)13-2-3-13)12-4-6-14(7-5-12)23-9-8-19-18(23)25/h4-11,13H,2-3H2,1H3,(H,19,25)(H2,20,21,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 2944-56 (2005)
Article DOI: 10.1021/jm0408870
BindingDB Entry DOI: 10.7270/Q2WQ020R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021653
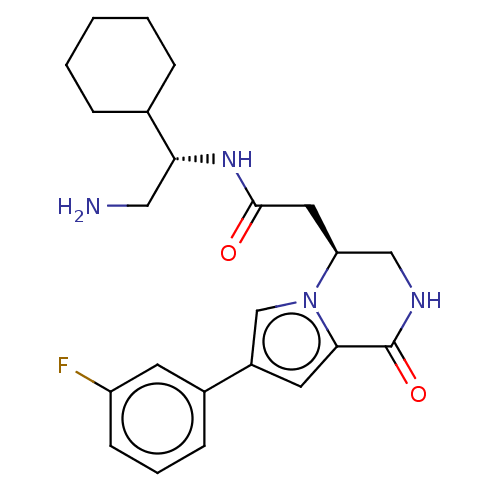
(CHEMBL3297759)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H29FN4O2/c24-18-8-4-7-16(9-18)17-10-21-23(30)26-13-19(28(21)14-17)11-22(29)27-20(12-25)15-5-2-1-3-6-15/h4,7-10,14-15,19-20H,1-3,5-6,11-13,25H2,(H,26,30)(H,27,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50021618
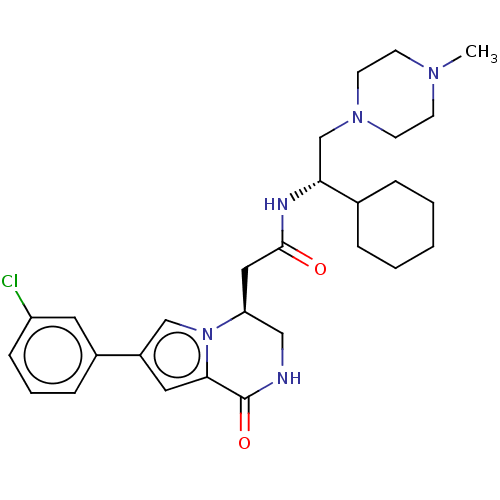
(CHEMBL3297762 | US9145418, 2)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2cccc(Cl)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C28H38ClN5O2/c1-32-10-12-33(13-11-32)19-25(20-6-3-2-4-7-20)31-27(35)16-24-17-30-28(36)26-15-22(18-34(24)26)21-8-5-9-23(29)14-21/h5,8-9,14-15,18,20,24-25H,2-4,6-7,10-13,16-17,19H2,1H3,(H,30,36)(H,31,35)/t24-,25+/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM3 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM12985
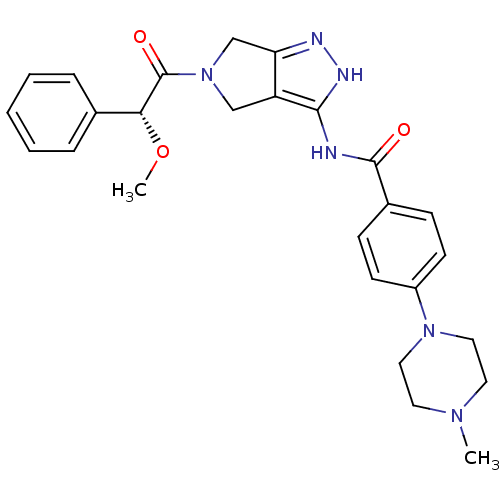
(5-Amido-pyrrolopyrazole 9d | CHEMBL402548 | N-{5-[...)Show SMILES CO[C@@H](C(=O)N1Cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2C1)c1ccccc1 |r| Show InChI InChI=1S/C26H30N6O3/c1-30-12-14-31(15-13-30)20-10-8-19(9-11-20)25(33)27-24-21-16-32(17-22(21)28-29-24)26(34)23(35-2)18-6-4-3-5-7-18/h3-11,23H,12-17H2,1-2H3,(H2,27,28,29,33)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 49: 7247-51 (2006)
