 Found 196 hits with Last Name = 'raza' and Initial = 'a'
Found 196 hits with Last Name = 'raza' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Homo sapiens (Human)) | BDBM92543

(Coumarin analogue, 3a)Show SMILES COc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:9.9| Show InChI InChI=1S/C20H15N3O3S/c1-25-15-7-4-5-13(9-15)11-21-23-20-22-17(12-27-20)16-10-14-6-2-3-8-18(14)26-19(16)24/h2-10,12H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92554
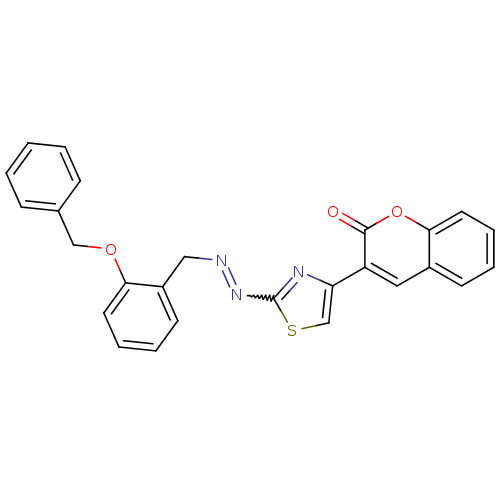
(Coumarin analogue, 3l)Show SMILES O=c1oc2ccccc2cc1-c1csc(N=NCc2ccccc2OCc2ccccc2)n1 |w:15.16| Show InChI InChI=1S/C26H19N3O3S/c30-25-21(14-19-10-4-7-13-24(19)32-25)22-17-33-26(28-22)29-27-15-20-11-5-6-12-23(20)31-16-18-8-2-1-3-9-18/h1-14,17H,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92545

(Coumarin analogue, 3c)Show SMILES Clc1ccccc1CN=Nc1nc(cs1)-c1cc2ccccc2oc1=O |w:9.10| Show InChI InChI=1S/C19H12ClN3O2S/c20-15-7-3-1-6-13(15)10-21-23-19-22-16(11-26-19)14-9-12-5-2-4-8-17(12)25-18(14)24/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92544
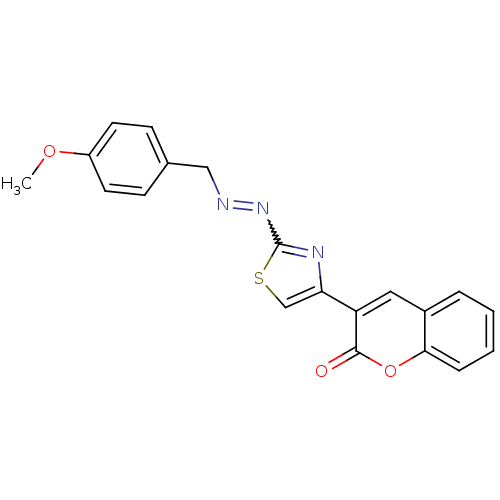
(Coumarin analogue, 3b)Show SMILES COc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:8.8| Show InChI InChI=1S/C20H15N3O3S/c1-25-15-8-6-13(7-9-15)11-21-23-20-22-17(12-27-20)16-10-14-4-2-3-5-18(14)26-19(16)24/h2-10,12H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92548
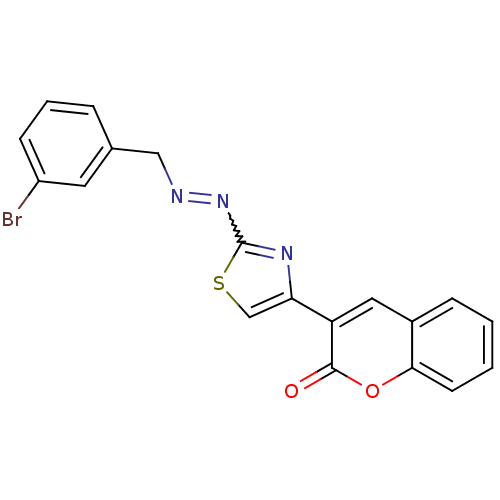
(Coumarin analogue, 3f)Show SMILES Brc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:8.8| Show InChI InChI=1S/C19H12BrN3O2S/c20-14-6-3-4-12(8-14)10-21-23-19-22-16(11-26-19)15-9-13-5-1-2-7-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92551

(Coumarin analogue, 3i)Show SMILES CN(C)c1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:9.9| Show InChI InChI=1S/C21H18N4O2S/c1-25(2)16-9-7-14(8-10-16)12-22-24-21-23-18(13-28-21)17-11-15-5-3-4-6-19(15)27-20(17)26/h3-11,13H,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92553

