

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50163152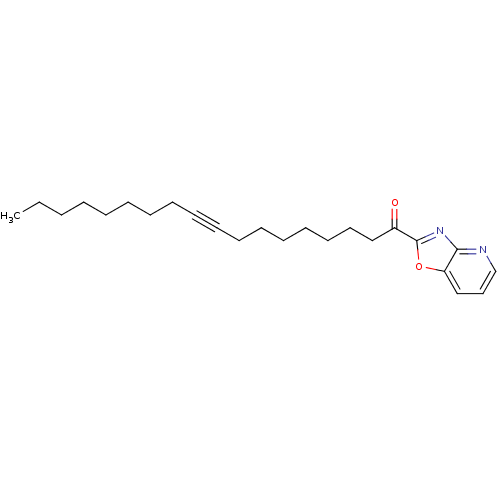 (1-Oxazolo[4,5-b]pyridin-2-yl-octadec-9-yn-1-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM23316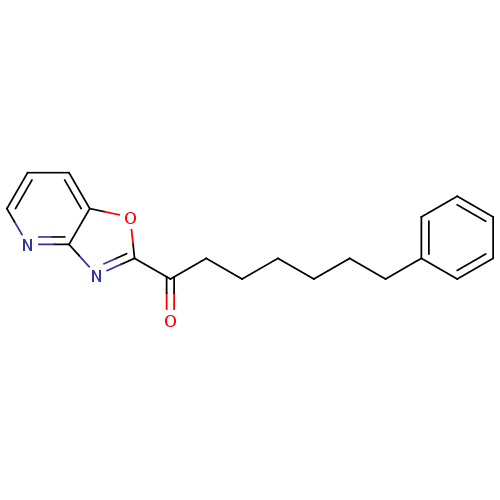 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitory constant determined against recombinant Fatty-acid amide hydrolase from rat expressed in Escherichia coli | J Med Chem 48: 1849-56 (2005) Article DOI: 10.1021/jm049614v BindingDB Entry DOI: 10.7270/Q2Q52P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM23316 (7-phenyl-1-{pyrido[2,3-d][1,3]oxazol-2-yl}heptan-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161528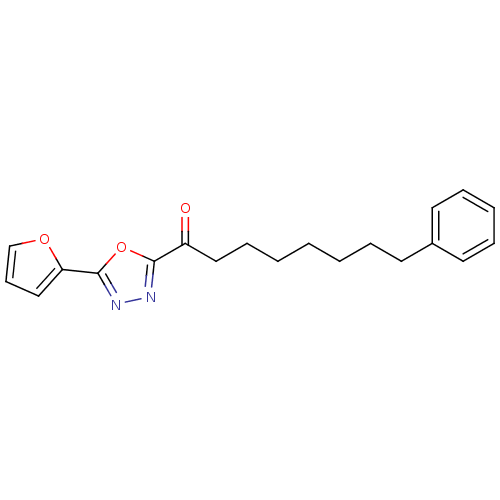 (8-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from alpha4beta2 nicotinic receptor expressed in human HEK293 cells | Bioorg Med Chem Lett 20: 4749-52 (2010) Article DOI: 10.1016/j.bmcl.2010.06.142 BindingDB Entry DOI: 10.7270/Q2QC04GV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169264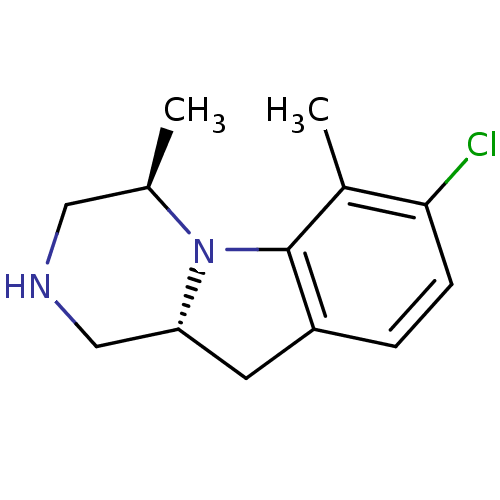 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human recombinant 5HT2C receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 