 Found 1520 hits with Last Name = 'mayweg' and Initial = 'a'
Found 1520 hits with Last Name = 'mayweg' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86998
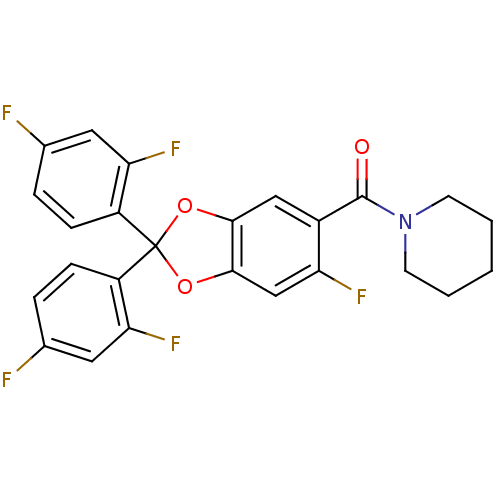
((2,2-bis(2,4-difluorophenyl)-6-fluorobenzo[d][1,3]...)Show SMILES Fc1ccc(c(F)c1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCCCC1)c1ccc(F)cc1F Show InChI InChI=1S/C25H18F5NO3/c26-14-4-6-17(20(29)10-14)25(18-7-5-15(27)11-21(18)30)33-22-12-16(19(28)13-23(22)34-25)24(32)31-8-2-1-3-9-31/h4-7,10-13H,1-3,8-9H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87007
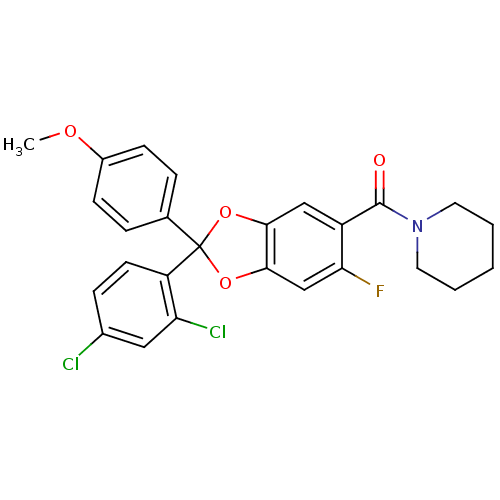
((2-(2,4-dichlorophenyl)-6-fluoro-2-(4-methoxypheny...)Show SMILES COc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCCCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C26H22Cl2FNO4/c1-32-18-8-5-16(6-9-18)26(20-10-7-17(27)13-21(20)28)33-23-14-19(22(29)15-24(23)34-26)25(31)30-11-3-2-4-12-30/h5-10,13-15H,2-4,11-12H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86989
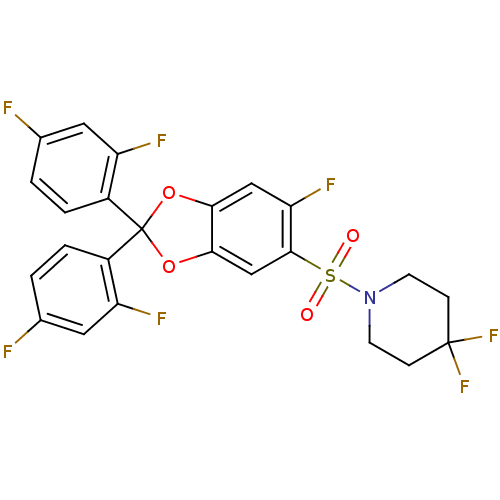
(1-(2,2-bis(2,4-difluorophenyl)-6-fluorobenzo[d][1,...)Show SMILES Fc1ccc(c(F)c1)C1(Oc2cc(F)c(cc2O1)S(=O)(=O)N1CCC(F)(F)CC1)c1ccc(F)cc1F Show InChI InChI=1S/C24H16F7NO4S/c25-13-1-3-15(17(27)9-13)24(16-4-2-14(26)10-18(16)28)35-20-11-19(29)22(12-21(20)36-24)37(33,34)32-7-5-23(30,31)6-8-32/h1-4,9-12H,5-8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM86984
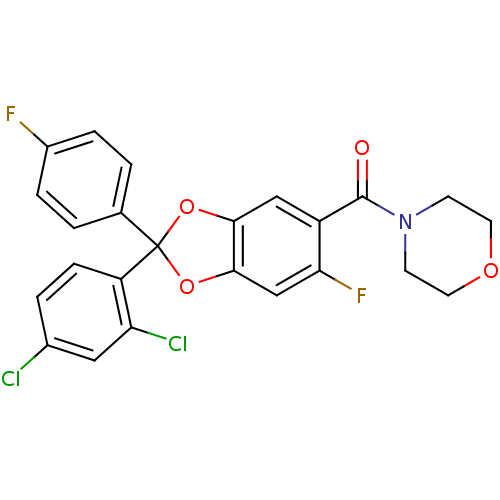
((R)-[2-(2,4-dichloro-phenyl)-6-fluoro-2-(4-fluoro-...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H17Cl2F2NO4/c25-15-3-6-18(19(26)11-15)24(14-1-4-16(27)5-2-14)32-21-12-17(20(28)13-22(21)33-24)23(30)29-7-9-31-10-8-29/h1-6,11-13H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86984

((R)-[2-(2,4-dichloro-phenyl)-6-fluoro-2-(4-fluoro-...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H17Cl2F2NO4/c25-15-3-6-18(19(26)11-15)24(14-1-4-16(27)5-2-14)32-21-12-17(20(28)13-22(21)33-24)23(30)29-7-9-31-10-8-29/h1-6,11-13H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87003
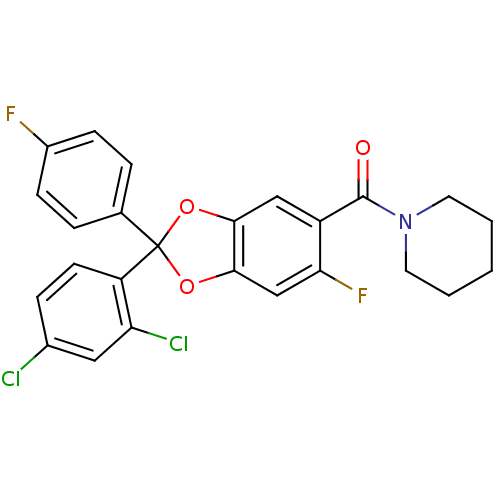
((2-(2,4-dichlorophenyl)-6-fluoro-2-(4-fluorophenyl...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCCCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C25H19Cl2F2NO3/c26-16-6-9-19(20(27)12-16)25(15-4-7-17(28)8-5-15)32-22-13-18(21(29)14-23(22)33-25)24(31)30-10-2-1-3-11-30/h4-9,12-14H,1-3,10-11H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86984