(Coumarin analogue, 3k)Show SMILES COc1cc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc(OC)c1O |w:7.7| Show InChI InChI=1S/C21H17N3O5S/c1-27-17-7-12(8-18(28-2)19(17)25)10-22-24-21-23-15(11-30-21)14-9-13-5-3-4-6-16(13)29-20(14)26/h3-9,11,25H,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92549
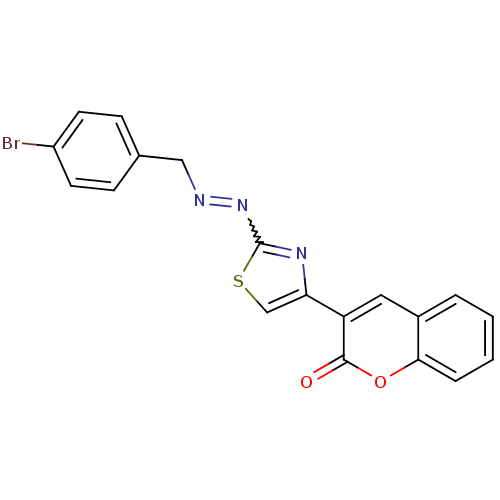
(Coumarin analogue, 3g)Show SMILES Brc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:7.7| Show InChI InChI=1S/C19H12BrN3O2S/c20-14-7-5-12(6-8-14)10-21-23-19-22-16(11-26-19)15-9-13-3-1-2-4-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92550
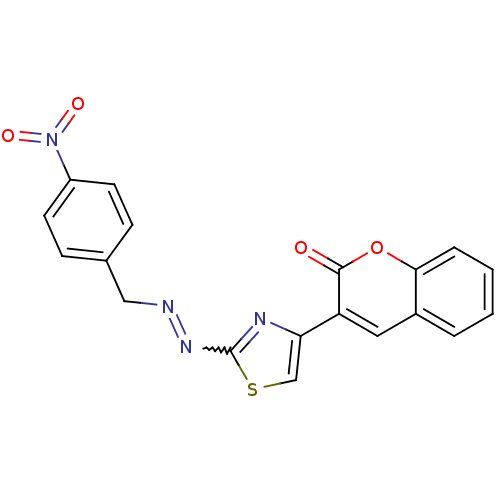
(Coumarin analogue, 3h)Show SMILES O=c1oc2ccccc2cc1-c1csc(N=NCc2ccc(cc2)N(=O)=O)n1 |w:15.16| Show InChI InChI=1S/C19H12N4O4S/c24-18-15(9-13-3-1-2-4-17(13)27-18)16-11-28-19(21-16)22-20-10-12-5-7-14(8-6-12)23(25)26/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92546
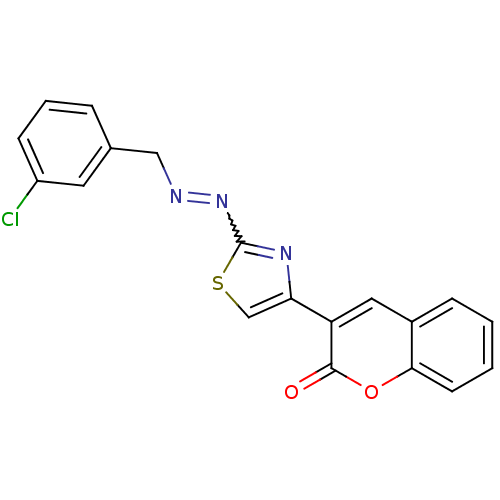
(Coumarin analogue, 3d)Show SMILES Clc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:8.8| Show InChI InChI=1S/C19H12ClN3O2S/c20-14-6-3-4-12(8-14)10-21-23-19-22-16(11-26-19)15-9-13-5-1-2-7-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92547

(Coumarin analogue, 3e)Show SMILES Clc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:7.7| Show InChI InChI=1S/C19H12ClN3O2S/c20-14-7-5-12(6-8-14)10-21-23-19-22-16(11-26-19)15-9-13-3-1-2-4-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM92552

(Coumarin analogue, 3j)Show SMILES COc1cc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc(OC)c1OC |w:7.7| Show InChI InChI=1S/C22H19N3O5S/c1-27-18-8-13(9-19(28-2)20(18)29-3)11-23-25-22-24-16(12-31-22)15-10-14-6-4-5-7-17(14)30-21(15)26/h4-10,12H,11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92548

(Coumarin analogue, 3f)Show SMILES Brc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:8.8| Show InChI InChI=1S/C19H12BrN3O2S/c20-14-6-3-4-12(8-14)10-21-23-19-22-16(11-26-19)15-9-13-5-1-2-7-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92544

(Coumarin analogue, 3b)Show SMILES COc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:8.8| Show InChI InChI=1S/C20H15N3O3S/c1-25-15-8-6-13(7-9-15)11-21-23-20-22-17(12-27-20)16-10-14-4-2-3-5-18(14)26-19(16)24/h2-10,12H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92553