Article DOI: 10.1021/jm060897w
BindingDB Entry DOI: 10.7270/Q2NS0S4R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021647
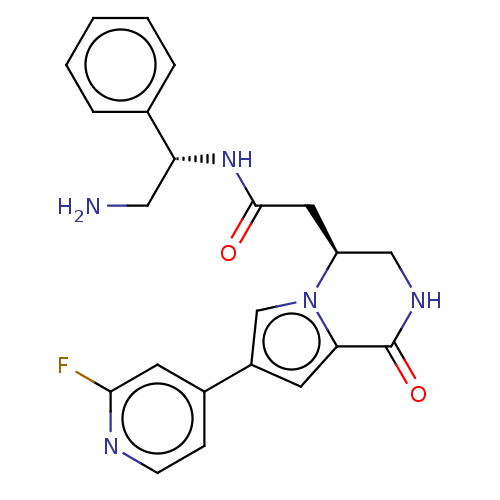
(CHEMBL3298889)Show SMILES NC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1ccnc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C22H22FN5O2/c23-20-9-15(6-7-25-20)16-8-19-22(30)26-12-17(28(19)13-16)10-21(29)27-18(11-24)14-4-2-1-3-5-14/h1-9,13,17-18H,10-12,24H2,(H,26,30)(H,27,29)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7191

(3-[4-(trifluoromethyl)phenyl]-3,4,10,11-tetraazatr...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)C(F)(F)F)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C16H10F3N5O/c17-16(18,19)8-1-3-9(4-2-8)24-14-10(13(23-24)15(20)25)5-6-12-11(14)7-21-22-12/h1-7H,(H2,20,25)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327915
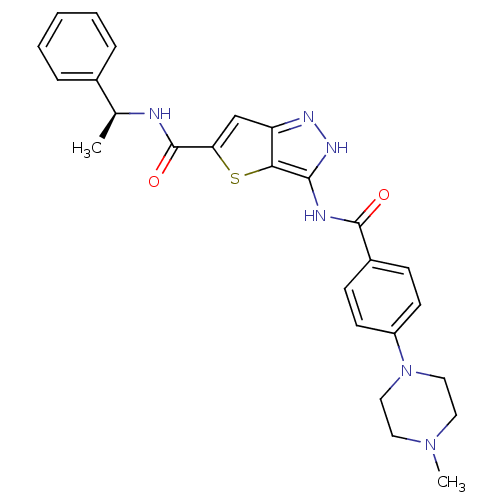
(3-[4-(4-Methyl-piperazin-1-yl)-benzoylamino]-1H-th...)Show SMILES C[C@H](NC(=O)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCN(C)CC3)c2s1)c1ccccc1 |r| Show InChI InChI=1S/C26H28N6O2S/c1-17(18-6-4-3-5-7-18)27-26(34)22-16-21-23(35-22)24(30-29-21)28-25(33)19-8-10-20(11-9-19)32-14-12-31(2)13-15-32/h3-11,16-17H,12-15H2,1-2H3,(H,27,34)(H2,28,29,30,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7121
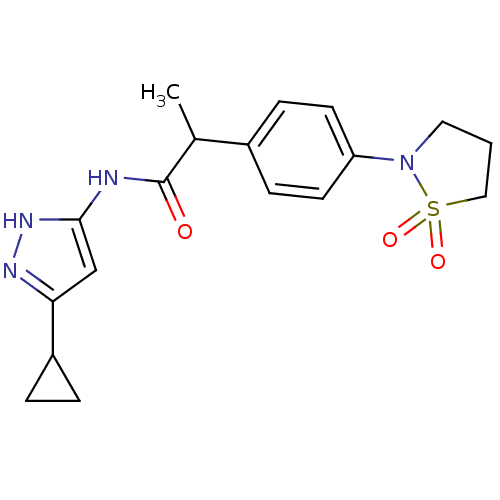
(3-Aminopyrazole deriv. 36 | N-(5-Cyclopropyl-1H-py...)Show SMILES CC(C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)N1CCCS1(=O)=O Show InChI InChI=1S/C18H22N4O3S/c1-12(18(23)19-17-11-16(20-21-17)14-3-4-14)13-5-7-15(8-6-13)22-9-2-10-26(22,24)25/h5-8,11-12,14H,2-4,9-10H2,1H3,(H2,19,20,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Pharmacia Italia