((R)-[2-(2,4-dichloro-phenyl)-6-fluoro-2-(4-fluoro-...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H17Cl2F2NO4/c25-15-3-6-18(19(26)11-15)24(14-1-4-16(27)5-2-14)32-21-12-17(20(28)13-22(21)33-24)23(30)29-7-9-31-10-8-29/h1-6,11-13H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86995
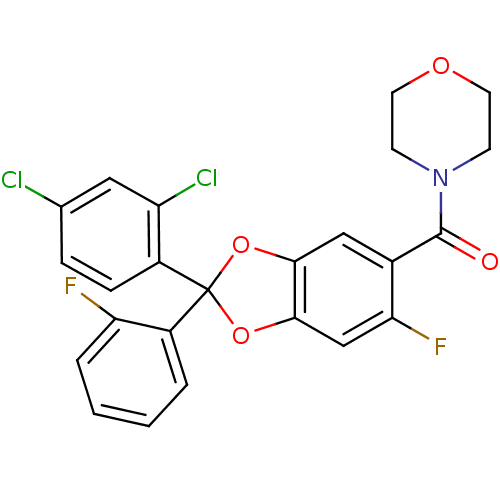
((2-(2,4-dichlorophenyl)-6-fluoro-2-(2-fluorophenyl...)Show SMILES Fc1ccccc1C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H17Cl2F2NO4/c25-14-5-6-16(18(26)11-14)24(17-3-1-2-4-19(17)27)32-21-12-15(20(28)13-22(21)33-24)23(30)29-7-9-31-10-8-29/h1-6,11-13H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86992
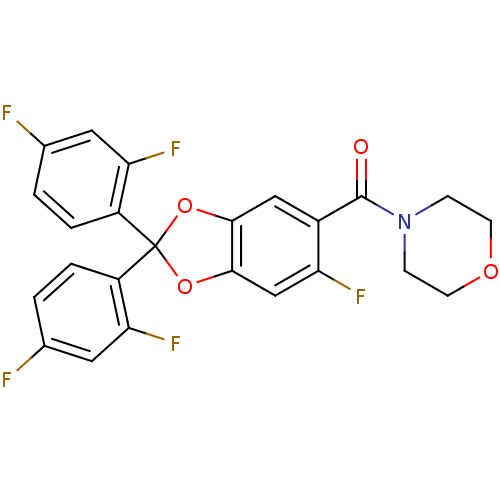
((2,2-bis(2,4-difluorophenyl)-6-fluorobenzo[d][1,3]...)Show SMILES Fc1ccc(c(F)c1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(F)cc1F Show InChI InChI=1S/C24H16F5NO4/c25-13-1-3-16(19(28)9-13)24(17-4-2-14(26)10-20(17)29)33-21-11-15(18(27)12-22(21)34-24)23(31)30-5-7-32-8-6-30/h1-4,9-12H,5-8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86997
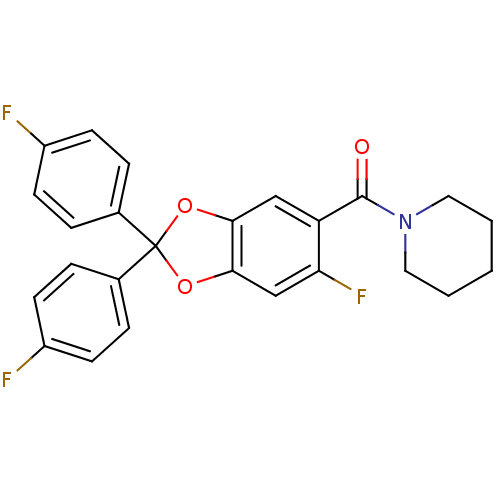
((6-fluoro-2,2-bis(4-fluorophenyl)benzo[d][1,3]diox...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCCCC1)c1ccc(F)cc1 Show InChI InChI=1S/C25H20F3NO3/c26-18-8-4-16(5-9-18)25(17-6-10-19(27)11-7-17)31-22-14-20(21(28)15-23(22)32-25)24(30)29-12-2-1-3-13-29/h4-11,14-15H,1-3,12-13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM29094
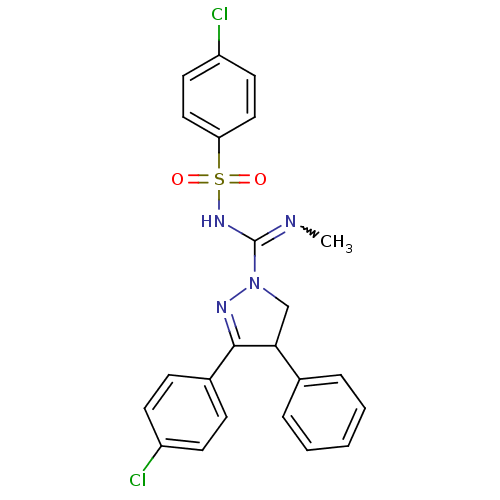
((+/-)-SLV319 | (S)-3-(4-chlorophenyl)-N-(4-chlorop...)Show SMILES CN=C(NS(=O)(=O)c1ccc(Cl)cc1)N1CC(C(=N1)c1ccc(Cl)cc1)c1ccccc1 |w:1.0,c:18| Show InChI InChI=1S/C23H20Cl2N4O2S/c1-26-23(28-32(30,31)20-13-11-19(25)12-14-20)29-15-21(16-5-3-2-4-6-16)22(27-29)17-7-9-18(24)10-8-17/h2-14,21H,15H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87009
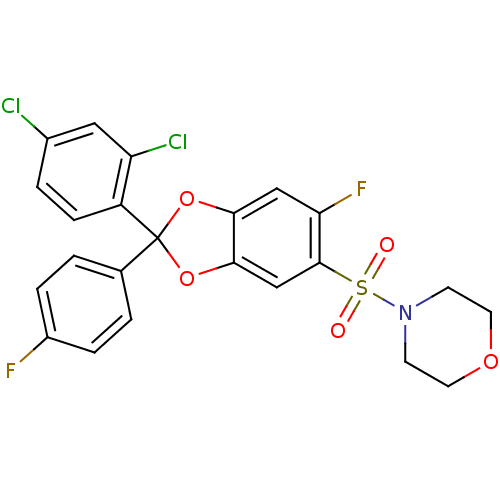
(4-(2-(2,4-dichlorophenyl)-6-fluoro-2-(4-fluorophen...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)S(=O)(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H17Cl2F2NO5S/c24-15-3-6-17(18(25)11-15)23(14-1-4-16(26)5-2-14)32-20-12-19(27)22(13-21(20)33-23)34(29,30)28-7-9-31-10-8-28/h1-6,11-13H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86986
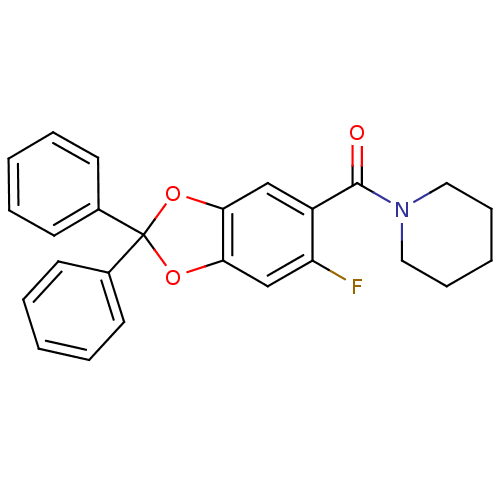
((6-fluoro-2,2-diphenylbenzo[d][1,3]dioxol-5-yl)(pi...)Show SMILES Fc1cc2OC(Oc2cc1C(=O)N1CCCCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22FNO3/c26-21-17-23-22(16-20(21)24(28)27-14-8-3-9-15-27)29-25(30-23,18-10-4-1-5-11-18)19-12-6-2-7-13-19/h1-2,4-7,10-13,16-17H,3,8-9,14-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86999
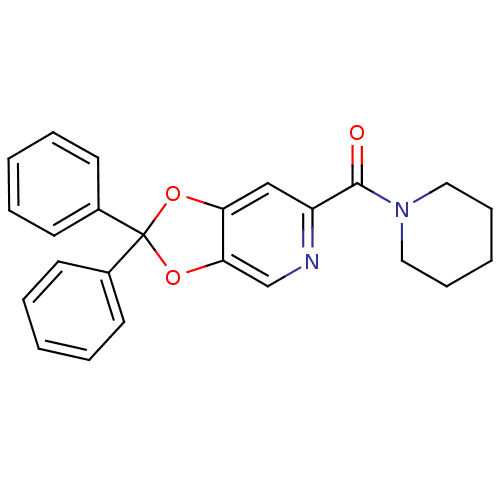