(Coumarin analogue, 3k)Show SMILES COc1cc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc(OC)c1O |w:7.7| Show InChI InChI=1S/C21H17N3O5S/c1-27-17-7-12(8-18(28-2)19(17)25)10-22-24-21-23-15(11-30-21)14-9-13-5-3-4-6-16(13)29-20(14)26/h3-9,11,25H,10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92547

(Coumarin analogue, 3e)Show SMILES Clc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:7.7| Show InChI InChI=1S/C19H12ClN3O2S/c20-14-7-5-12(6-8-14)10-21-23-19-22-16(11-26-19)15-9-13-3-1-2-4-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92550

(Coumarin analogue, 3h)Show SMILES O=c1oc2ccccc2cc1-c1csc(N=NCc2ccc(cc2)N(=O)=O)n1 |w:15.16| Show InChI InChI=1S/C19H12N4O4S/c24-18-15(9-13-3-1-2-4-17(13)27-18)16-11-28-19(21-16)22-20-10-12-5-7-14(8-6-12)23(25)26/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92549

(Coumarin analogue, 3g)Show SMILES Brc1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:7.7| Show InChI InChI=1S/C19H12BrN3O2S/c20-14-7-5-12(6-8-14)10-21-23-19-22-16(11-26-19)15-9-13-3-1-2-4-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92546

(Coumarin analogue, 3d)Show SMILES Clc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:8.8| Show InChI InChI=1S/C19H12ClN3O2S/c20-14-6-3-4-12(8-14)10-21-23-19-22-16(11-26-19)15-9-13-5-1-2-7-17(13)25-18(15)24/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92545

(Coumarin analogue, 3c)Show SMILES Clc1ccccc1CN=Nc1nc(cs1)-c1cc2ccccc2oc1=O |w:9.10| Show InChI InChI=1S/C19H12ClN3O2S/c20-15-7-3-1-6-13(15)10-21-23-19-22-16(11-26-19)14-9-12-5-2-4-8-17(12)25-18(14)24/h1-9,11H,10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92552

(Coumarin analogue, 3j)Show SMILES COc1cc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc(OC)c1OC |w:7.7| Show InChI InChI=1S/C22H19N3O5S/c1-27-18-8-13(9-19(28-2)20(18)29-3)11-23-25-22-24-16(12-31-22)15-10-14-6-4-5-7-17(14)30-21(15)26/h4-10,12H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92543

(Coumarin analogue, 3a)Show SMILES COc1cccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)c1 |w:9.9| Show InChI InChI=1S/C20H15N3O3S/c1-25-15-7-4-5-13(9-15)11-21-23-20-22-17(12-27-20)16-10-14-6-2-3-8-18(14)26-19(16)24/h2-10,12H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92554

(Coumarin analogue, 3l)Show SMILES O=c1oc2ccccc2cc1-c1csc(N=NCc2ccccc2OCc2ccccc2)n1 |w:15.16| Show InChI InChI=1S/C26H19N3O3S/c30-25-21(14-19-10-4-7-13-24(19)32-25)22-17-33-26(28-22)29-27-15-20-11-5-6-12-23(20)31-16-18-8-2-1-3-9-18/h1-14,17H,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM92551

(Coumarin analogue, 3i)Show SMILES CN(C)c1ccc(CN=Nc2nc(cs2)-c2cc3ccccc3oc2=O)cc1 |w:9.9| Show InChI InChI=1S/C21H18N4O2S/c1-25(2)16-9-7-14(8-10-16)12-22-24-21-23-18(13-28-21)17-11-15-5-3-4-6-19(15)27-20(17)26/h3-11,13H,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50022775

