 Found 481 hits with Last Name = 'motani' and Initial = 'a'
Found 481 hits with Last Name = 'motani' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human MCHR1 |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM28681

(5-[(4-{2-[methyl(pyridin-2-yl)amino]ethoxy}phenyl)...)Show InChI InChI=1S/C18H19N3O3S/c1-21(16-4-2-3-9-19-16)10-11-24-14-7-5-13(6-8-14)12-15-17(22)20-18(23)25-15/h2-9,22H,10-12H2,1H3,(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-rosiglitazone from GST-tagged PPARgammaLBD (unknown origin) after 1 hr by scintillation proximity assay |
Bioorg Med Chem 21: 979-92 (2013)
Article DOI: 10.1016/j.bmc.2012.11.058
BindingDB Entry DOI: 10.7270/Q23F4R13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193846
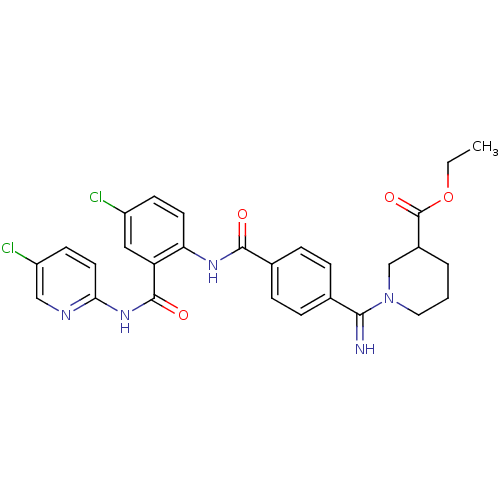
(1-({4-[4-chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES CCOC(=O)C1CCCN(C1)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C28H27Cl2N5O4/c1-2-39-28(38)19-4-3-13-35(16-19)25(31)17-5-7-18(8-6-17)26(36)33-23-11-9-20(29)14-22(23)27(37)34-24-12-10-21(30)15-32-24/h5-12,14-15,19,31H,2-4,13,16H2,1H3,(H,33,36)(H,32,34,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human Erg |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT2C receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin transporter |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT1A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to serotonin 5HT2A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to norepinephrine transporter |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M3 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to muscarinic M2 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha2A receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to adrenergic alpha2C receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to opioid mu receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D1 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50383522