((2,2-diphenyl-[1,3]dioxolo[4,5-c]pyridin-6-yl)-pip...)Show SMILES O=C(N1CCCCC1)c1cc2OC(Oc2cn1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H22N2O3/c27-23(26-14-8-3-9-15-26)20-16-21-22(17-25-20)29-24(28-21,18-10-4-1-5-11-18)19-12-6-2-7-13-19/h1-2,4-7,10-13,16-17H,3,8-9,14-15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87000
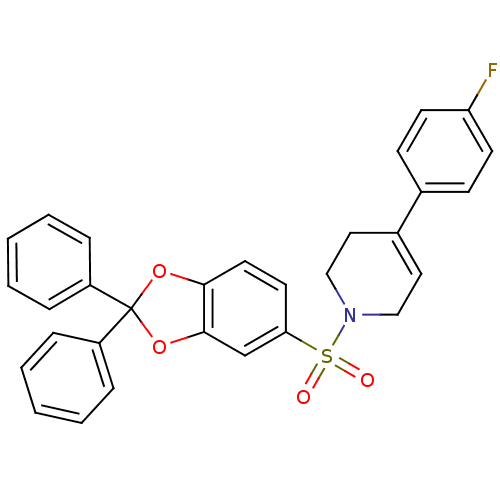
(1-(2,2-diphenyl-benzo[1,3]dioxole-5-sulfonyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1=CCN(CC1)S(=O)(=O)c1ccc2OC(Oc2c1)(c1ccccc1)c1ccccc1 |t:8| Show InChI InChI=1S/C30H24FNO4S/c31-26-13-11-22(12-14-26)23-17-19-32(20-18-23)37(33,34)27-15-16-28-29(21-27)36-30(35-28,24-7-3-1-4-8-24)25-9-5-2-6-10-25/h1-17,21H,18-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86993
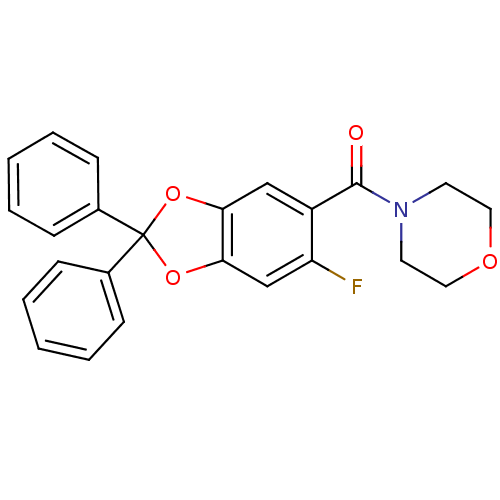
((6-fluoro-2,2-diphenyl-benzo[1,3]dioxol-5-yl)-morp...)Show SMILES Fc1cc2OC(Oc2cc1C(=O)N1CCOCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H20FNO4/c25-20-16-22-21(15-19(20)23(27)26-11-13-28-14-12-26)29-24(30-22,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,15-16H,11-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87006
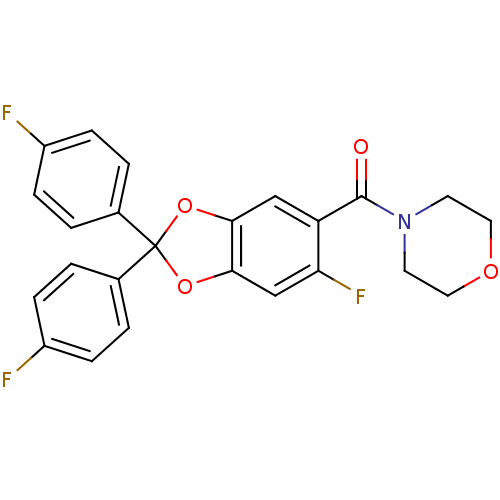
((6-fluoro-2,2-bis(4-fluorophenyl)benzo[d][1,3]diox...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(F)cc1 Show InChI InChI=1S/C24H18F3NO4/c25-17-5-1-15(2-6-17)24(16-3-7-18(26)8-4-16)31-21-13-19(20(27)14-22(21)32-24)23(29)28-9-11-30-12-10-28/h1-8,13-14H,9-12H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87012
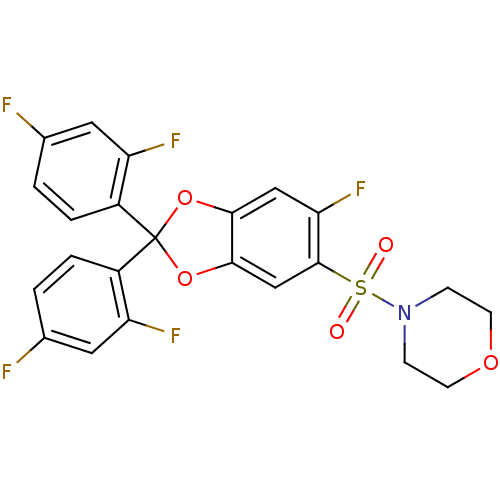
(4-(2,2-bis(2,4-difluorophenyl)-6-fluorobenzo[d][1,...)Show SMILES Fc1ccc(c(F)c1)C1(Oc2cc(F)c(cc2O1)S(=O)(=O)N1CCOCC1)c1ccc(F)cc1F Show InChI InChI=1S/C23H16F5NO5S/c24-13-1-3-15(17(26)9-13)23(16-4-2-14(25)10-18(16)27)33-20-11-19(28)22(12-21(20)34-23)35(30,31)29-5-7-32-8-6-29/h1-4,9-12H,5-8H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86991
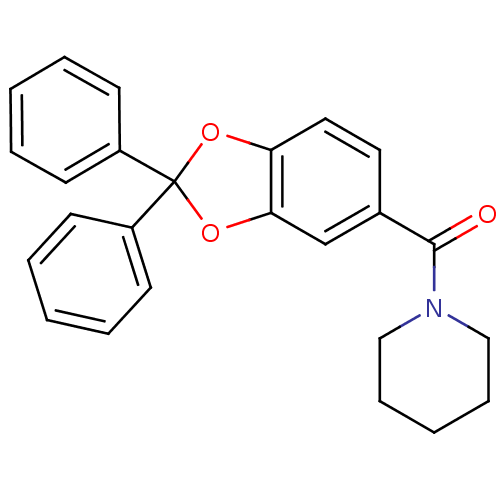
((2,2-diphenylbenzo[d][1,3]dioxol-5-yl)(piperidin-1...)Show SMILES O=C(N1CCCCC1)c1ccc2OC(Oc2c1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H23NO3/c27-24(26-16-8-3-9-17-26)19-14-15-22-23(18-19)29-25(28-22,20-10-4-1-5-11-20)21-12-6-2-7-13-21/h1-2,4-7,10-15,18H,3,8-9,16-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87001
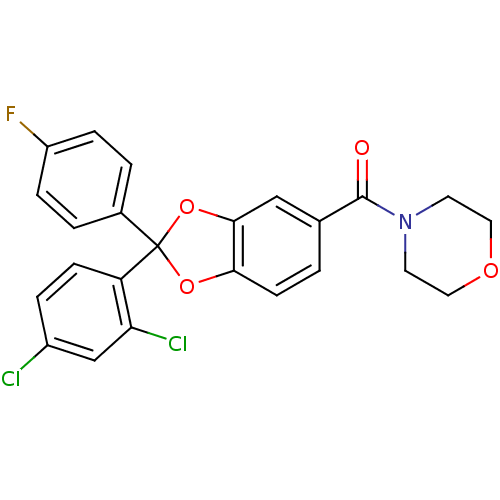
((2-(2,4-dichlorophenyl)-2-(4-fluorophenyl)benzo[d]...)Show SMILES Fc1ccc(cc1)C1(Oc2ccc(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H18Cl2FNO4/c25-17-4-7-19(20(26)14-17)24(16-2-5-18(27)6-3-16)31-21-8-1-15(13-22(21)32-24)23(29)28-9-11-30-12-10-28/h1-8,13-14H,9-12H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86984