((m-Hydroxyphenyl)trimethylammonium dimethylcarbama...)Show InChI InChI=1S/C12H19N2O2/c1-13(2)12(15)16-11-8-6-7-10(9-11)14(3,4)5/h6-9H,1-5H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology
| Assay Description
Inhibition assay using AChE and BuChE. |
Chem Biol Drug Des 80: 605-15 (2012)
Article DOI: 10.1111/j.1747-0285.2012.01435.x
BindingDB Entry DOI: 10.7270/Q28P5Z3T |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199266
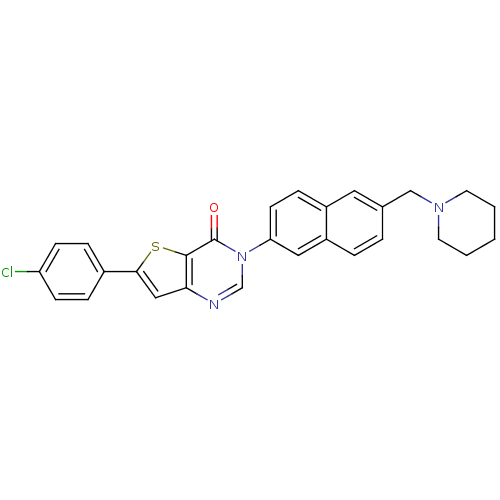
(6-(4-chlorophenyl)-3-[6-(1-piperidinylmethyl)-2-na...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4cc(CN5CCCCC5)ccc4c3)c(=O)c2s1 Show InChI InChI=1S/C28H24ClN3OS/c29-23-9-6-20(7-10-23)26-16-25-27(34-26)28(33)32(18-30-25)24-11-8-21-14-19(4-5-22(21)15-24)17-31-12-2-1-3-13-31/h4-11,14-16,18H,1-3,12-13,17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50067593
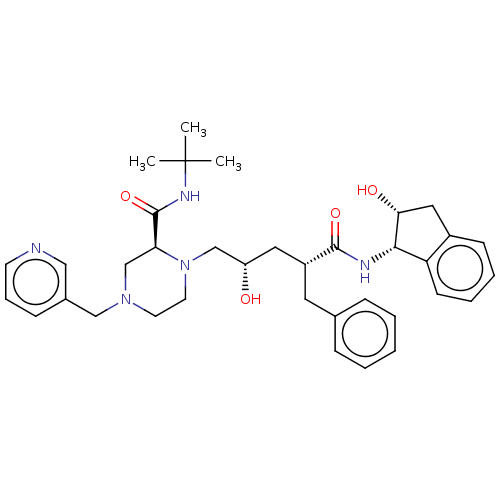
(CHEBI:44032 | Crixivan | Indinavir | L-735524 | MK...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 18: 5406-10 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.044
BindingDB Entry DOI: 10.7270/Q29K4F1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50241083
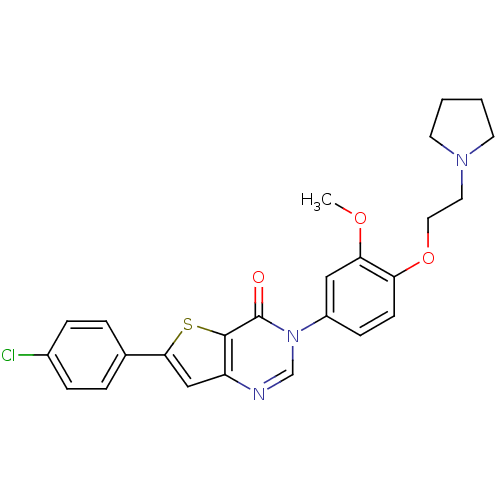
(6-(4-chlorophenyl)-3-(3-methoxy-4-(2-(pyrrolidin-1...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClN3O3S/c1-31-22-14-19(8-9-21(22)32-13-12-28-10-2-3-11-28)29-16-27-20-15-23(33-24(20)25(29)30)17-4-6-18(26)7-5-17/h4-9,14-16H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 16: 4723-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.008
BindingDB Entry DOI: 10.7270/Q24B32MB |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50422052