(CHEMBL2032049)Show SMILES C[C@@H]1[C@H]2CN(CCC3(CCCCC3)C(O)=O)CC[C@@H]2Cc2[nH]c3ccc(cc3c12)C(F)(F)F |r| Show InChI InChI=1S/C26H33F3N2O2/c1-16-20-15-31(12-10-25(24(32)33)8-3-2-4-9-25)11-7-17(20)13-22-23(16)19-14-18(26(27,28)29)5-6-21(19)30-22/h5-6,14,16-17,20,30H,2-4,7-13,15H2,1H3,(H,32,33)/t16-,17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50193857
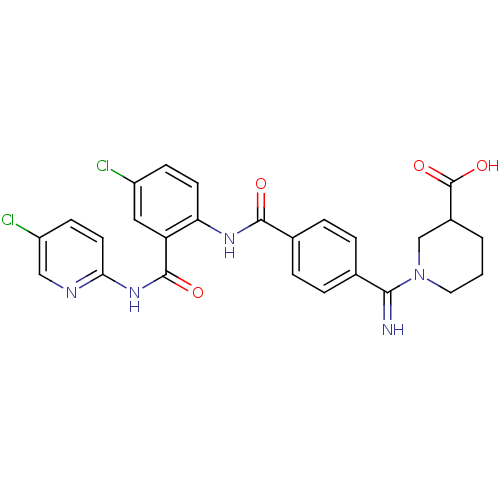
(1-({4-[4-chloro-2-(5-chloro-pyridin-2-ylcarbamoyl)...)Show SMILES OC(=O)C1CCCN(C1)C(=N)c1ccc(cc1)C(=O)Nc1ccc(Cl)cc1C(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C26H23Cl2N5O4/c27-18-7-9-21(20(12-18)25(35)32-22-10-8-19(28)13-30-22)31-24(34)16-5-3-15(4-6-16)23(29)33-11-1-2-17(14-33)26(36)37/h3-10,12-13,17,29H,1-2,11,14H2,(H,31,34)(H,36,37)(H,30,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen San Francisco
Curated by ChEMBL
| Assay Description
Binding affinity to human Erg |
Bioorg Med Chem Lett 22: 3781-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.006
BindingDB Entry DOI: 10.7270/Q2542PMN |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312776
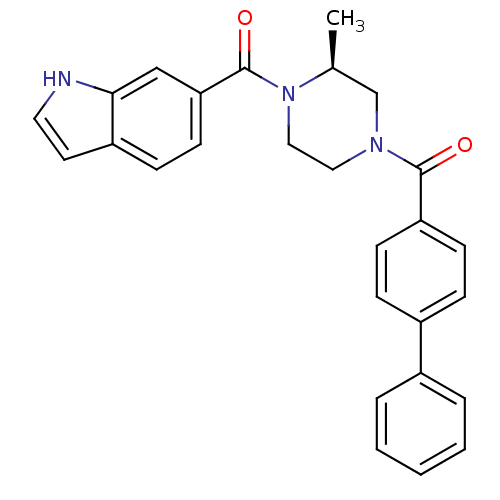
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O2/c1-19-18-29(26(31)23-10-7-21(8-11-23)20-5-3-2-4-6-20)15-16-30(19)27(32)24-12-9-22-13-14-28-25(22)17-24/h2-14,17,19,28H,15-16,18H2,1H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312777