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 47: 3367-80 (2004)
Article DOI: 10.1021/jm031145u
BindingDB Entry DOI: 10.7270/Q2RX998G |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021621
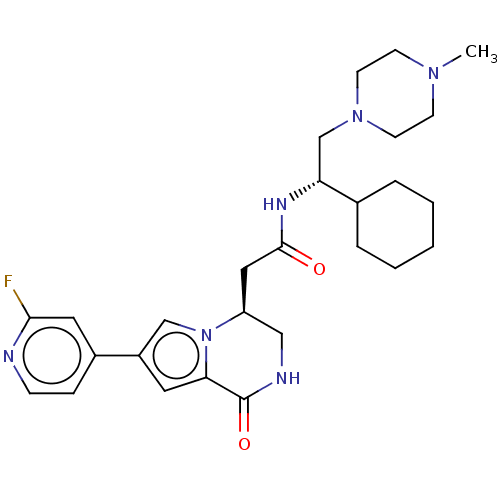
(CHEMBL3297765)Show SMILES CN1CCN(C[C@@H](NC(=O)C[C@H]2CNC(=O)c3cc(cn23)-c2ccnc(F)c2)C2CCCCC2)CC1 |r| Show InChI InChI=1S/C27H37FN6O2/c1-32-9-11-33(12-10-32)18-23(19-5-3-2-4-6-19)31-26(35)15-22-16-30-27(36)24-13-21(17-34(22)24)20-7-8-29-25(28)14-20/h7-8,13-14,17,19,22-23H,2-6,9-12,15-16,18H2,1H3,(H,30,36)(H,31,35)/t22-,23+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7181

(3-phenyl-3,4,10,11-tetraazatricyclo[7.3.0.0^{2,6}]...)Show InChI InChI=1S/C15H11N5O/c16-15(21)13-10-6-7-12-11(8-17-18-12)14(10)20(19-13)9-4-2-1-3-5-9/h1-8H,(H2,16,21)(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50327912
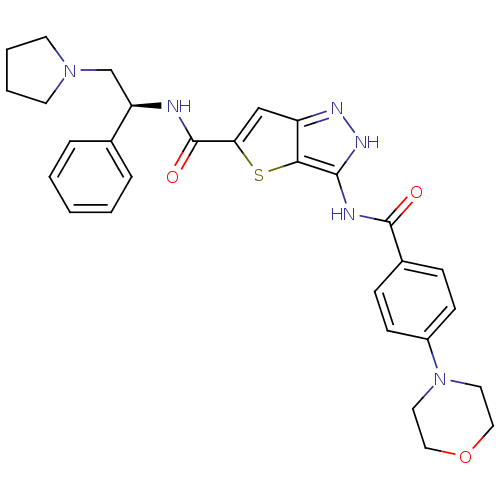
(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of FGFR1 |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7183

(3-(4-methylphenyl)-3,4,10,11-tetraazatricyclo[7.3....)Show InChI InChI=1S/C16H13N5O/c1-9-2-4-10(5-3-9)21-15-11(14(20-21)16(17)22)6-7-13-12(15)8-18-19-13/h2-8H,1H3,(H2,17,22)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50021650
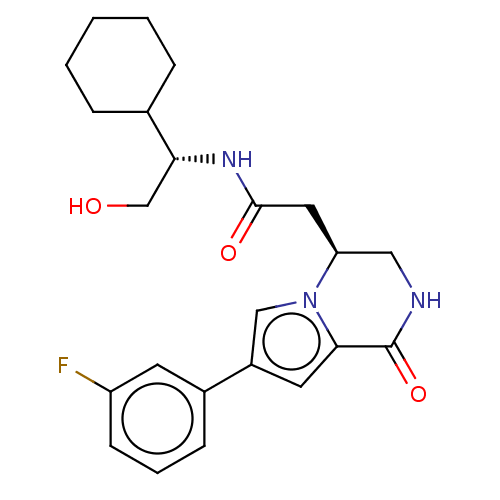
