

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50070824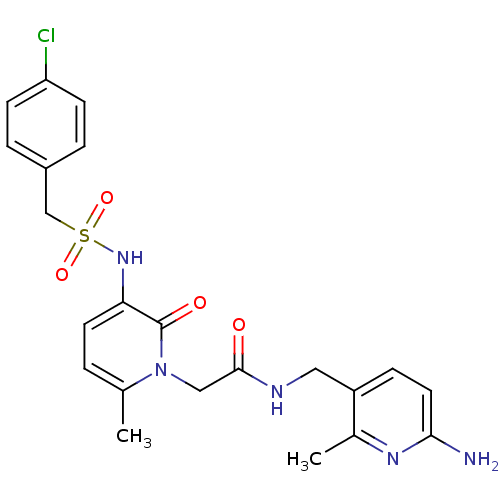 (CHEMBL47920 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060708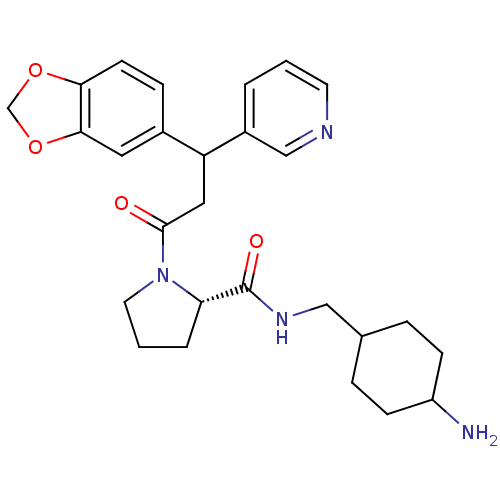 ((S)-1-(3-Benzo[1,3]dioxol-5-yl-3-pyridin-3-yl-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066331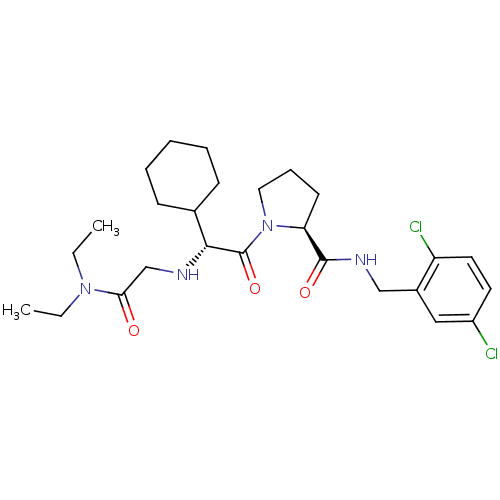 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067795 (CHEMBL138855 | N-(6-Amino-2,4-dimethyl-pyridin-3-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066338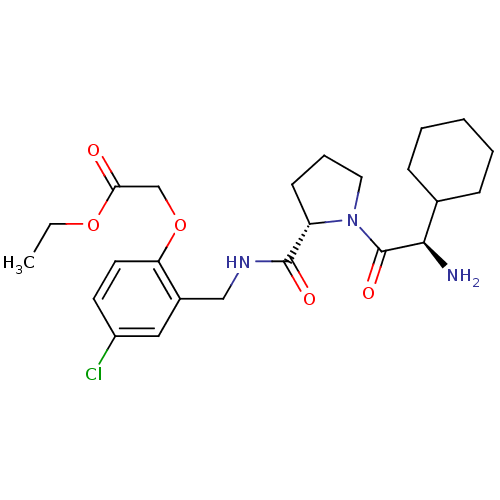 (CHEMBL327115 | [2-({[(S)-1-((R)-2-Amino-2-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796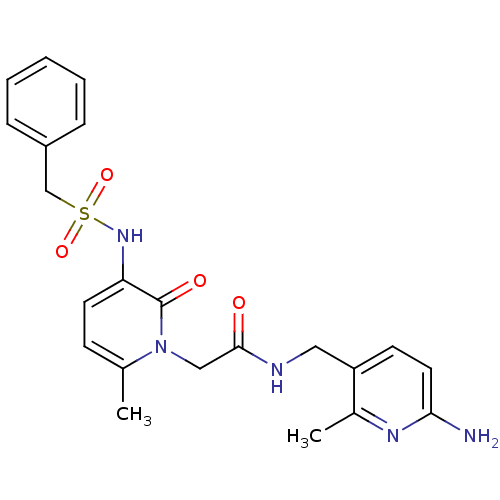 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067796 (CHEMBL11157 | L-374087 | N-((6-amino-2-methylpyrid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066332 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067798 (CHEMBL336438 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066333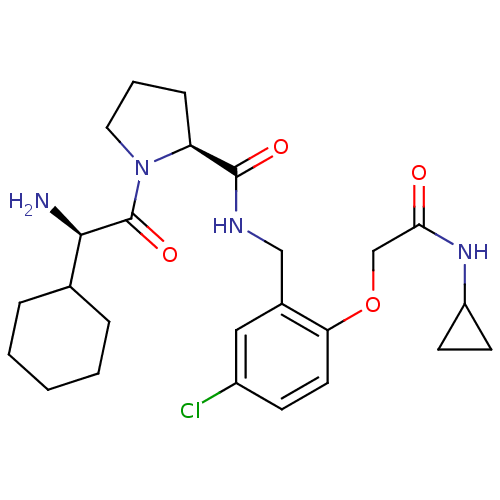 