1207-11 (2006) Article DOI: 10.1016/j.bmcl.2005.11.083 BindingDB Entry DOI: 10.7270/Q21R6Q3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50169264 ((4R,10aR)-7-Chloro-4,6-dimethyl-1,2,3,4,10,10a-hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis Research Ltd. Curated by ChEMBL | Assay Description Binding affinity toward 5-HT2C receptor evaluated by displacement of [3H]-5-HT radioligand | Bioorg Med Chem Lett 15: 3604-8 (2005) Article DOI: 10.1016/j.bmcl.2005.05.074 BindingDB Entry DOI: 10.7270/Q2GQ6X87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23079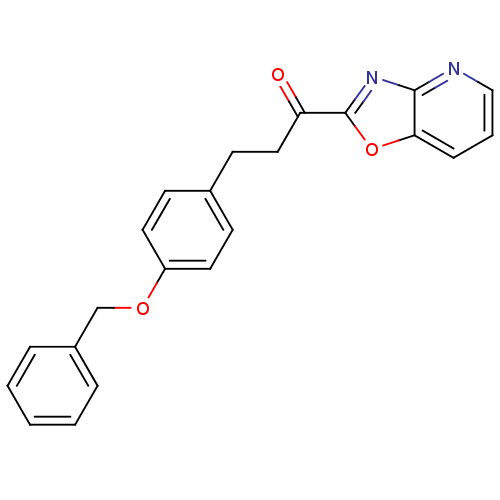 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.380 | -53.8 | 0.300 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161520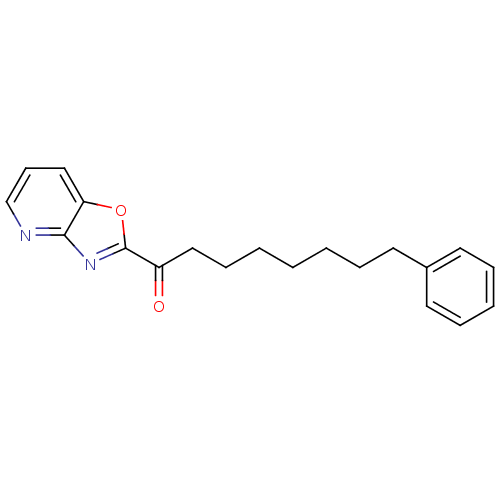 (1-(oxazolo[4,5-b]pyridin-2-yl)-8-phenyloctan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23069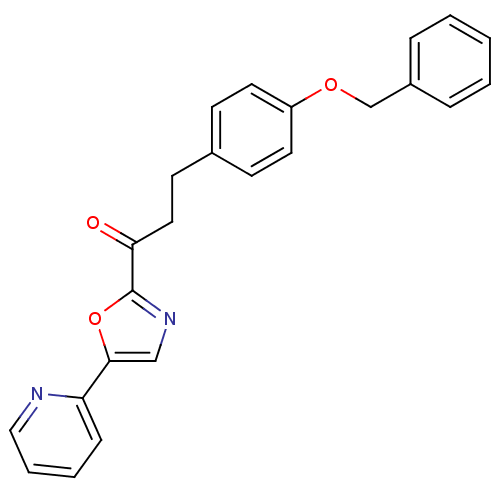 (3-[4-(benzyloxy)phenyl]-1-[5-(pyridin-2-yl)-1,3-ox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | -53.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161525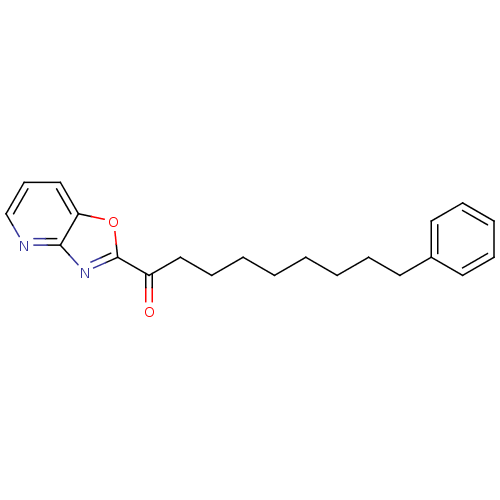 (1-(oxazolo[4,5-b]pyridin-2-yl)-9-phenylnonan-1-one...