(CHEMBL3298892)Show SMILES OC[C@@H](NC(=O)C[C@H]1CNC(=O)c2cc(cn12)-c1cccc(F)c1)C1CCCCC1 |r| Show InChI InChI=1S/C23H28FN3O3/c24-18-8-4-7-16(9-18)17-10-21-23(30)25-12-19(27(21)13-17)11-22(29)26-20(14-28)15-5-2-1-3-6-15/h4,7-10,13,15,19-20,28H,1-3,5-6,11-12,14H2,(H,25,30)(H,26,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of human PIM1 using ARK-RERTYSFGHHA as substrate incubated for 60 mins prior to substrate addition by topcount scintillation counting anal... |
Bioorg Med Chem 21: 7364-80 (2013)
Article DOI: 10.1016/j.bmc.2013.09.054
BindingDB Entry DOI: 10.7270/Q23F4R7S |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7118
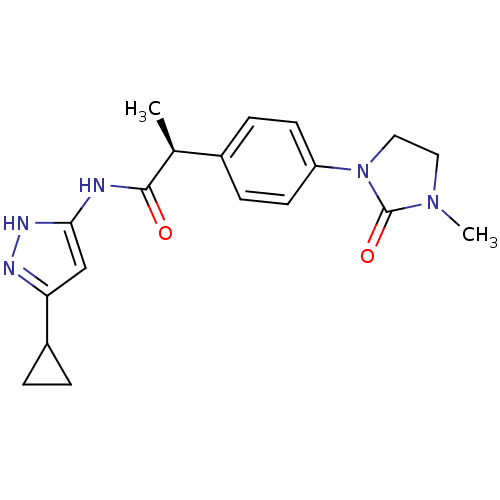
((2S)-N-(5-Cyclopropyl-1H-pyrazol-3-yl)-2-[4-(3-met...)Show SMILES C[C@H](C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)N1CCN(C)C1=O |r| Show InChI InChI=1S/C19H23N5O2/c1-12(18(25)20-17-11-16(21-22-17)14-3-4-14)13-5-7-15(8-6-13)24-10-9-23(2)19(24)26/h5-8,11-12,14H,3-4,9-10H2,1-2H3,(H2,20,21,22,25)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 2944-56 (2005)
Article DOI: 10.1021/jm0408870
BindingDB Entry DOI: 10.7270/Q2WQ020R |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7112
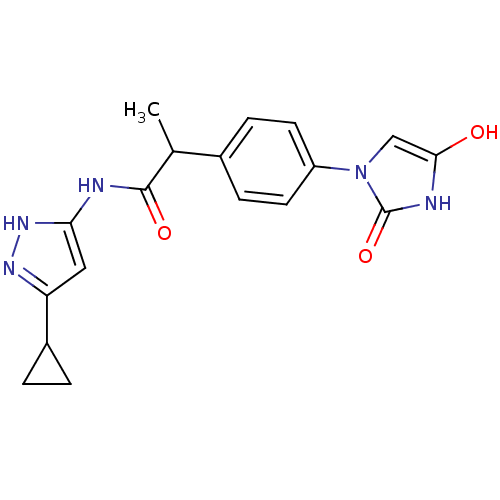
(3-Aminopyrazole deriv. 25 | N-(5-cyclopropyl-1H-py...)Show SMILES CC(C(=O)Nc1cc(n[nH]1)C1CC1)c1ccc(cc1)-n1cc(O)[nH]c1=O Show InChI InChI=1S/C18H19N5O3/c1-10(17(25)19-15-8-14(21-22-15)12-2-3-12)11-4-6-13(7-5-11)23-9-16(24)20-18(23)26/h4-10,12,24H,2-3H2,1H3,(H,20,26)(H2,19,21,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
J Med Chem 48: 2944-56 (2005)
Article DOI: 10.1021/jm0408870
BindingDB Entry DOI: 10.7270/Q2WQ020R |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50327912