((R)-[2-(2,4-dichloro-phenyl)-6-fluoro-2-(4-fluoro-...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(cc2O1)C(=O)N1CCOCC1)c1ccc(Cl)cc1Cl Show InChI InChI=1S/C24H17Cl2F2NO4/c25-15-3-6-18(19(26)11-15)24(14-1-4-16(27)5-2-14)32-21-12-17(20(28)13-22(21)33-24)23(30)29-7-9-31-10-8-29/h1-6,11-13H,7-10H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87004
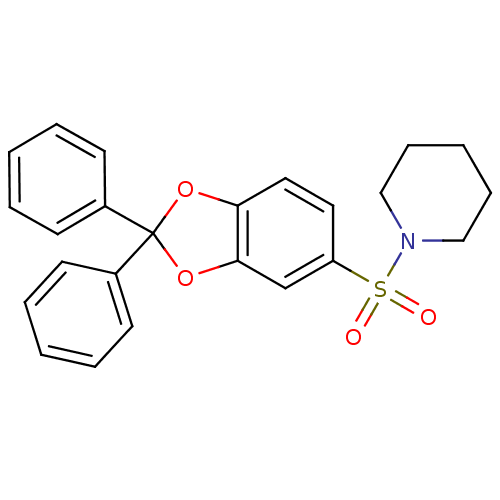
(1-(2,2-diphenyl-benzo[1,3]dioxole-5-sulfonyl)-pipe...)Show SMILES O=S(=O)(N1CCCCC1)c1ccc2OC(Oc2c1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H23NO4S/c26-30(27,25-16-8-3-9-17-25)21-14-15-22-23(18-21)29-24(28-22,19-10-4-1-5-11-19)20-12-6-2-7-13-20/h1-2,4-7,10-15,18H,3,8-9,16-17H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87011
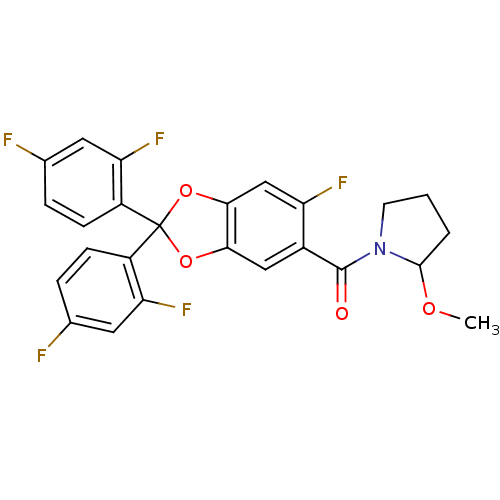
((S)-(2,2-bis(2,4-difluorophenyl)-6-fluorobenzo[d][...)Show SMILES COC1CCCN1C(=O)c1cc2OC(Oc2cc1F)(c1ccc(F)cc1F)c1ccc(F)cc1F Show InChI InChI=1S/C25H18F5NO4/c1-33-23-3-2-8-31(23)24(32)15-11-21-22(12-18(15)28)35-25(34-21,16-6-4-13(26)9-19(16)29)17-7-5-14(27)10-20(17)30/h4-7,9-12,23H,2-3,8H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87008
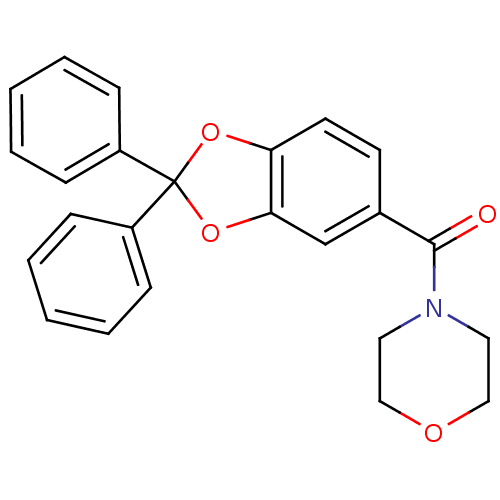
((2,2-diphenylbenzo[d][1,3]dioxol-5-yl)(morpholino)...)Show SMILES O=C(N1CCOCC1)c1ccc2OC(Oc2c1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H21NO4/c26-23(25-13-15-27-16-14-25)18-11-12-21-22(17-18)29-24(28-21,19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-12,17H,13-16H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM87005
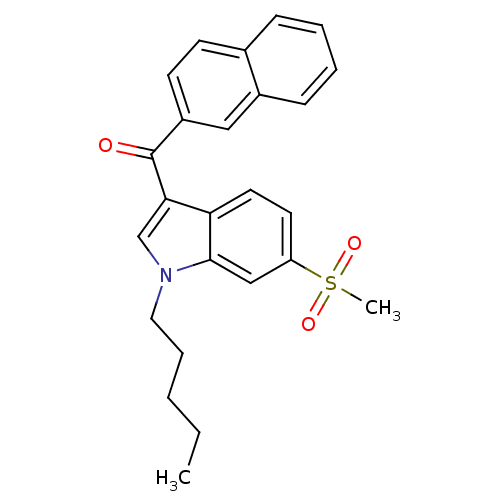
((6-(methylsulfonyl)-1-pentyl-1H-indol-3-yl)(naphth...)Show SMILES CCCCCn1cc(C(=O)c2ccc3ccccc3c2)c2ccc(cc12)S(C)(=O)=O Show InChI InChI=1S/C25H25NO3S/c1-3-4-7-14-26-17-23(22-13-12-21(16-24(22)26)30(2,28)29)25(27)20-11-10-18-8-5-6-9-19(18)15-20/h5-6,8-13,15-17H,3-4,7,14H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86988
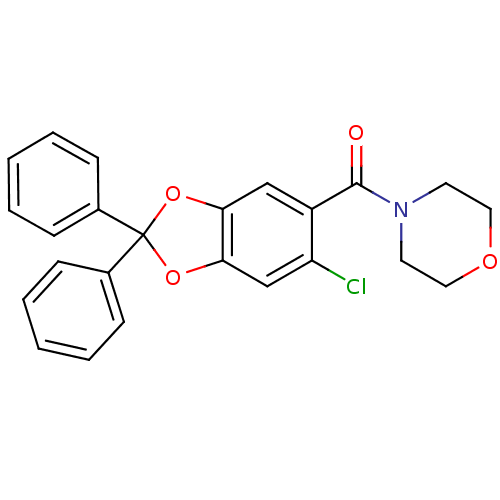
((6-chloro-2,2-diphenyl-benzo[1,3]dioxol-5-yl)-morp...)Show SMILES Clc1cc2OC(Oc2cc1C(=O)N1CCOCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H20ClNO4/c25-20-16-22-21(15-19(20)23(27)26-11-13-28-14-12-26)29-24(30-22,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,15-16H,11-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86990