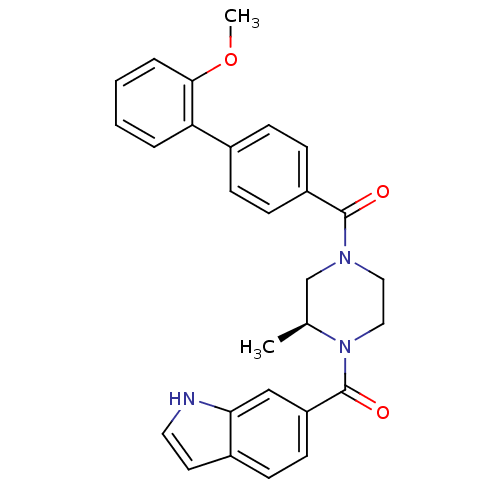
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccccc1-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)23-12-9-21-13-14-29-25(21)17-23)27(32)22-10-7-20(8-11-22)24-5-3-4-6-26(24)34-2/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312778
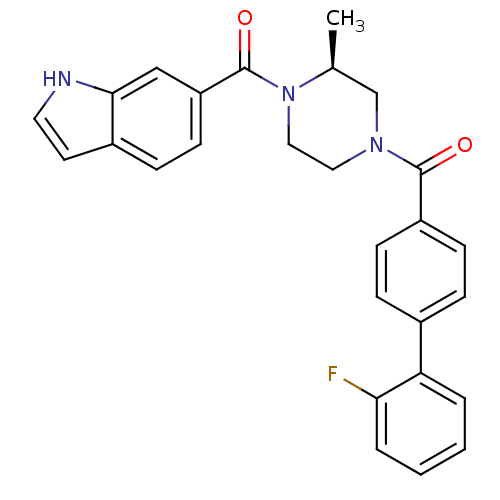
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1F |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312779
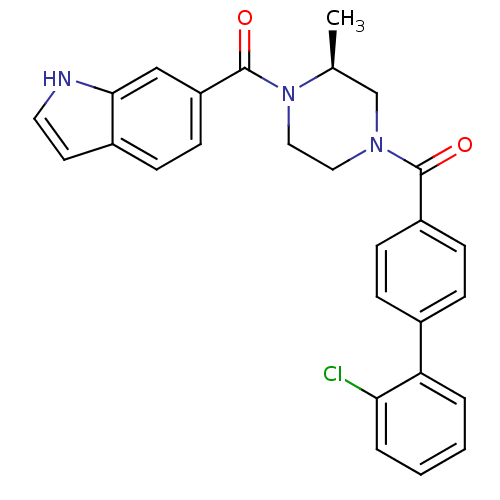
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C27H24ClN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312780
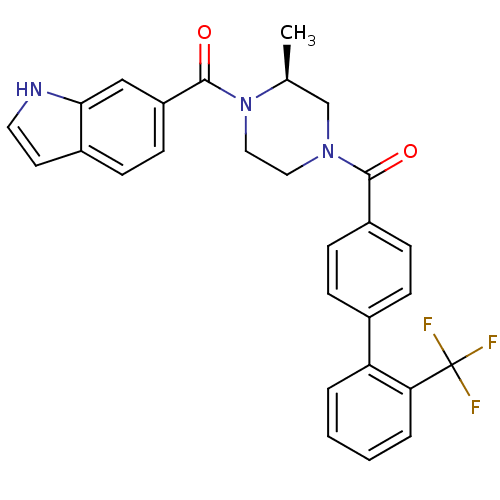
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C28H24F3N3O2/c1-18-17-33(14-15-34(18)27(36)22-11-8-20-12-13-32-25(20)16-22)26(35)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312766
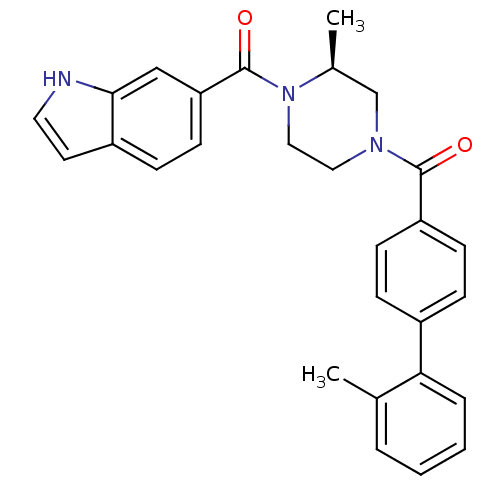
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C |r| Show InChI InChI=1S/C28H27N3O2/c1-19-5-3-4-6-25(19)21-7-10-23(11-8-21)27(32)30-15-16-31(20(2)18-30)28(33)24-12-9-22-13-14-29-26(22)17-24/h3-14,17,20,29H,15-16,18H2,1-2H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312767
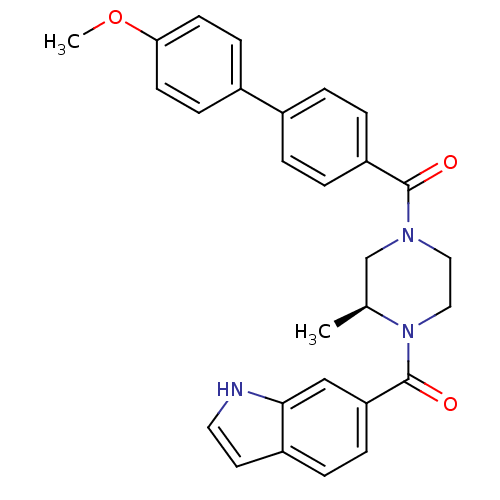