(3-(4-Morpholin-4-yl-benzoylamino)-1H-thieno[3,2-c]...)Show SMILES O=C(N[C@H](CN1CCCC1)c1ccccc1)c1cc2n[nH]c(NC(=O)c3ccc(cc3)N3CCOCC3)c2s1 |r| Show InChI InChI=1S/C29H32N6O3S/c36-28(21-8-10-22(11-9-21)35-14-16-38-17-15-35)31-27-26-23(32-33-27)18-25(39-26)29(37)30-24(19-34-12-4-5-13-34)20-6-2-1-3-7-20/h1-3,6-11,18,24H,4-5,12-17,19H2,(H,30,37)(H2,31,32,33,36)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences-Oncology
Curated by ChEMBL
| Assay Description
Inhibition of AurA |
Bioorg Med Chem 18: 7113-20 (2010)
Article DOI: 10.1016/j.bmc.2010.07.048
BindingDB Entry DOI: 10.7270/Q2Q240GM |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7212
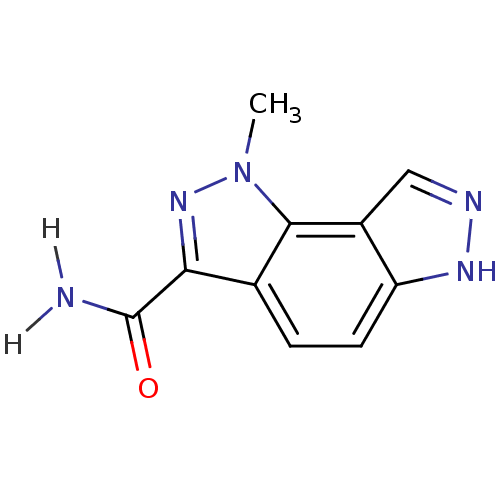
(3-methyl-3,4,10,11-tetraazatricyclo[7.3.0.0^{2,6}]...)Show InChI InChI=1S/C10H9N5O/c1-15-9-5(8(14-15)10(11)16)2-3-7-6(9)4-12-13-7/h2-4H,1H3,(H2,11,16)(H,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM7197

(3-[4-(morpholin-4-yl)phenyl]-3,4,10,11-tetraazatri...)Show SMILES NC(=O)c1nn(-c2ccc(cc2)N2CCOCC2)c2c1ccc1[nH]ncc21 Show InChI InChI=1S/C19H18N6O2/c20-19(26)17-14-5-6-16-15(11-21-22-16)18(14)25(23-17)13-3-1-12(2-4-13)24-7-9-27-10-8-24/h1-6,11H,7-10H2,(H2,20,26)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
| Assay Description
The biochemical activity of compounds was determined by incubation with specific enzymes and substrates in the presence ATP/[gamma-33P] ATP. After in... |
Bioorg Med Chem Lett 15: 1315-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.01.023
BindingDB Entry DOI: 10.7270/Q2N58JKH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data