(CHEMBL109952 | L-700417)Show SMILES OC(C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C39H42N2O5/c42-31(21-29(19-25-11-3-1-4-12-25)38(45)40-36-32-17-9-7-15-27(32)23-34(36)43)22-30(20-26-13-5-2-6-14-26)39(46)41-37-33-18-10-8-16-28(33)24-35(37)44/h1-18,29-31,34-37,42-44H,19-24H2,(H,40,45)(H,41,46)/t29-,30-,34-,35-,36+,37+/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
Bioorg Med Chem Lett 18: 5406-10 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.044
BindingDB Entry DOI: 10.7270/Q29K4F1M |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199205
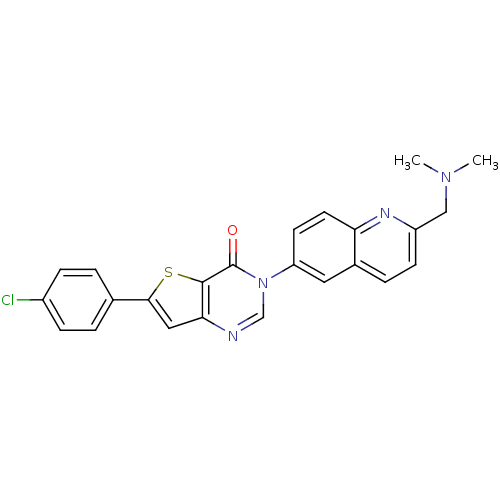
(6-(4-chlorophenyl)-3-{2-[(dimethylamino)methyl]-6-...)Show SMILES CN(C)Cc1ccc2cc(ccc2n1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H19ClN4OS/c1-28(2)13-18-8-5-16-11-19(9-10-20(16)27-18)29-14-26-21-12-22(31-23(21)24(29)30)15-3-6-17(25)7-4-15/h3-12,14H,13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7095-107 (2006)
Article DOI: 10.1021/jm060572f
BindingDB Entry DOI: 10.7270/Q2P26XS8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199251
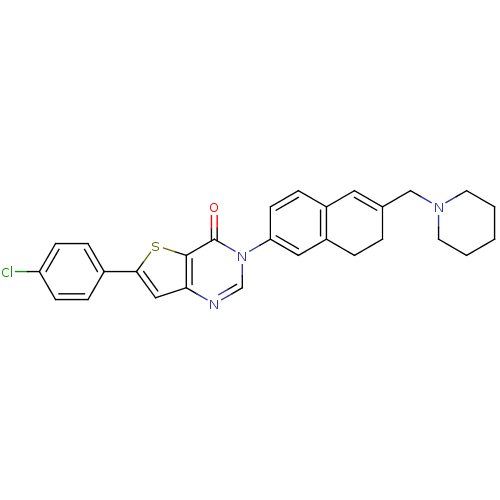
(6-(4-chlorophenyl)-3-[6-(1-piperidinylmethyl)-7,8-...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4C=C(CN5CCCCC5)CCc4c3)c(=O)c2s1 |t:18| Show InChI InChI=1S/C28H26ClN3OS/c29-23-9-6-20(7-10-23)26-16-25-27(34-26)28(33)32(18-30-25)24-11-8-21-14-19(4-5-22(21)15-24)17-31-12-2-1-3-13-31/h6-11,14-16,18H,1-5,12-13,17H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199249
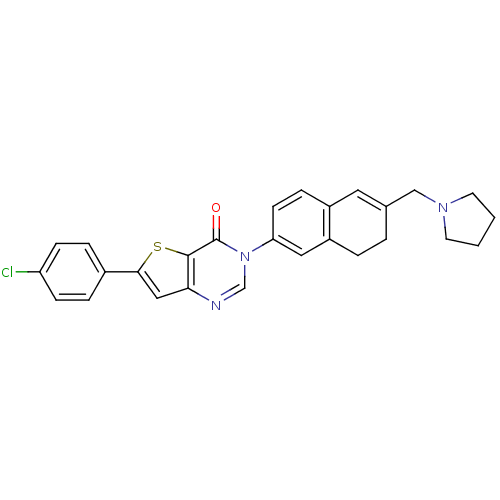
(6-(4-chlorophenyl)-3-[6-(1-pyrrolidinylmethyl)-7,8...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4C=C(CN5CCCC5)CCc4c3)c(=O)c2s1 |t:18| Show InChI InChI=1S/C27H24ClN3OS/c28-22-8-5-19(6-9-22)25-15-24-26(33-25)27(32)31(17-29-24)23-10-7-20-13-18(3-4-21(20)14-23)16-30-11-1-2-12-30/h5-10,13-15,17H,1-4,11-12,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199202
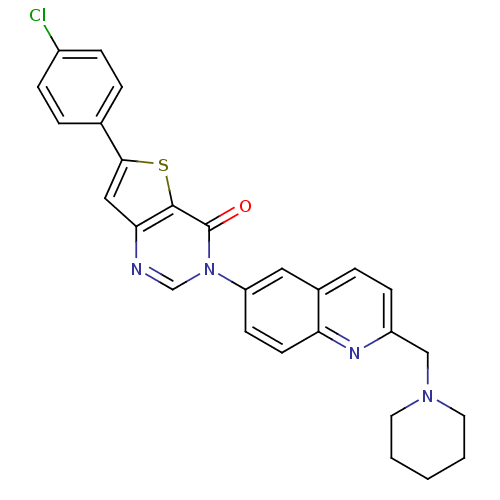
(6-(4-chlorophenyl)-3-[2-(1-piperidinylmethyl)-6-qu...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4nc(CN5CCCCC5)ccc4c3)c(=O)c2s1 Show InChI InChI=1S/C27H23ClN4OS/c28-20-7-4-18(5-8-20)25-15-24-26(34-25)27(33)32(17-29-24)22-10-11-23-19(14-22)6-9-21(30-23)16-31-12-2-1-3-13-31/h4-11,14-15,17H,1-3,12-13,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7095-107 (2006)
Article DOI: 10.1021/jm060572f
BindingDB Entry DOI: 10.7270/Q2P26XS8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199254
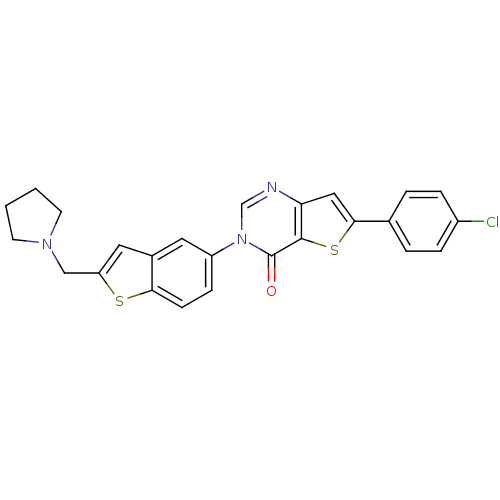
(6-(4-chlorophenyl)-3-[2-(1-pyrrolidinylmethyl)-1-b...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4sc(CN5CCCC5)cc4c3)c(=O)c2s1 Show InChI InChI=1S/C25H20ClN3OS2/c26-18-5-3-16(4-6-18)23-13-21-24(32-23)25(30)29(15-27-21)19-7-8-22-17(11-19)12-20(31-22)14-28-9-1-2-10-28/h3-8,11-13,15H,1-2,9-10,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199219
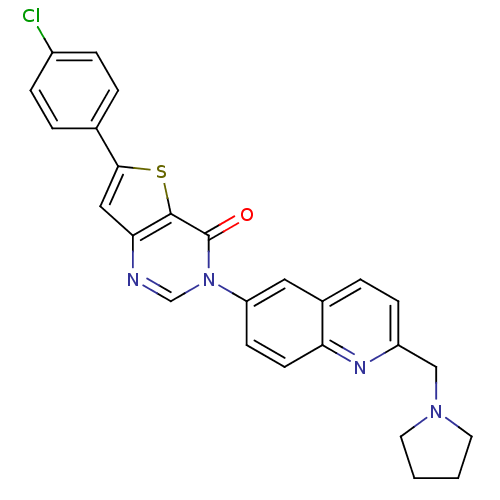
(6-(4-chlorophenyl)-3-[2-(1-pyrrolidinylmethyl)-6-q...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4nc(CN5CCCC5)ccc4c3)c(=O)c2s1 Show InChI InChI=1S/C26H21ClN4OS/c27-19-6-3-17(4-7-19)24-14-23-25(33-24)26(32)31(16-28-23)21-9-10-22-18(13-21)5-8-20(29-22)15-30-11-1-2-12-30/h3-10,13-14,16H,1-2,11-12,15H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7095-107 (2006)
Article DOI: 10.1021/jm060572f
BindingDB Entry DOI: 10.7270/Q2P26XS8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50422940