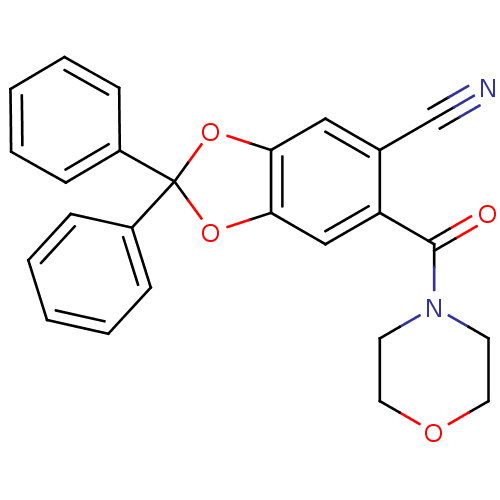
(6-(morpholine-4-carbonyl)-2,2-diphenyl-benzo[1,3]d...)Show SMILES O=C(N1CCOCC1)c1cc2OC(Oc2cc1C#N)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H20N2O4/c26-17-18-15-22-23(16-21(18)24(28)27-11-13-29-14-12-27)31-25(30-22,19-7-3-1-4-8-19)20-9-5-2-6-10-20/h1-10,15-16H,11-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 277 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM86994
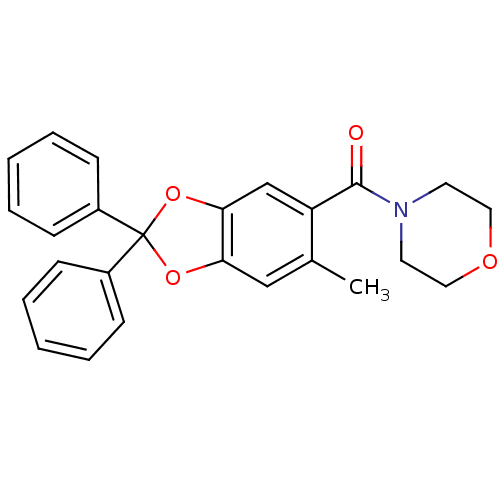
((6-methyl-2,2-diphenyl-benzo[1,3]dioxol-5-yl)-morp...)Show SMILES Cc1cc2OC(Oc2cc1C(=O)N1CCOCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H23NO4/c1-18-16-22-23(17-21(18)24(27)26-12-14-28-15-13-26)30-25(29-22,19-8-4-2-5-9-19)20-10-6-3-7-11-20/h2-11,16-17H,12-15H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 509 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
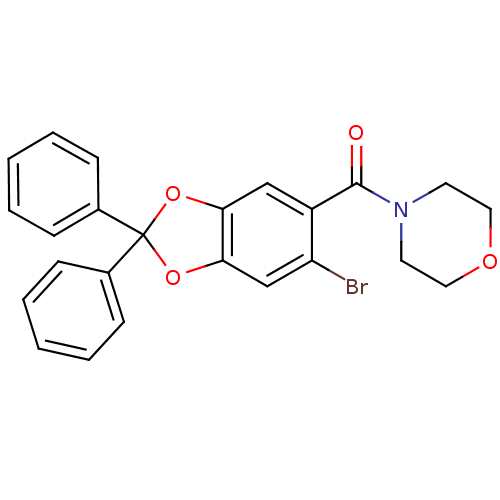
(Homo sapiens (Human)) | BDBM86985
((6-bromo-2,2-diphenyl-benzo[1,3]dioxol-5-yl)-morph...)Show SMILES Brc1cc2OC(Oc2cc1C(=O)N1CCOCC1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C24H20BrNO4/c25-20-16-22-21(15-19(20)23(27)26-11-13-28-14-12-26)29-24(30-22,17-7-3-1-4-8-17)18-9-5-2-6-10-18/h1-10,15-16H,11-14H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
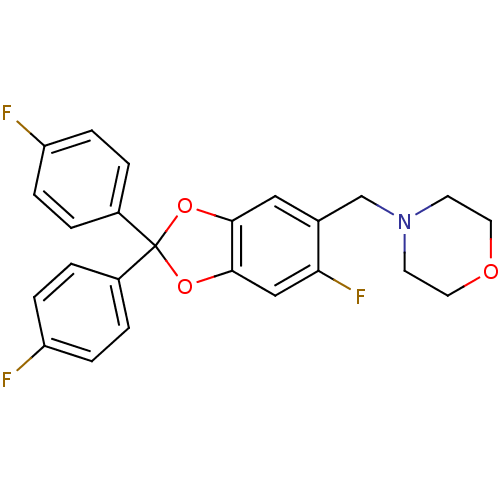
(Homo sapiens (Human)) | BDBM86996
(4-[6-fluoro-2,2-bis-(4-fluoro-phenyl)-benzo[1,3]di...)Show SMILES Fc1ccc(cc1)C1(Oc2cc(F)c(CN3CCOCC3)cc2O1)c1ccc(F)cc1 Show InChI InChI=1S/C24H20F3NO3/c25-19-5-1-17(2-6-19)24(18-3-7-20(26)8-4-18)30-22-13-16(21(27)14-23(22)31-24)15-28-9-11-29-12-10-28/h1-8,13-14H,9-12,15H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
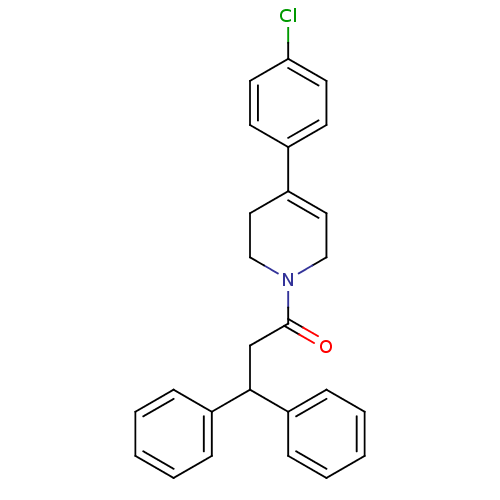
(Homo sapiens (Human)) | BDBM87010
(1-(4-(4-chlorophenyl)-5,6-dihydropyridin-1(2H)-yl)...)Show SMILES Clc1ccc(cc1)C1=CCN(CC1)C(=O)CC(c1ccccc1)c1ccccc1 |t:8| Show InChI InChI=1S/C26H24ClNO/c27-24-13-11-20(12-14-24)21-15-17-28(18-16-21)26(29)19-25(22-7-3-1-4-8-22)23-9-5-2-6-10-23/h1-15,25H,16-19H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM87000