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)24-8-5-22-13-14-29-26(22)17-24)27(32)23-6-3-20(4-7-23)21-9-11-25(34-2)12-10-21/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312768
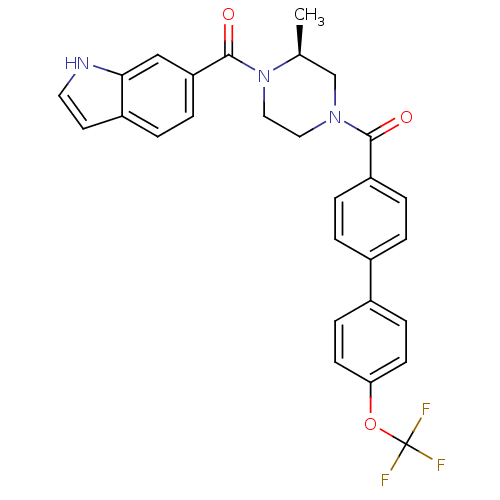
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C28H24F3N3O3/c1-18-17-33(14-15-34(18)27(36)23-7-4-21-12-13-32-25(21)16-23)26(35)22-5-2-19(3-6-22)20-8-10-24(11-9-20)37-28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50312769
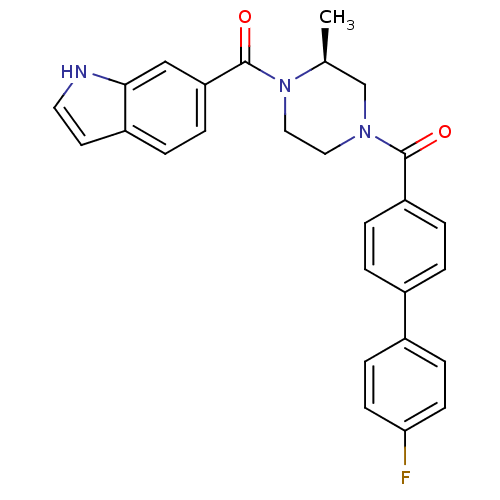
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(14-15-31(18)27(33)23-7-4-21-12-13-29-25(21)16-23)26(32)22-5-2-19(3-6-22)20-8-10-24(28)11-9-20/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of NET |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312770
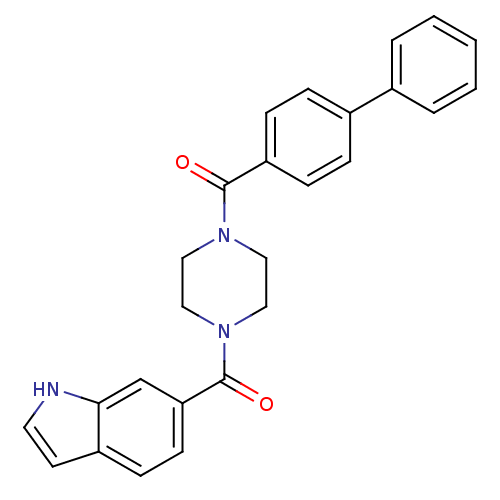
((4-(1H-indole-6-carbonyl)piperazin-1-yl)(biphenyl-...)Show SMILES O=C(N1CCN(CC1)C(=O)c1ccc2cc[nH]c2c1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C26H23N3O2/c30-25(22-9-6-20(7-10-22)19-4-2-1-3-5-19)28-14-16-29(17-15-28)26(31)23-11-8-21-12-13-27-24(21)18-23/h1-13,18,27H,14-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312771
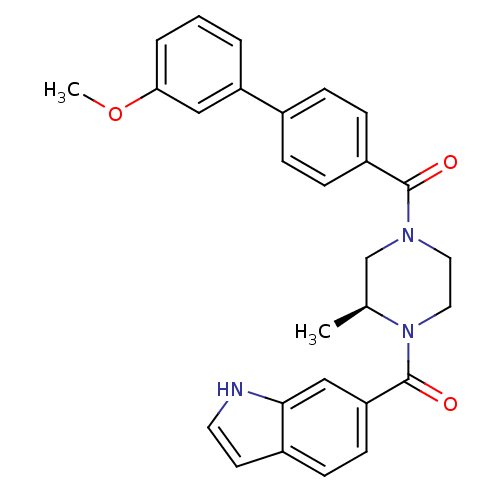
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(14-15-31(19)28(33)24-11-8-21-12-13-29-26(21)17-24)27(32)22-9-6-20(7-10-22)23-4-3-5-25(16-23)34-2/h3-13,16-17,19,29H,14-15,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312772