(CHEMBL213008)Show SMILES COc1ccc(cc1)-c1cc2ncn(-c3ccc(OCCN4CCCC4)c(OC)c3)c(=O)c2s1 Show InChI InChI=1S/C26H27N3O4S/c1-31-20-8-5-18(6-9-20)24-16-21-25(34-24)26(30)29(17-27-21)19-7-10-22(23(15-19)32-2)33-14-13-28-11-3-4-12-28/h5-10,15-17H,3-4,11-14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 16: 4723-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.008
BindingDB Entry DOI: 10.7270/Q24B32MB |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199248

(6-(4-methylphenyl)-3-[2-(pyrrolidin-1-ylmethyl)-1-...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4oc(CN5CCCC5)cc4c3)c(=O)c2s1 Show InChI InChI=1S/C25H20ClN3O2S/c26-18-5-3-16(4-6-18)23-13-21-24(32-23)25(30)29(15-27-21)19-7-8-22-17(11-19)12-20(31-22)14-28-9-1-2-10-28/h3-8,11-13,15H,1-2,9-10,14H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199258
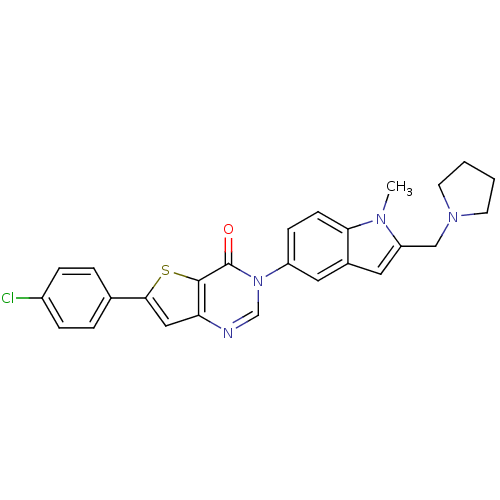
(6-(4-chlorophenyl)-3-[1-methyl-2-(pyrrolidin-1-ylm...)Show SMILES Cn1c(CN2CCCC2)cc2cc(ccc12)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H23ClN4OS/c1-29-21(15-30-10-2-3-11-30)13-18-12-20(8-9-23(18)29)31-16-28-22-14-24(33-25(22)26(31)32)17-4-6-19(27)7-5-17/h4-9,12-14,16H,2-3,10-11,15H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199256

(6-(4-chlorophenyl)-3-{6-[(dimethylamino)methyl]-5,...)Show SMILES CN(C)CC1CCc2cc(ccc2C1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClN3OS/c1-28(2)14-16-3-4-19-12-21(10-7-18(19)11-16)29-15-27-22-13-23(31-24(22)25(29)30)17-5-8-20(26)9-6-17/h5-10,12-13,15-16H,3-4,11,14H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199253