(1-(2,2-diphenyl-benzo[1,3]dioxole-5-sulfonyl)-4-(4...)Show SMILES Fc1ccc(cc1)C1=CCN(CC1)S(=O)(=O)c1ccc2OC(Oc2c1)(c1ccccc1)c1ccccc1 |t:8| Show InChI InChI=1S/C30H24FNO4S/c31-26-13-11-22(12-14-26)23-17-19-32(20-18-23)37(33,34)27-15-16-28-29(21-27)36-30(35-28,24-7-3-1-4-8-24)25-9-5-2-6-10-25/h1-17,21H,18-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by PDSP Ki Database
| |
J Med Chem 51: 2115-27 (2008)
Article DOI: 10.1021/jm701487t
BindingDB Entry DOI: 10.7270/Q26H4G0Z |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541792

(CHEMBL4635011)Show SMILES Cn1cc(cn1)C(=O)N1CC(C1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C23H22ClN5O3/c1-23(2)20-6-15(24)4-5-19(20)22(31)29(23)16-7-17(10-25-9-16)32-18-12-28(13-18)21(30)14-8-26-27(3)11-14/h4-11,18H,12-13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432614
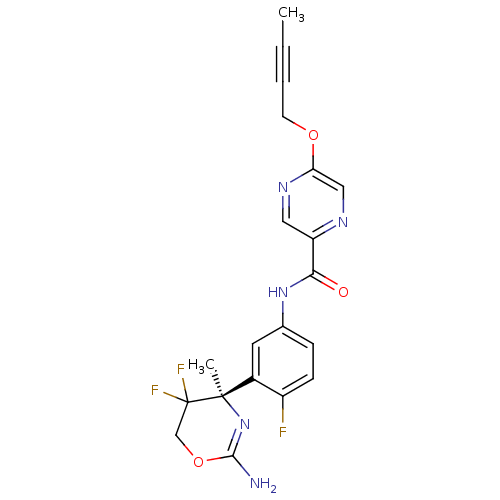
(CHEMBL2347198)Show SMILES CC#CCOc1cnc(cn1)C(=O)Nc1ccc(F)c(c1)[C@@]1(C)N=C(N)OCC1(F)F |r,t:25| Show InChI InChI=1S/C20H18F3N5O3/c1-3-4-7-30-16-10-25-15(9-26-16)17(29)27-12-5-6-14(21)13(8-12)19(2)20(22,23)11-31-18(24)28-19/h5-6,8-10H,7,11H2,1-2H3,(H2,24,28)(H,27,29)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... |
J Med Chem 56: 3980-95 (2013)
Article DOI: 10.1021/jm400225m
BindingDB Entry DOI: 10.7270/Q2RX9DFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432632