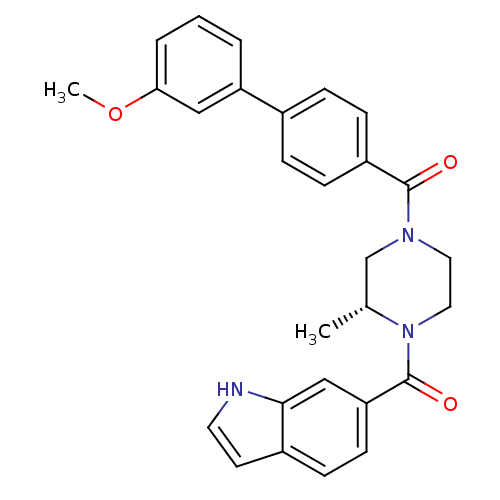
((R)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1cccc(c1)-c1ccc(cc1)C(=O)N1CCN([C@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(14-15-31(19)28(33)24-11-8-21-12-13-29-26(21)17-24)27(32)22-9-6-20(7-10-22)23-4-3-5-25(16-23)34-2/h3-13,16-17,19,29H,14-15,18H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312773
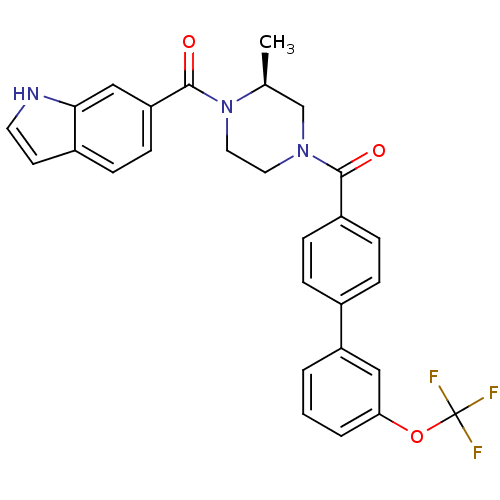
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1cccc(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C28H24F3N3O3/c1-18-17-33(13-14-34(18)27(36)23-10-7-20-11-12-32-25(20)16-23)26(35)21-8-5-19(6-9-21)22-3-2-4-24(15-22)37-28(29,30)31/h2-12,15-16,18,32H,13-14,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312774
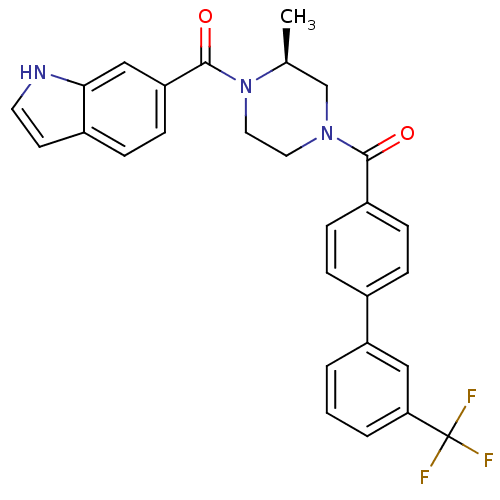
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H24F3N3O2/c1-18-17-33(13-14-34(18)27(36)23-10-7-20-11-12-32-25(20)16-23)26(35)21-8-5-19(6-9-21)22-3-2-4-24(15-22)28(29,30)31/h2-12,15-16,18,32H,13-14,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312775
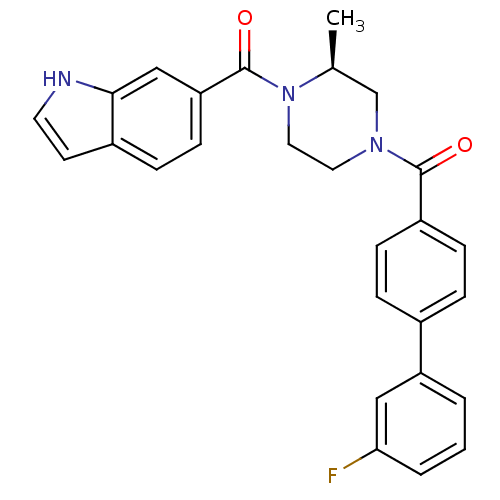
((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(13-14-31(18)27(33)23-10-7-20-11-12-29-25(20)16-23)26(32)21-8-5-19(6-9-21)22-3-2-4-24(28)15-22/h2-12,15-16,18,29H,13-14,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312776