(6-(4-chlorophenyl)-3-[6-(1-pyrrolidinylmethyl)-5,6...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4CC(CN5CCCC5)CCc4c3)c(=O)c2s1 Show InChI InChI=1S/C27H26ClN3OS/c28-22-8-5-19(6-9-22)25-15-24-26(33-25)27(32)31(17-29-24)23-10-7-20-13-18(3-4-21(20)14-23)16-30-11-1-2-12-30/h5-10,14-15,17-18H,1-4,11-13,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199229
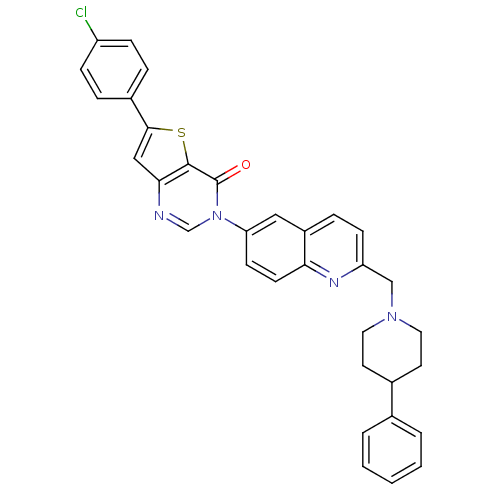
(6-(4-chlorophenyl)-3-{2-[(4-phenyl-1-piperidinyl)m...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4nc(CN5CCC(CC5)c5ccccc5)ccc4c3)c(=O)c2s1 Show InChI InChI=1S/C33H27ClN4OS/c34-26-9-6-24(7-10-26)31-19-30-32(40-31)33(39)38(21-35-30)28-12-13-29-25(18-28)8-11-27(36-29)20-37-16-14-23(15-17-37)22-4-2-1-3-5-22/h1-13,18-19,21,23H,14-17,20H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7095-107 (2006)
Article DOI: 10.1021/jm060572f
BindingDB Entry DOI: 10.7270/Q2P26XS8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50422936
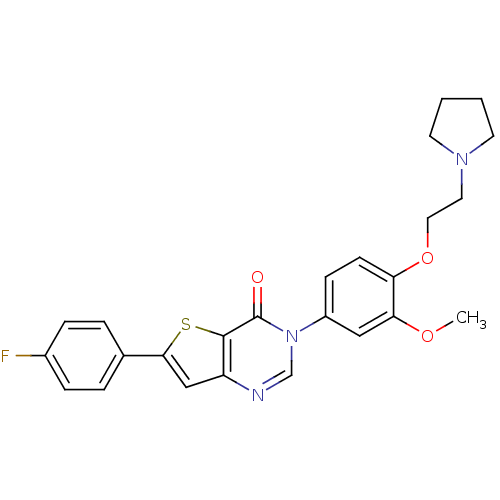
(CHEMBL212657)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnc2cc(sc2c1=O)-c1ccc(F)cc1 Show InChI InChI=1S/C25H24FN3O3S/c1-31-22-14-19(8-9-21(22)32-13-12-28-10-2-3-11-28)29-16-27-20-15-23(33-24(20)25(29)30)17-4-6-18(26)7-5-17/h4-9,14-16H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 16: 4723-7 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.008
BindingDB Entry DOI: 10.7270/Q24B32MB |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199260
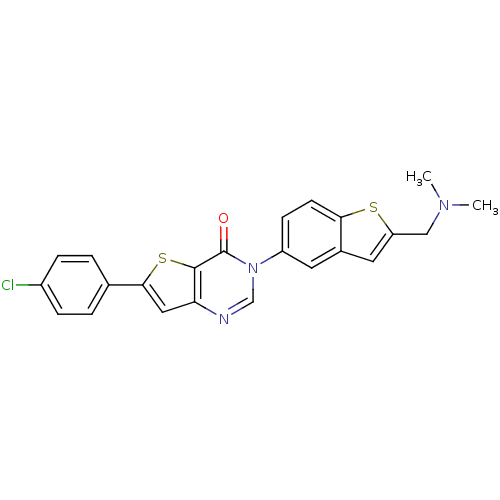
(6-(4-chlorophenyl)-3-{2-[(dimethylamino)methyl]-1-...)Show SMILES CN(C)Cc1cc2cc(ccc2s1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H18ClN3OS2/c1-26(2)12-18-10-15-9-17(7-8-20(15)29-18)27-13-25-19-11-21(30-22(19)23(27)28)14-3-5-16(24)6-4-14/h3-11,13H,12H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199265