(CHEMBL2347211)Show SMILES C[C@]1(CCSC(N)=N1)c1cc(NC(=O)c2ccc(Cl)cn2)ccc1F |r,c:6| Show InChI InChI=1S/C17H16ClFN4OS/c1-17(6-7-25-16(20)23-17)12-8-11(3-4-13(12)19)22-15(24)14-5-2-10(18)9-21-14/h2-5,8-9H,6-7H2,1H3,(H2,20,23)(H,22,24)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... |
J Med Chem 56: 3980-95 (2013)
Article DOI: 10.1021/jm400225m
BindingDB Entry DOI: 10.7270/Q2RX9DFZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541786
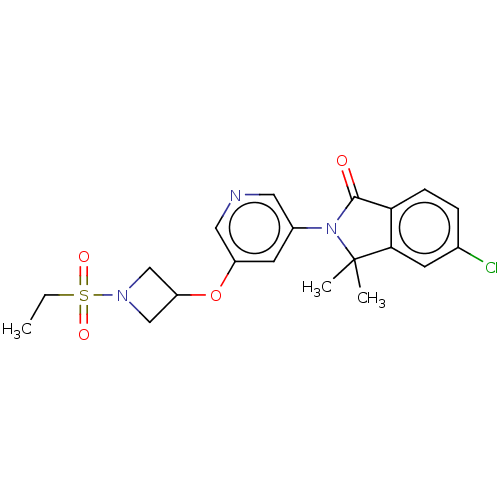
(CHEMBL4647543)Show SMILES CCS(=O)(=O)N1CC(C1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C20H22ClN3O4S/c1-4-29(26,27)23-11-16(12-23)28-15-8-14(9-22-10-15)24-19(25)17-6-5-13(21)7-18(17)20(24,2)3/h5-10,16H,4,11-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in HEK293 cell assessed as reduction in 18-hyroxycorticosterone by LC/MS-MS method |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541786