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O2/c1-19-18-29(26(31)23-10-7-21(8-11-23)20-5-3-2-4-6-20)15-16-30(19)27(32)24-12-9-22-13-14-28-25(22)17-24/h2-14,17,19,28H,15-16,18H2,1H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312777

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccccc1-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)23-12-9-21-13-14-29-25(21)17-23)27(32)22-10-7-20(8-11-22)24-5-3-4-6-26(24)34-2/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312778

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1F |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312779

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C27H24ClN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312780

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C28H24F3N3O2/c1-18-17-33(14-15-34(18)27(36)22-11-8-20-12-13-32-25(20)16-22)26(35)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312766

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C |r| Show InChI InChI=1S/C28H27N3O2/c1-19-5-3-4-6-25(19)21-7-10-23(11-8-21)27(32)30-15-16-31(20(2)18-30)28(33)24-12-9-22-13-14-29-26(22)17-24/h3-14,17,20,29H,15-16,18H2,1-2H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312767

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)24-8-5-22-13-14-29-26(22)17-24)27(32)23-6-3-20(4-7-23)21-9-11-25(34-2)12-10-21/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312768

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C28H24F3N3O3/c1-18-17-33(14-15-34(18)27(36)23-7-4-21-12-13-32-25(21)16-23)26(35)22-5-2-19(3-6-22)20-8-10-24(11-9-20)37-28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50312769

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(14-15-31(18)27(33)23-7-4-21-12-13-29-25(21)16-23)26(32)22-5-2-19(3-6-22)20-8-10-24(28)11-9-20/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of histamine H2 receptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312774

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H24F3N3O2/c1-18-17-33(13-14-34(18)27(36)23-10-7-20-11-12-32-25(20)16-23)26(35)21-8-5-19(6-9-21)22-3-2-4-24(15-22)28(29,30)31/h2-12,15-16,18,32H,13-14,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312775

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(13-14-31(18)27(33)23-10-7-20-11-12-29-25(20)16-23)26(32)21-8-5-19(6-9-21)22-3-2-4-24(28)15-22/h2-12,15-16,18,29H,13-14,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312776

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C27H25N3O2/c1-19-18-29(26(31)23-10-7-21(8-11-23)20-5-3-2-4-6-20)15-16-30(19)27(32)24-12-9-22-13-14-28-25(22)17-24/h2-14,17,19,28H,15-16,18H2,1H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312777

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccccc1-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)23-12-9-21-13-14-29-25(21)17-23)27(32)22-10-7-20(8-11-22)24-5-3-4-6-26(24)34-2/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312778

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1F |r| Show InChI InChI=1S/C27H24FN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312779

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1Cl |r| Show InChI InChI=1S/C27H24ClN3O2/c1-18-17-30(14-15-31(18)27(33)22-11-8-20-12-13-29-25(20)16-22)26(32)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28/h2-13,16,18,29H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312780

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C28H24F3N3O2/c1-18-17-33(14-15-34(18)27(36)22-11-8-20-12-13-32-25(20)16-22)26(35)21-9-6-19(7-10-21)23-4-2-3-5-24(23)28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312766

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccccc1C |r| Show InChI InChI=1S/C28H27N3O2/c1-19-5-3-4-6-25(19)21-7-10-23(11-8-21)27(32)30-15-16-31(20(2)18-30)28(33)24-12-9-22-13-14-29-26(22)17-24/h3-14,17,20,29H,15-16,18H2,1-2H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312767

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)N1CCN([C@@H](C)C1)C(=O)c1ccc2cc[nH]c2c1 |r| Show InChI InChI=1S/C28H27N3O3/c1-19-18-30(15-16-31(19)28(33)24-8-5-22-13-14-29-26(22)17-24)27(32)23-6-3-20(4-7-23)21-9-11-25(34-2)12-10-21/h3-14,17,19,29H,15-16,18H2,1-2H3/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50312768

((S)-(4-(1H-indole-6-carbonyl)-3-methylpiperazin-1-...)Show SMILES C[C@H]1CN(CCN1C(=O)c1ccc2cc[nH]c2c1)C(=O)c1ccc(cc1)-c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C28H24F3N3O3/c1-18-17-33(14-15-34(18)27(36)23-7-4-21-12-13-32-25(21)16-23)26(35)22-5-2-19(3-6-22)20-8-10-24(11-9-20)37-28(29,30)31/h2-13,16,18,32H,14-15,17H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of dopamine D2 rceptor |
Bioorg Med Chem Lett 20: 1758-62 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.043
BindingDB Entry DOI: 10.7270/Q2WM1DJD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data