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066334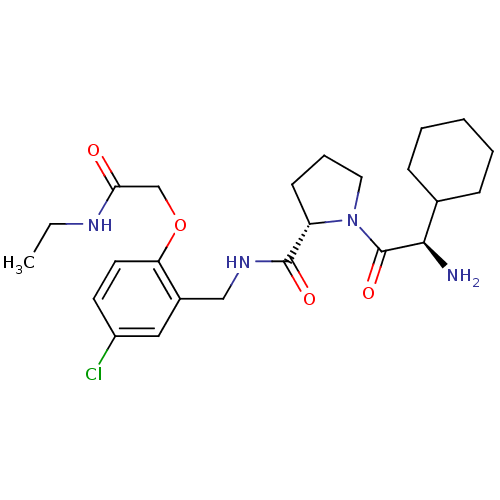 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067797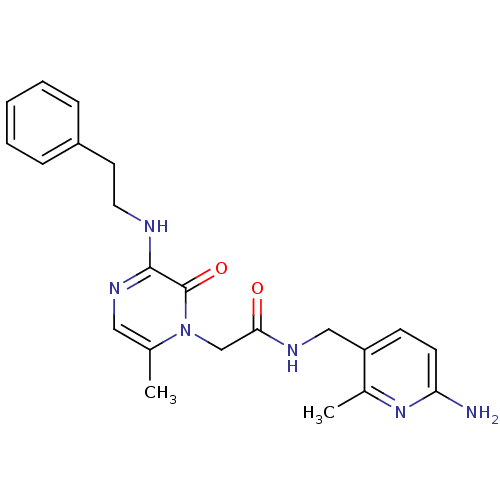 (CHEMBL19080 | L-37378 | N-(6-Amino-2-methyl-pyridi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070823 (CHEMBL45480 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50200045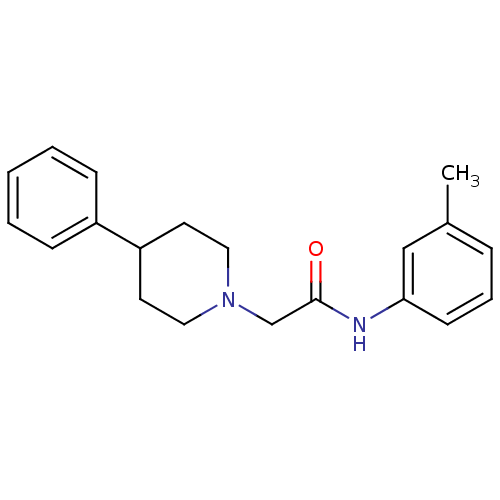 (2-(4-phenylpiperidin-1-yl)-N-(m-tolyl)acetamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523474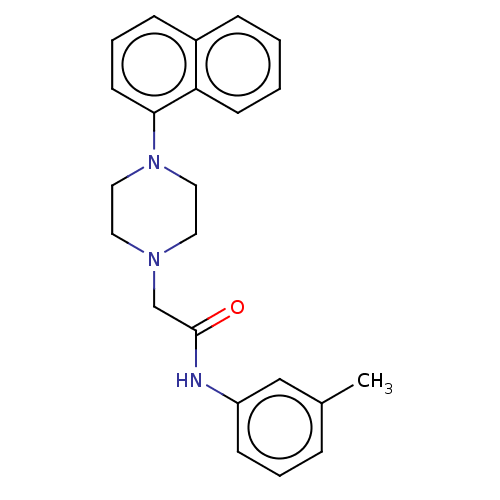 (CHEMBL4473652) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523487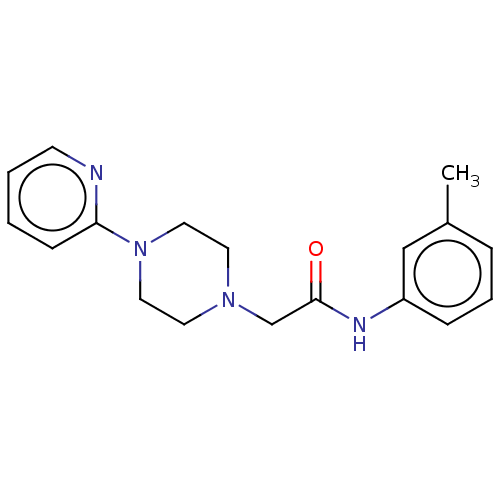 (CHEMBL4448320) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060705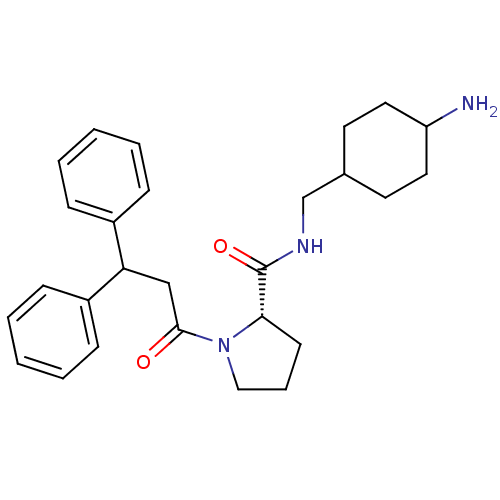 ((S)-1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carbo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523476 (CHEMBL4442548) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066340 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070827 (CHEMBL46135 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060704 ((S)-1-(2-5H-Dibenzo[a,d]cyclohepten-5-yl-acetyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066337 ((S)-1-(2-Amino-3,3-diphenyl-propionyl)-pyrrolidine...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060703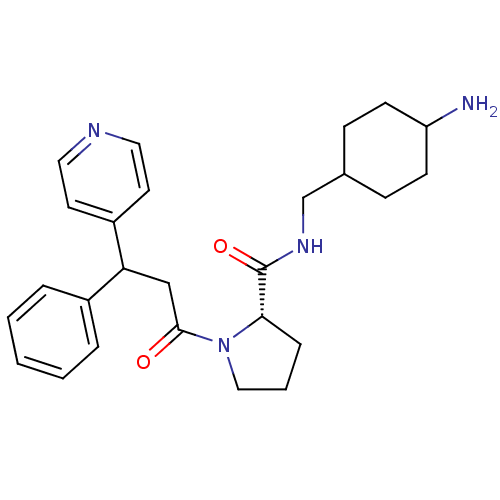 ((S)-1-(3-Phenyl-3-pyridin-4-yl-propionyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | 1.55E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50200037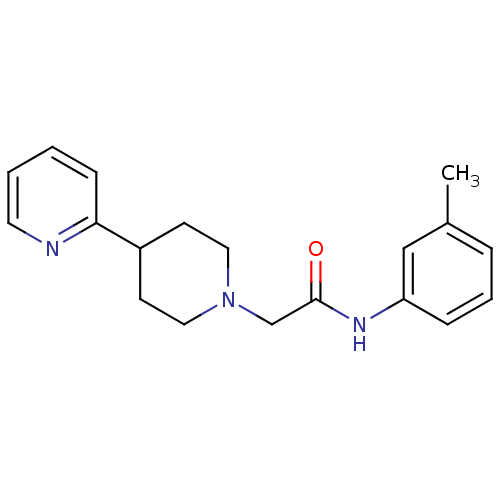 (2-(3',4',-5',6'-tetrahydro-2'H-[2,4'-bipyridine]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070825 (CHEMBL49457 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066336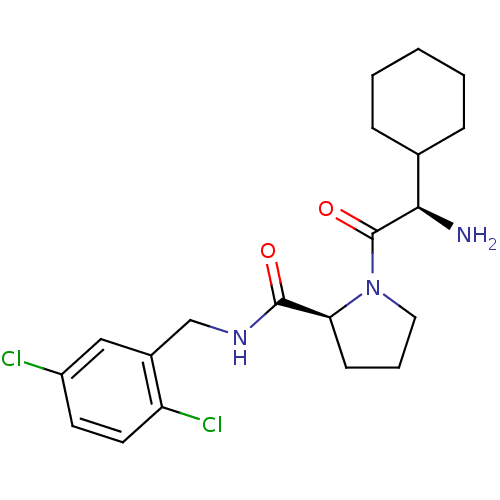 ((S)-1-((R)-2-Amino-2-cyclohexyl-acetyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523471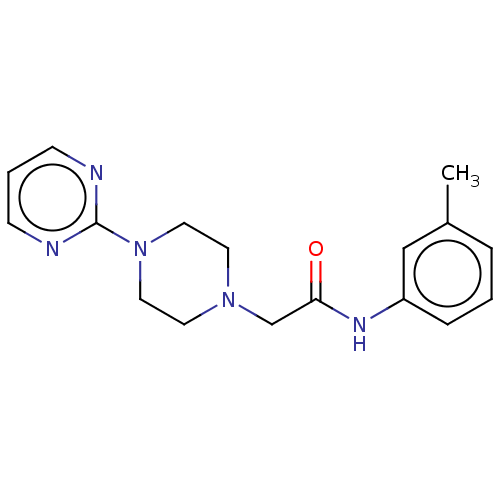 (CHEMBL4443054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523480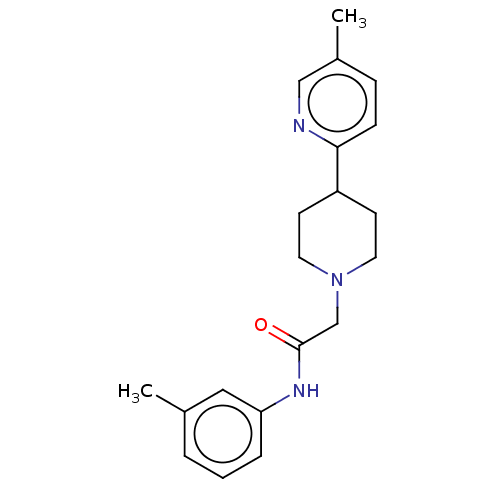 (CHEMBL4587925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067791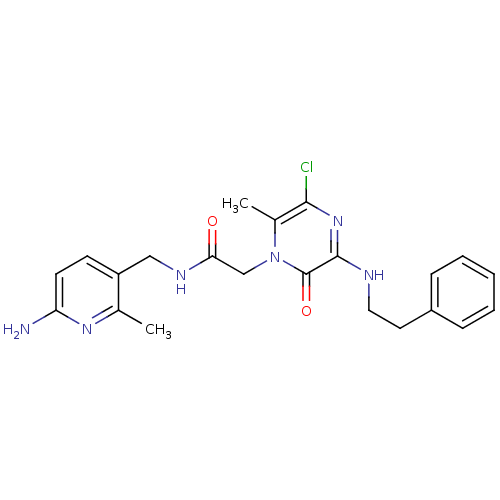 (CHEMBL140885 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060706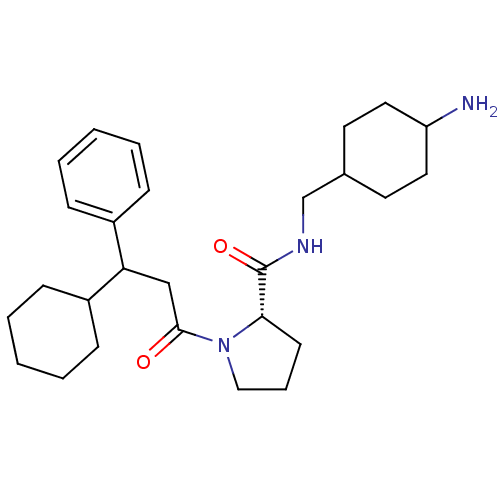 ((S)-1-(3-Cyclohexyl-3-phenyl-propionyl)-pyrrolidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067793 (CHEMBL139331 | N-(6-Amino-pyridin-3-ylmethyl)-2-(6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060707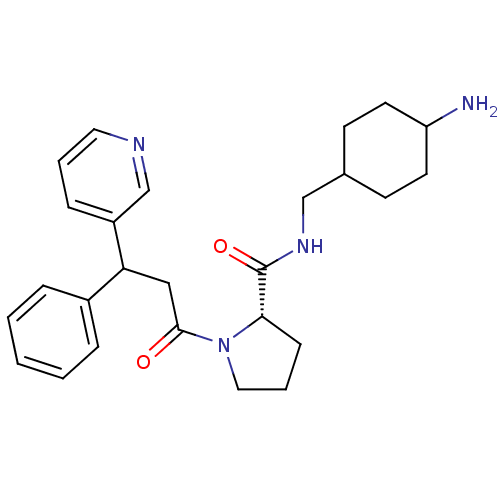 ((S)-1-(3-Phenyl-3-pyridin-3-yl-propionyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | 2.10E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060709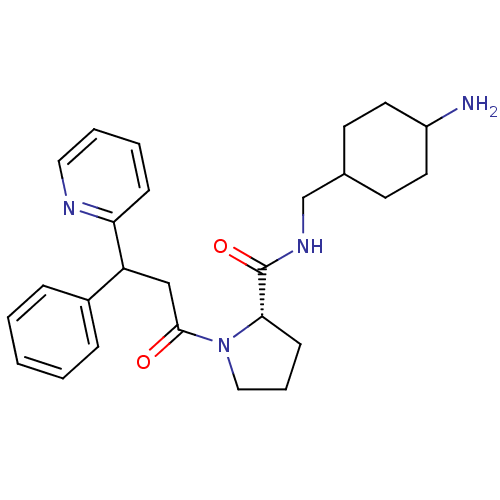 ((S)-1-(3-Phenyl-3-pyridin-2-yl-propionyl)-pyrrolid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070826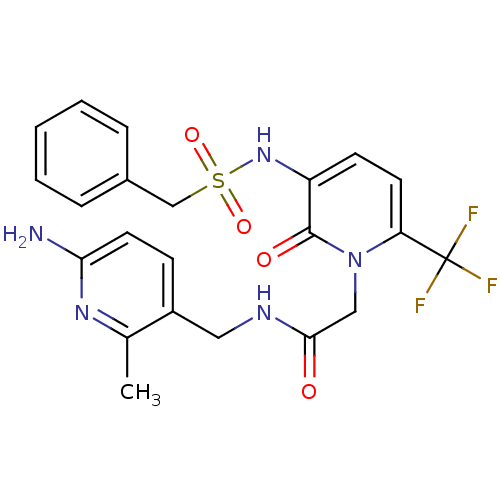 (CHEMBL46331 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523481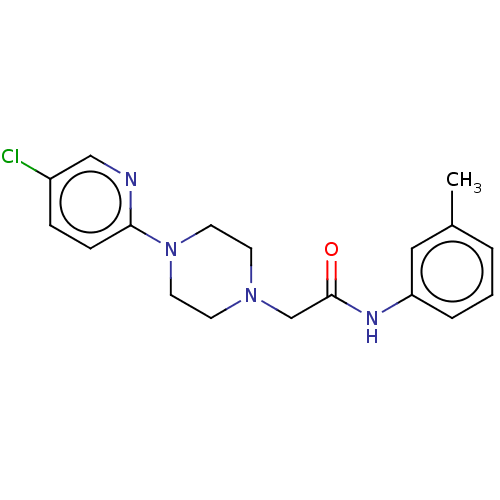 (CHEMBL4572245) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060711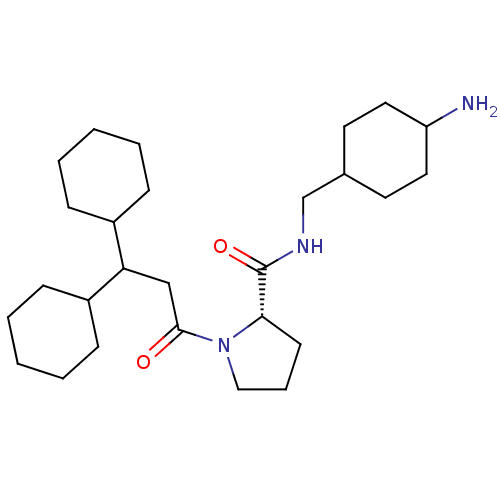 ((S)-1-(3,3-Dicyclohexyl-propionyl)-pyrrolidine-2-c...