(CHEMBL4647543)Show SMILES CCS(=O)(=O)N1CC(C1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C20H22ClN3O4S/c1-4-29(26,27)23-11-16(12-23)28-15-8-14(9-22-10-15)24-19(25)17-6-5-13(21)7-18(17)20(24,2)3/h5-10,16H,4,11-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CYP11B2 in HEK293 cell assessed as reduction in aldosterone by LC/MS-MS method |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432608

(CHEMBL2347204 | US8754075, 4)Show SMILES C[C@@]1(N=C(N)OC[C@@H]1F)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,t:2| Show InChI InChI=1S/C18H15F2N5O2/c1-18(15(20)9-27-17(22)25-18)12-6-11(3-4-13(12)19)24-16(26)14-5-2-10(7-21)8-23-14/h2-6,8,15H,9H2,1H3,(H2,22,25)(H,24,26)/t15-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... |
J Med Chem 56: 3980-95 (2013)
Article DOI: 10.1021/jm400225m
BindingDB Entry DOI: 10.7270/Q2RX9DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432615
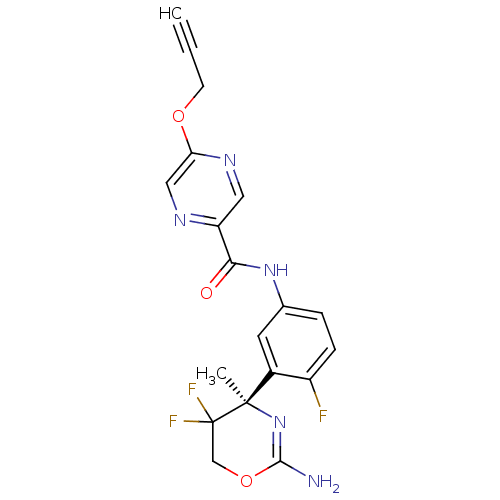
(CHEMBL2347197)Show SMILES C[C@@]1(N=C(N)OCC1(F)F)c1cc(NC(=O)c2cnc(OCC#C)cn2)ccc1F |r,t:2| Show InChI InChI=1S/C19H16F3N5O3/c1-3-6-29-15-9-24-14(8-25-15)16(28)26-11-4-5-13(20)12(7-11)18(2)19(21,22)10-30-17(23)27-18/h1,4-5,7-9H,6,10H2,2H3,(H2,23,27)(H,26,28)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... |
J Med Chem 56: 3980-95 (2013)
Article DOI: 10.1021/jm400225m
BindingDB Entry DOI: 10.7270/Q2RX9DFZ |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50432601
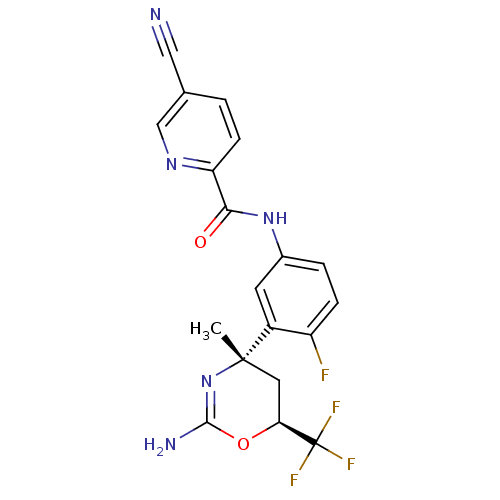
(CHEMBL2347208 | US9296734, 102)Show SMILES C[C@]1(C[C@H](OC(N)=N1)C(F)(F)F)c1cc(NC(=O)c2ccc(cn2)C#N)ccc1F |r,c:6| Show InChI InChI=1S/C19H15F4N5O2/c1-18(7-15(19(21,22)23)30-17(25)28-18)12-6-11(3-4-13(12)20)27-16(29)14-5-2-10(8-24)9-26-14/h2-6,9,15H,7H2,1H3,(H2,25,28)(H,27,29)/t15-,18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 in HEK293 cells transfected with wild type APP assessed as reduction of amyloid beta40 level after 18 to 20 hrs by AlphaLIS... |
J Med Chem 56: 3980-95 (2013)
Article DOI: 10.1021/jm400225m
BindingDB Entry DOI: 10.7270/Q2RX9DFZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM279728
(5-Chloro-3,3-dimethyl-2-[5-[2-(1-methylpyrazole-4-...)Show SMILES Cn1cc(cn1)C(=O)N1CC2(C1)CN(C2)c1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C25H25ClN6O2/c1-24(2)21-6-17(26)4-5-20(21)23(34)32(24)19-7-18(9-27-10-19)30-12-25(13-30)14-31(15-25)22(33)16-8-28-29(3)11-16/h4-11H,12-15H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541785

(CHEMBL4635517)Show SMILES CC1(C)N(C(=O)c2ccc(Cl)cc12)c1cncc(c1)N1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C20H23ClN4O3S/c1-20(2)18-10-14(21)4-5-17(18)19(26)25(20)16-11-15(12-22-13-16)23-6-8-24(9-7-23)29(3,27)28/h4-5,10-13H,6-9H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50444549

(CHEMBL3099695)Show InChI InChI=1S/C13H10FN3/c14-12-5-9(6-15)1-3-11(12)13-4-2-10-7-16-8-17(10)13/h1,3,5,7-8,13H,2,4H2/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharma Research and Early Development (pRED)
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in renal leiomyoblastoma cells |
J Med Chem 58: 8054-65 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00851
BindingDB Entry DOI: 10.7270/Q2K0763C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-C/Cyclin-dependent kinase 8
(Homo sapiens (Human)) | BDBM50260952

(CHEMBL4104489)Show InChI InChI=1S/C11H8F3N3O/c12-11(13,14)8-7(5-17-9(8)10(15)18)6-1-3-16-4-2-6/h1-5,17H,(H2,15,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medicinal Chemistry, Roche Innovation Center Shanghai, Bldg 5, 720 Cailun Road, Shanghai 201203, China. Electronic address: cyrus.han@roche.com.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length His-tagged human CDK8/Cyclin C expressed in baculovirus expression system using Ulight-GS peptide as substrate ... |
Bioorg Med Chem Lett 27: 4488-4492 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.080
BindingDB Entry DOI: 10.7270/Q2NP26W9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541783

(CHEMBL4642895)Show SMILES CCS(=O)(=O)N1CCC(CC1)c1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C22H26ClN3O3S/c1-4-30(28,29)25-9-7-15(8-10-25)16-11-18(14-24-13-16)26-21(27)19-6-5-17(23)12-20(19)22(26,2)3/h5-6,11-15H,4,7-10H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541764

(CHEMBL4645158)Show SMILES C[C@@H]1N(C(=O)c2ccc(Cl)cc12)c1cncc2[C@@H](CCCc12)NC(C)=O |r| Show InChI InChI=1S/C20H20ClN3O2/c1-11-16-8-13(21)6-7-15(16)20(26)24(11)19-10-22-9-17-14(19)4-3-5-18(17)23-12(2)25/h6-11,18H,3-5H2,1-2H3,(H,23,25)/t11-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541791

(CHEMBL4642138)Show SMILES Cc1ccncc1C(=O)N1CC(C1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C25H23ClN4O3/c1-15-6-7-27-12-21(15)23(31)29-13-19(14-29)33-18-9-17(10-28-11-18)30-24(32)20-5-4-16(26)8-22(20)25(30,2)3/h4-12,19H,13-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541789

(CHEMBL4646980)Show SMILES CC(=O)N1CCC(CC1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C22H24ClN3O3/c1-14(27)25-8-6-17(7-9-25)29-18-11-16(12-24-13-18)26-21(28)19-5-4-15(23)10-20(19)22(26,2)3/h4-5,10-13,17H,6-9H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM50541786

(CHEMBL4647543)Show SMILES CCS(=O)(=O)N1CC(C1)Oc1cncc(c1)N1C(=O)c2ccc(Cl)cc2C1(C)C Show InChI InChI=1S/C20H22ClN3O4S/c1-4-29(26,27)23-11-16(12-23)28-15-8-14(9-22-10-15)24-19(25)17-6-5-13(21)7-18(17)20(24,2)3/h5-10,16H,4,11-12H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human CYP11B2 expressed in human renal leiomyoblastoma cells harboring FDXR/FDX using 1-deoxycorticosterone as substrate by HTRF assay |
J Med Chem 63: 6876-6897 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00233
BindingDB Entry DOI: 10.7270/Q2H998RZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data