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161511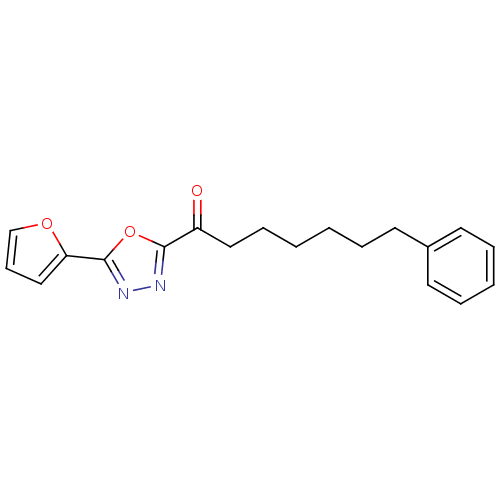 (1-(5-(Furan-2-yl)-1,3,4-oxadiazol-2-yl)-heptan-1-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description In vitro inhibition constant for Aurora-A | J Med Chem 49: 955-70 (2006) Article DOI: 10.1021/jm050786h BindingDB Entry DOI: 10.7270/Q24J0FXV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM23079 (3-[4-(benzyloxy)phenyl]-1-{pyrido[2,3-d][1,3]oxazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23078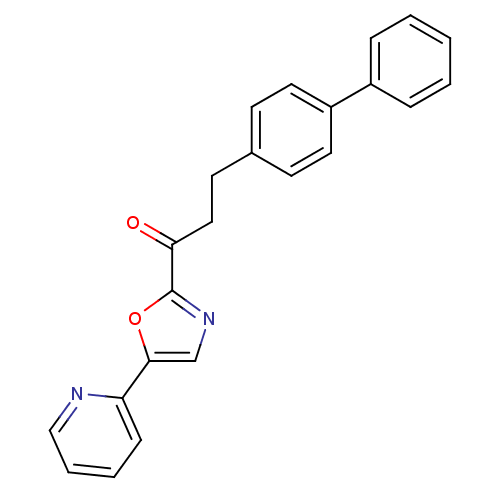 (3-(4-phenylphenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | -52.1 | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50179077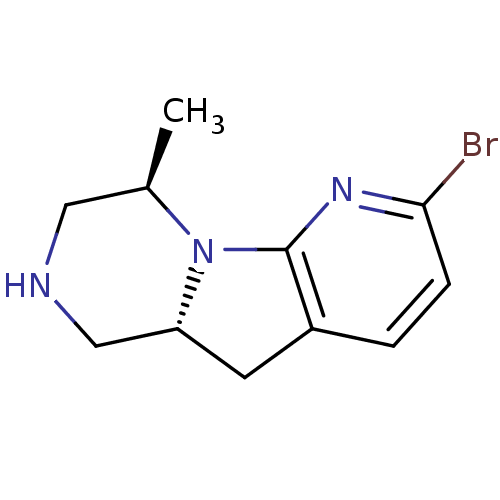 ((4R,9aR)-6-bromo-4-methyl-1,2,3,4,9,9a-hexahydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-5HT from human recombinant 5HT2C receptor expressed in CHO cells | Bioorg Med Chem Lett 16: 1207-11 (2006) Article DOI: 10.1016/j.bmcl.2005.11.083 BindingDB Entry DOI: 10.7270/Q21R6Q3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161513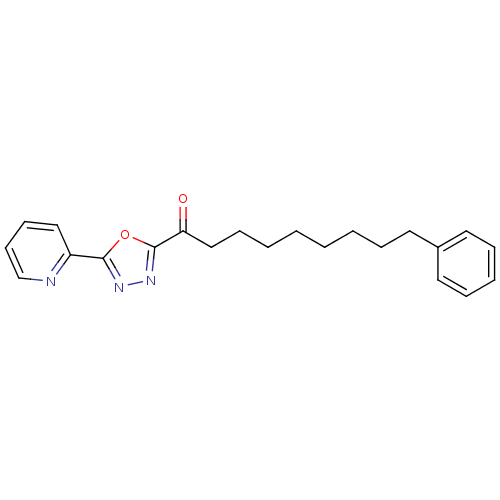 (7-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161513 (7-Phenyl-1-(5-pyridin-2-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23061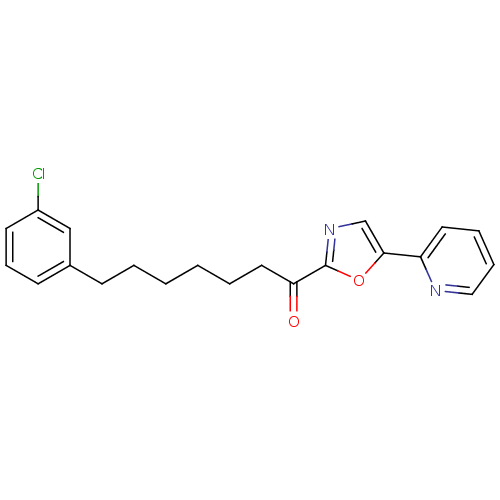 (7-(3-chlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23063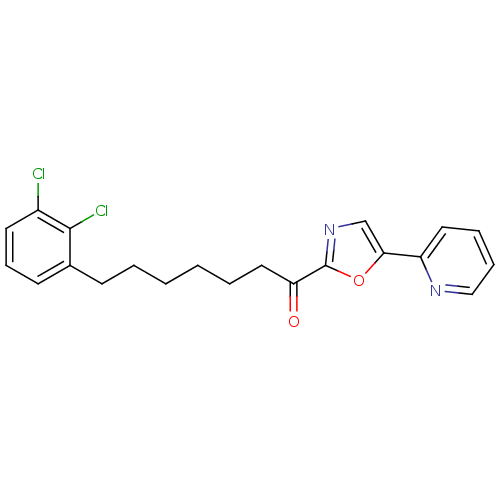 (7-(2,3-dichlorophenyl)-1-[5-(pyridin-2-yl)-1,3-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | -51.6 | 7 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23055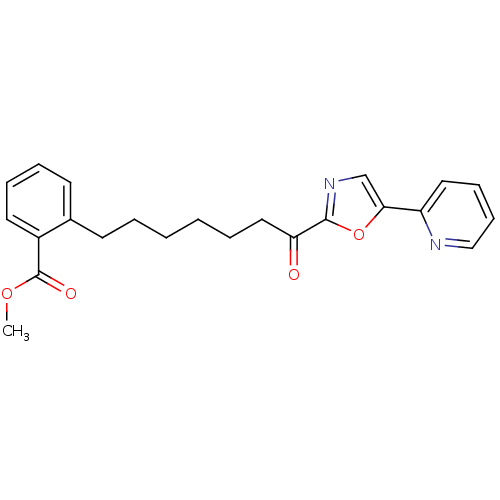 (alpha-ketooxazole, 5aa | methyl 2-{7-oxo-7-[5-(pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 0.400 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23073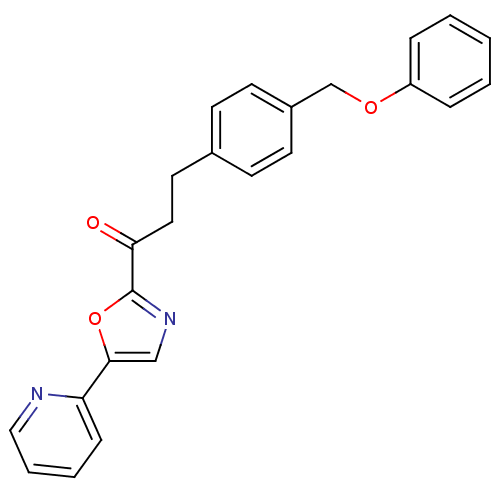 (3-[4-(phenoxymethyl)phenyl]-1-[5-(pyridin-2-yl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -51.4 | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA ligase 1 (Homo sapiens (Human)) | BDBM23053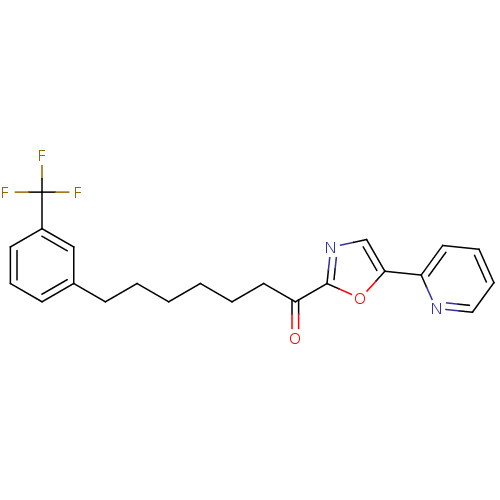 (1-[5-(pyridin-2-yl)-1,3-oxazol-2-yl]-7-[3-(trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -50.9 | 20 | n/a | n/a | n/a | n/a | 9.0 | 22 |