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50070828 (CHEMBL431535 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060710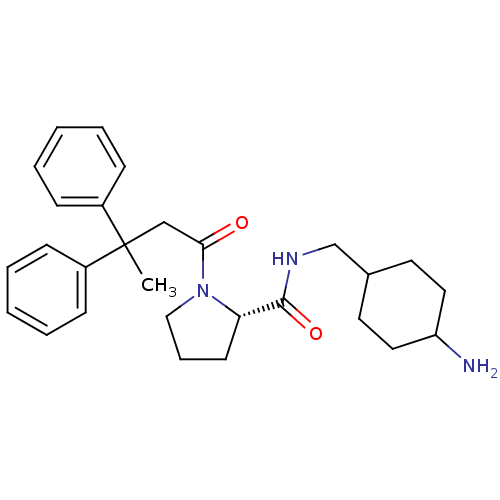 ((S)-1-(3,3-Diphenyl-butyryl)-pyrrolidine-2-carboxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50060712 ((S)-1-(2-10,11-Dihydro-5H-dibenzo[a,d]cyclohepten-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of Thrombin | J Med Chem 40: 3687-93 (1997) Article DOI: 10.1021/jm970397q BindingDB Entry DOI: 10.7270/Q20Z73ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066339 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50066341 ((S)-1-[(R)-2-Cyclohexyl-2-(diethylcarbamoylmethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was evaluated on serine protease thrombin. | J Med Chem 41: 3210-9 (1998) Article DOI: 10.1021/jm9801713 BindingDB Entry DOI: 10.7270/Q24Q7T4G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067794 (CHEMBL139178 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50067792 (CHEMBL141266 | N-(6-Amino-2-methyl-pyridin-3-ylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound against thrombin (IIa) was determined | J Med Chem 41: 4466-74 (1998) Article DOI: 10.1021/jm980368v BindingDB Entry DOI: 10.7270/Q2KK99X0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069192 (CHEMBL48929 | N-(6-Amino-2-methyl-pyridin-3-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated to inhibit the thrombin enzyme | Bioorg Med Chem Lett 8: 1719-24 (1999) BindingDB Entry DOI: 10.7270/Q2319V13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50200045 (2-(4-phenylpiperidin-1-yl)-N-(m-tolyl)acetamide | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D4.4 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523474 (CHEMBL4473652) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D4.4 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523477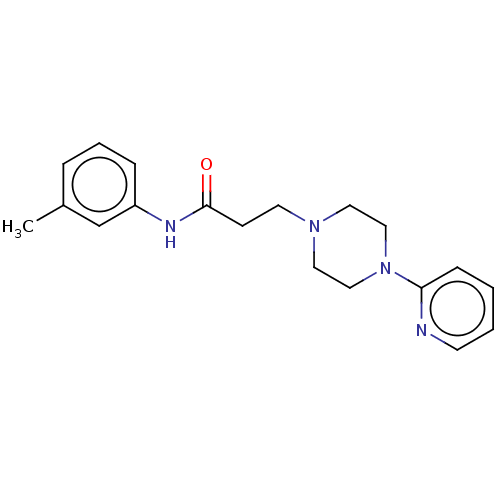 (CHEMBL4454068) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523473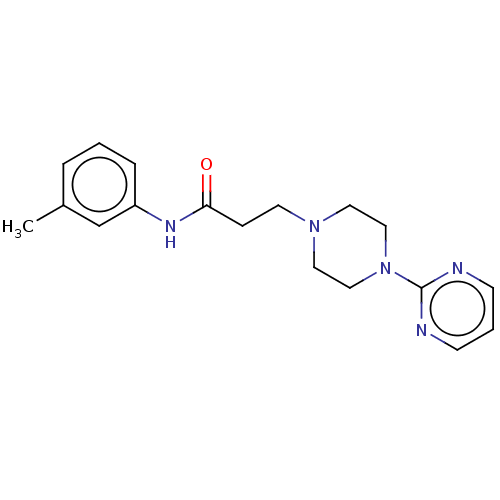 (CHEMBL4588808) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H](R)-(+)-7-OH-DPAT from human D4.4 receptor expressed in HEK293 cell membranes measured after 90 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50523480 (CHEMBL4587925) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rowan University Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D4.4 receptor expressed in HEK293 cell membranes measured after 60 mins by scintillation counting me... | J Med Chem 62: 3722-3740 (2019) Article DOI: 10.1021/acs.jmedchem.9b00231 BindingDB Entry DOI: 10.7270/Q20C505J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50114942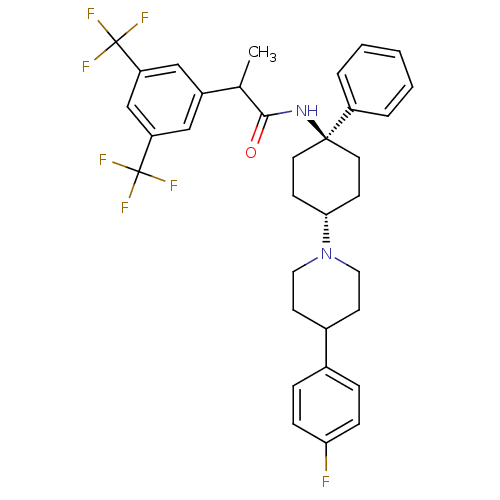 (2-(3,5-Bis-trifluoromethyl-phenyl)-N-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Compound was evaluated for the displacement of labelled MK-499 from the cloned channel expressed in HEK cells (Ikrchannel) | Bioorg Med Chem Lett 12: 1759-62 (2002) BindingDB Entry DOI: 10.7270/Q2P55MV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1221 total ) | Next | Last >> |