(6-(4-chlorophenyl)-3-(2-{[(2R)-2-(methoxymethyl)py...)Show SMILES COC[C@H]1CCCN1Cc1cc2cc(ccc2n1C)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H27ClN4O2S/c1-31-23(15-32-11-3-4-22(32)16-35-2)13-19-12-21(9-10-25(19)31)33-17-30-24-14-26(36-27(24)28(33)34)18-5-7-20(29)8-6-18/h5-10,12-14,17,22H,3-4,11,15-16H2,1-2H3/t22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCH1 receptor stably-expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7108-18 (2006)
Article DOI: 10.1021/jm060814b
BindingDB Entry DOI: 10.7270/Q2JD4WFT |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50199223
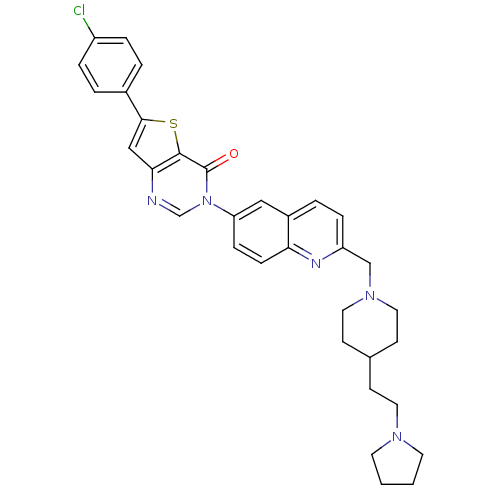
(6-(4-chlorophenyl)-3-[2-({4-[2-(1-pyrrolidinyl)eth...)Show SMILES Clc1ccc(cc1)-c1cc2ncn(-c3ccc4nc(CN5CCC(CCN6CCCC6)CC5)ccc4c3)c(=O)c2s1 Show InChI InChI=1S/C33H34ClN5OS/c34-26-6-3-24(4-7-26)31-20-30-32(41-31)33(40)39(22-35-30)28-9-10-29-25(19-28)5-8-27(36-29)21-38-17-12-23(13-18-38)11-16-37-14-1-2-15-37/h3-10,19-20,22-23H,1-2,11-18,21H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against human MCHR1 expressed in CHO cells by Gal4/Elk1-Luc reporter assay |
J Med Chem 49: 7095-107 (2006)
Article DOI: 10.1021/jm060572f
BindingDB Entry DOI: 10.7270/Q2P26XS8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50422890
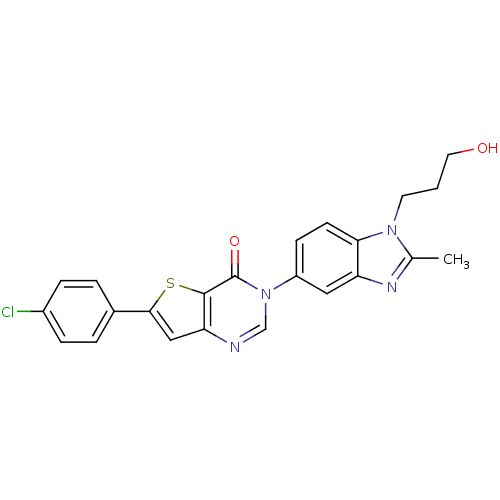
(CHEMBL213813)Show SMILES Cc1nc2cc(ccc2n1CCCO)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C23H19ClN4O2S/c1-14-26-18-11-17(7-8-20(18)27(14)9-2-10-29)28-13-25-19-12-21(31-22(19)23(28)30)15-3-5-16(24)6-4-15/h3-8,11-13,29H,2,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against MCH1R expressed in CHO cells assessed as inhibition of MCH-induced EC80 thrombin response by luciferase reporter gene ass... |
Bioorg Med Chem Lett 16: 4994-5000 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.054
BindingDB Entry DOI: 10.7270/Q2833T97 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50422916
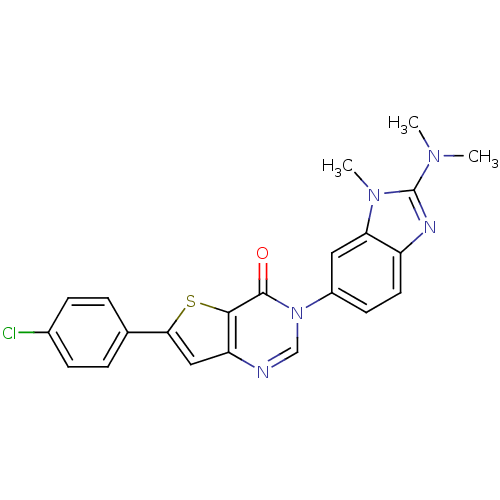
(CHEMBL215643)Show SMILES CN(C)c1nc2ccc(cc2n1C)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H18ClN5OS/c1-26(2)22-25-16-9-8-15(10-18(16)27(22)3)28-12-24-17-11-19(30-20(17)21(28)29)13-4-6-14(23)7-5-13/h4-12H,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity against MCH1R expressed in CHO cells assessed as inhibition of MCH-induced EC80 thrombin response by luciferase reporter gene ass... |
Bioorg Med Chem Lett 16: 4994-5000 (2006)
Article DOI: 10.1016/j.bmcl.2006.07.054
BindingDB Entry DOI: 10.7270/Q2833T97 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data