The Scripps Research Institute | Assay Description The inhibition assays were performed by incubating enzyme, 14C-labeled oleamide in reaction buffer at room temperature in the presence of three diffe... | J Med Chem 50: 3359-68 (2007) Article DOI: 10.1021/jm061414r BindingDB Entry DOI: 10.7270/Q2DR2SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26295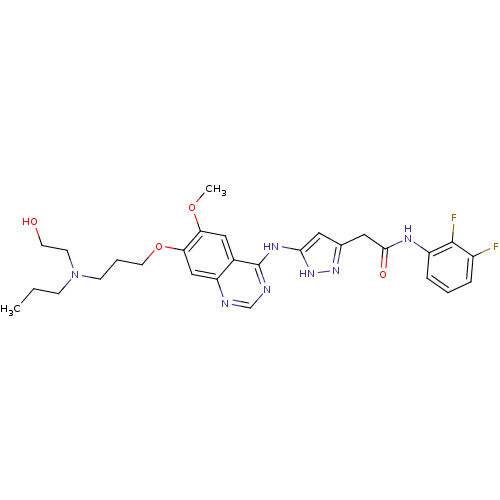 (CHEMBL214849 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Mus musculus) | BDBM50514105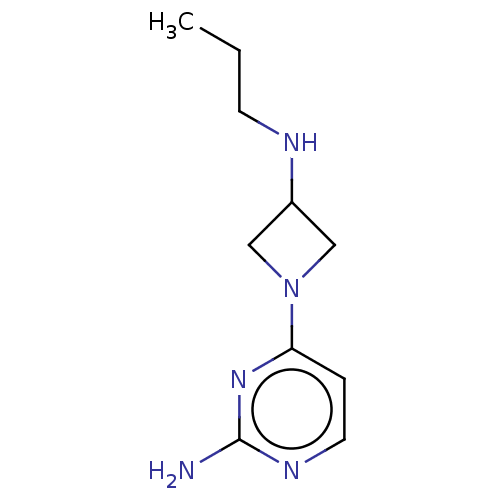 (CHEMBL4441731) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Amsterdam Curated by ChEMBL | Assay Description Displacement of [3H]NAMH from mouse H3R expressed in HEK293T cells incubated for 2 hrs by microbeta scintillation counting analysis | J Med Chem 62: 10848-10866 (2019) Article DOI: 10.1021/acs.jmedchem.9b01462 BindingDB Entry DOI: 10.7270/Q2M61PKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377558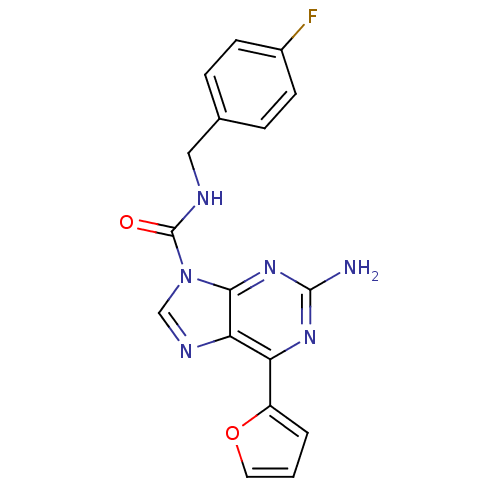 (CHEMBL429144) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377557 (CHEMBL264432) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377556 (CHEMBL411034) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377567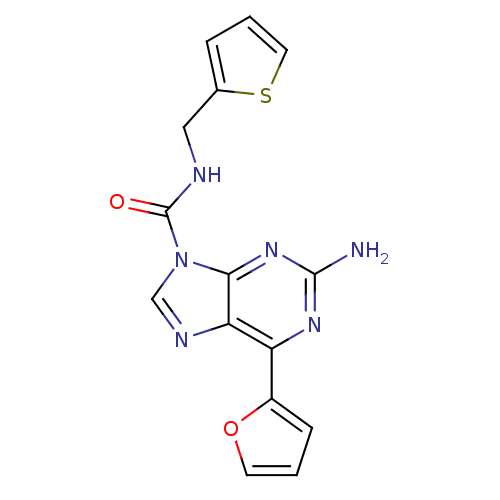 (CHEMBL409915) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377566 (CHEMBL259049) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377543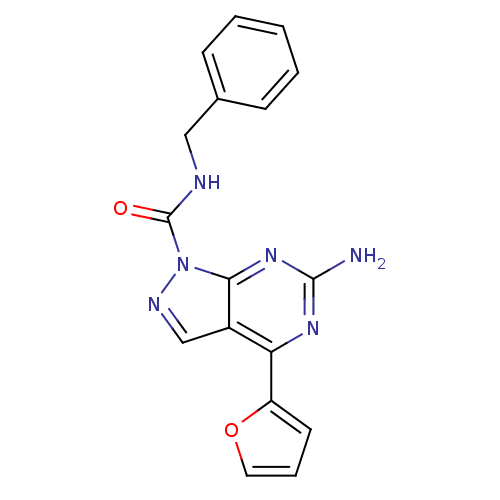 (CHEMBL260146) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50377538 (CHEMBL257757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50239036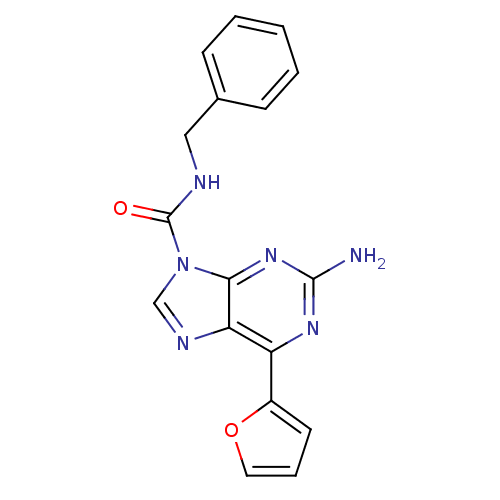 (2-amino-N-benzyl-6-(furan-2-yl)-9H-purine-9-carbox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vernalis (R&D) Ltd Curated by ChEMBL | Assay Description Binding affinity at human adenosine A2A receptor | Bioorg Med Chem Lett 18: 2924-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.072 BindingDB Entry DOI: 10.7270/Q23779KZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161524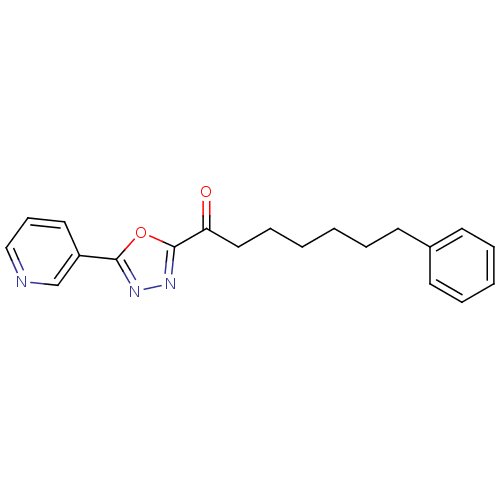 (7-Phenyl-1-(5-pyridin-3-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50161514 (7-Phenyl-1-(5-pyridin-4-yl-[1,3,4]oxadiazol-2-yl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibitor affinity of the compound towards enzymes of class serine hydrolase was determined using biotin or fluorescent as radioligand (FP-biotin or ... | Bioorg Med Chem Lett 15: 1423-8 (2005) Article DOI: 10.1016/j.bmcl.2004.12.085 BindingDB Entry DOI: 10.7270/Q2PK0FNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26296 (CHEMBL216053 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26294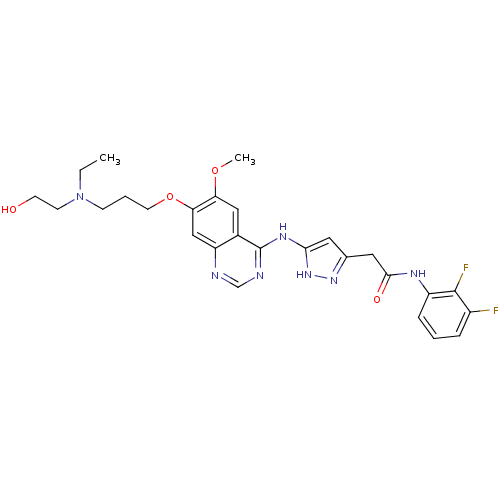 (CHEMBL214848 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26292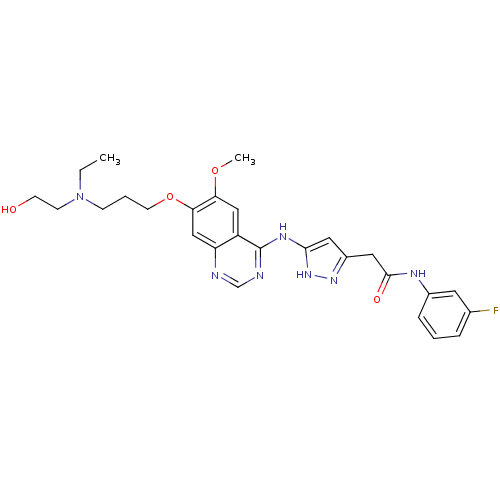 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26291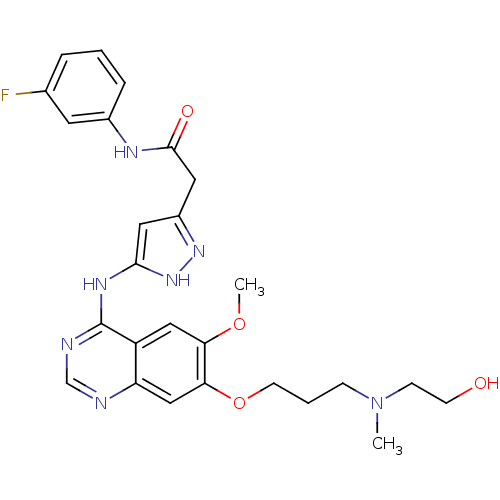 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)(m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26290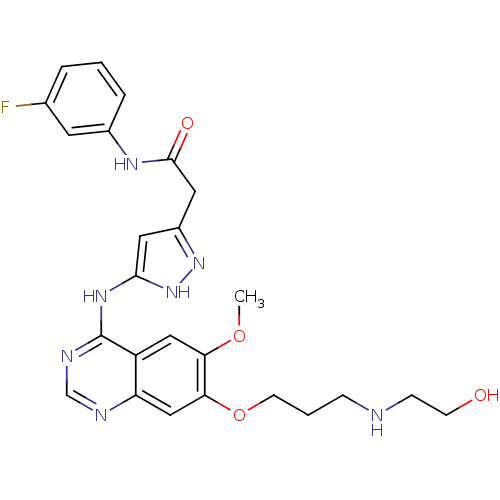 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethyl)am...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26289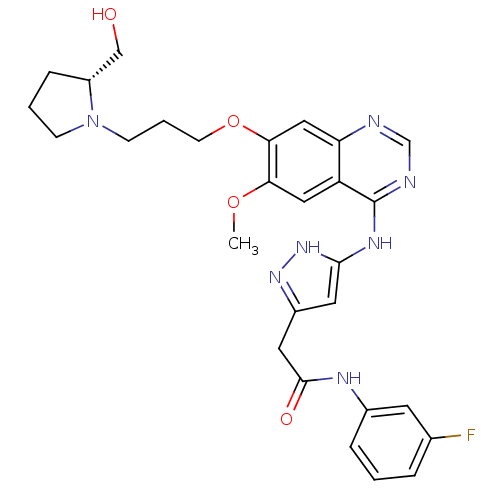 (N-(3-fluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydroxymet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26288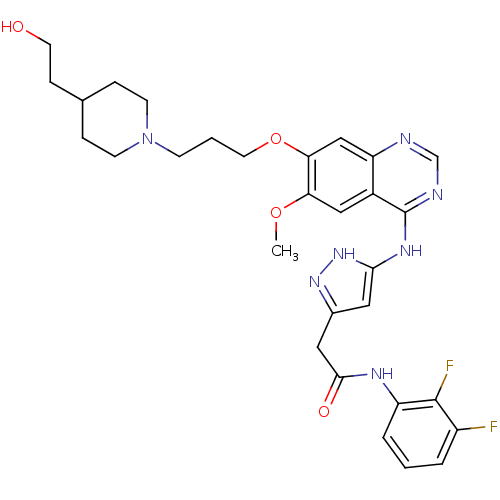 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[4-(2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26287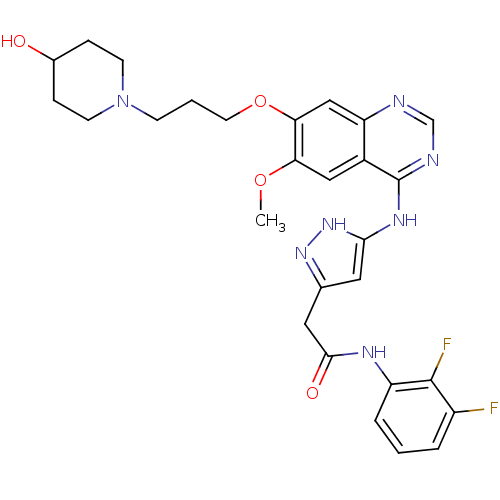 (N-(2,3-difluorophenyl)-2-[3-({7-[3-(4-hydroxypiper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26286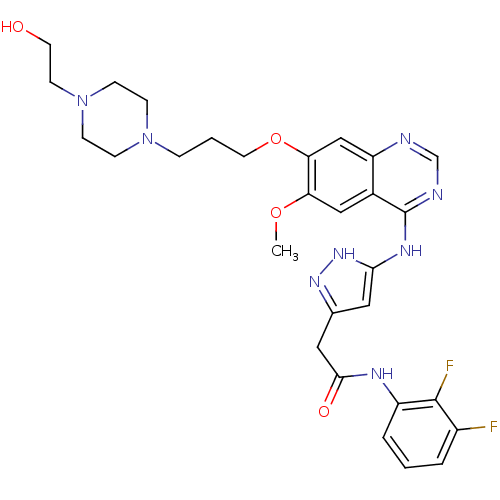 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[4-(2-hydroxyet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26297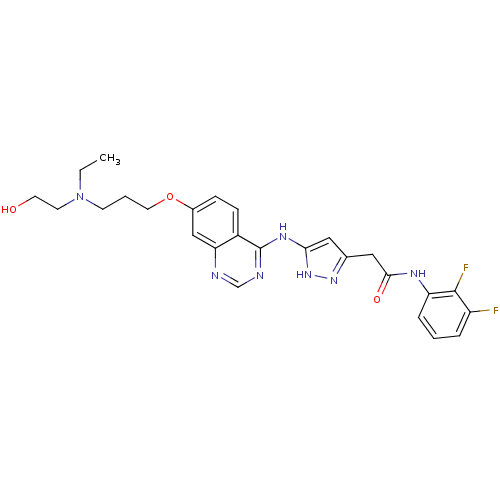 (CHEMBL215322 | N-(2,3-difluorophenyl)-2-{3-[(7-{3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26298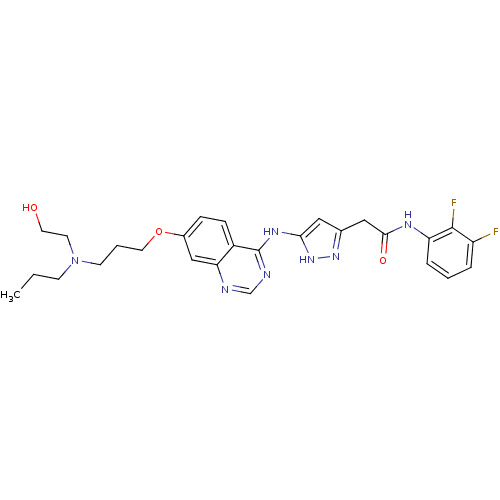 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2-hydroxyethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26299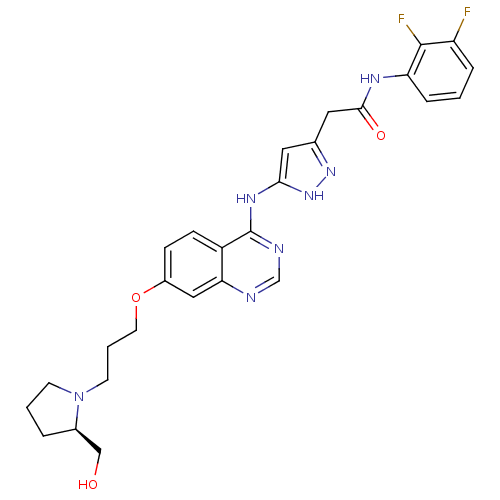 (N-(2,3-difluorophenyl)-2-{3-[(7-{3-[(2R)-2-(hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM26300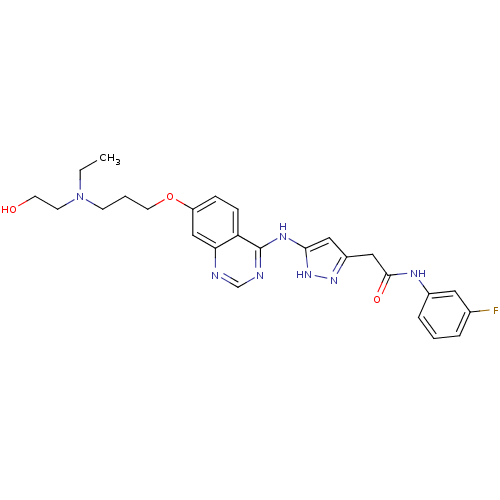 (2-{3-[(7-{3-[ethyl(2-hydroxyethyl)amino]propoxy}qu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AstraZeneca | Assay Description In vitro kinase assay using recombinant Aurora B bound to INCENP was incubated at room temperature with substrate, and test compounds in the presence... | J Med Chem 50: 2213-24 (2007) Article DOI: 10.1021/jm061335f BindingDB Entry DOI: 10.7270/Q2GQ6W24 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2996 total ) | Next | Last >> |