

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (MOUSE) | BDBM50033531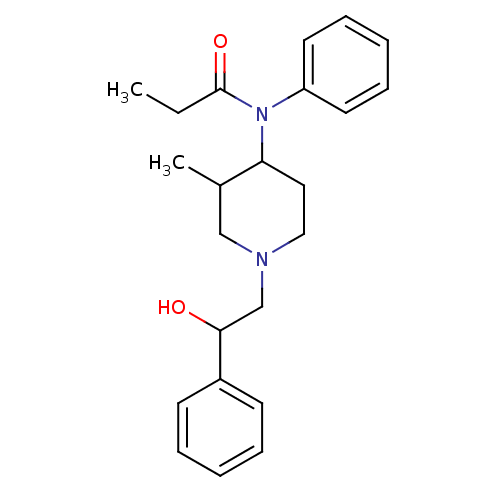 (CHEMBL333410 | N-[1-(2-Hydroxy-2-phenyl-ethyl)-3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Materia Medica Curated by ChEMBL | Assay Description Inhibition against Opioid receptor mu 1 using [3H]- DAMGO radioligand. | J Med Chem 38: 3652-9 (1995) BindingDB Entry DOI: 10.7270/Q2BP01V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M4 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M3 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50103430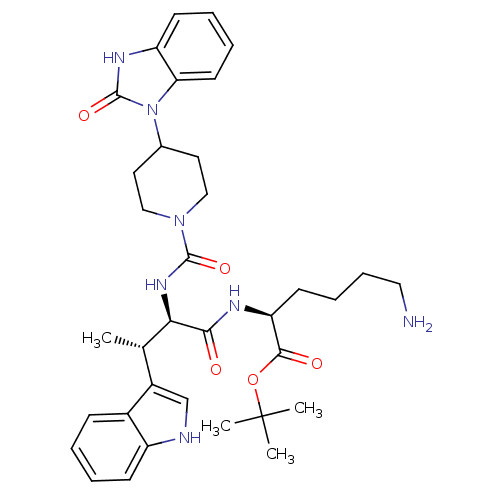 (6-Amino-2-(3-(1H-indol-3-yl)-2-{[4-(2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibition of [125 I -Tyr]SRIF-14 binding to membranes isolated from CHO-K1 cells expressing each of the cloned human SRIF receptor (sst-2) subtypes | J Med Chem 44: 2990-3000 (2001) BindingDB Entry DOI: 10.7270/Q2DB82JT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M1 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030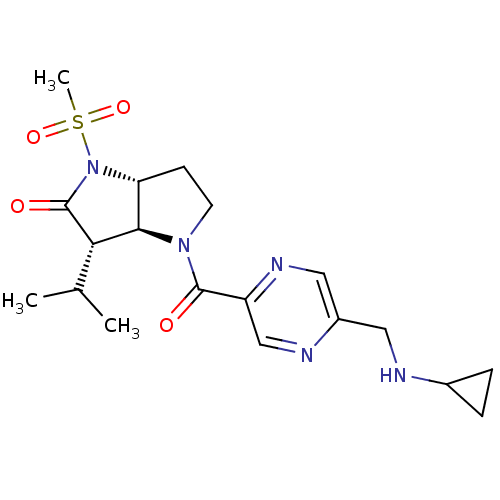 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320811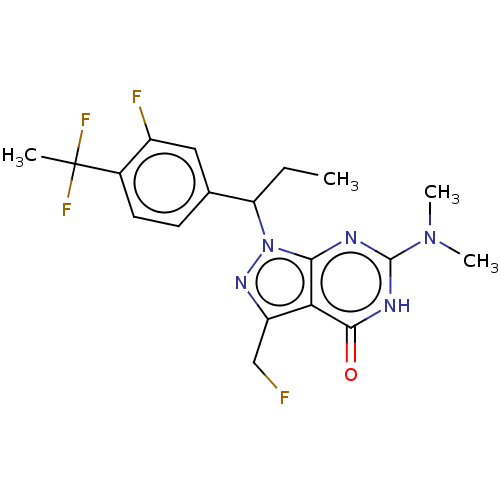 ((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320710 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-3-fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320946 ((R)- or (S)-1- (Cyclopropyl(4-(1,1- difluoroethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Non-receptor tyrosine-protein kinase TYK2 (Homo sapiens (Human)) | BDBM50507816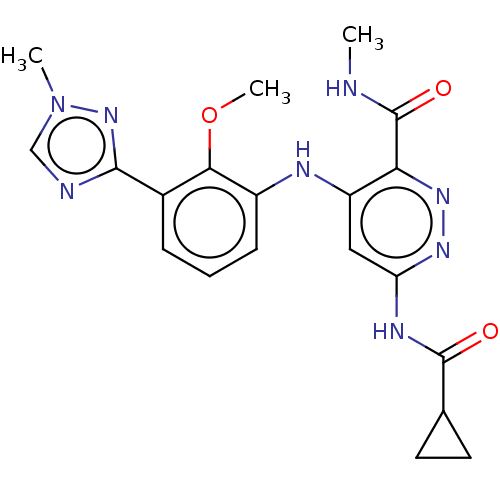 (Bms-986165 | Deucravacitinib) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... | J Med Chem 62: 8973-8995 (2019) Article DOI: 10.1021/acs.jmedchem.9b00444 BindingDB Entry DOI: 10.7270/Q2930XJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M2 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320813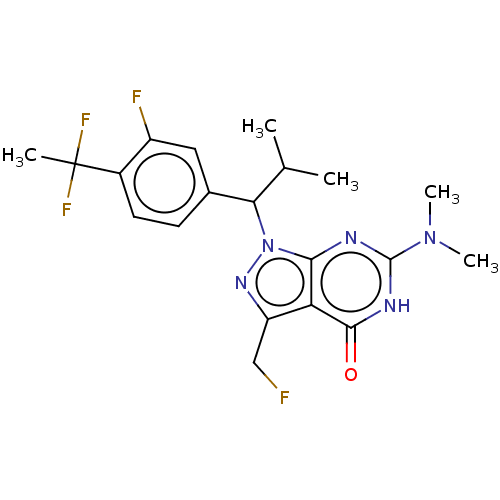 ((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50148931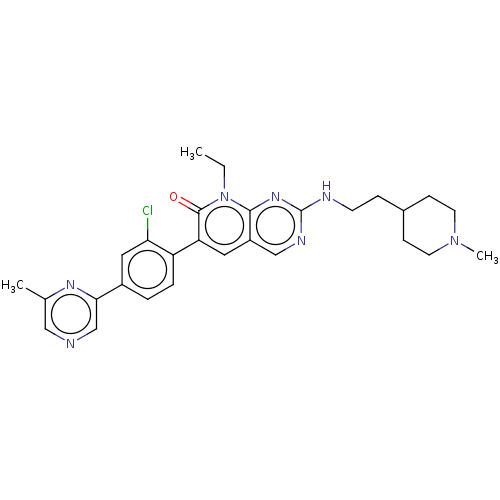 (CHEMBL3770186) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112517 BindingDB Entry DOI: 10.7270/Q2Q243W7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320712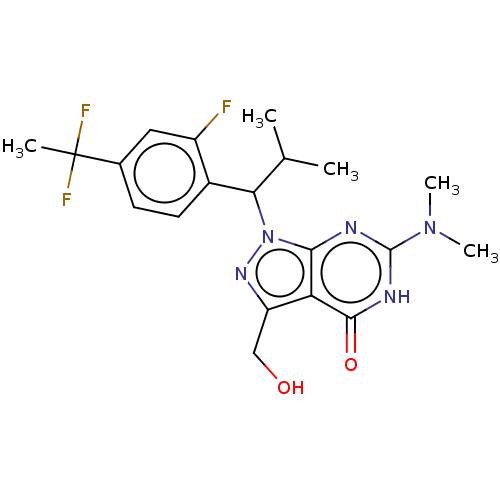 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-2-fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341575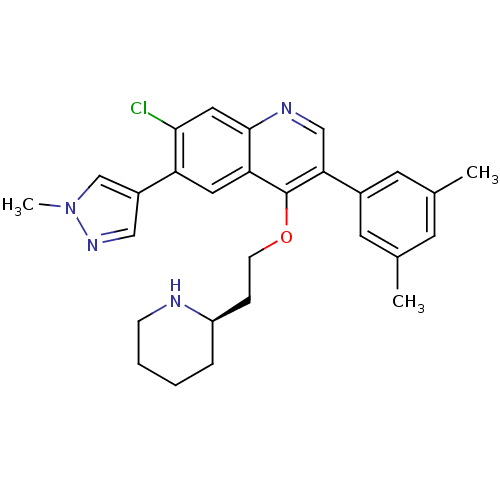 (7-Chloro-3-(3,5-dimethylphenyl)-6-(1-methyl-1H-pyr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320710 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-3-fluorop...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341578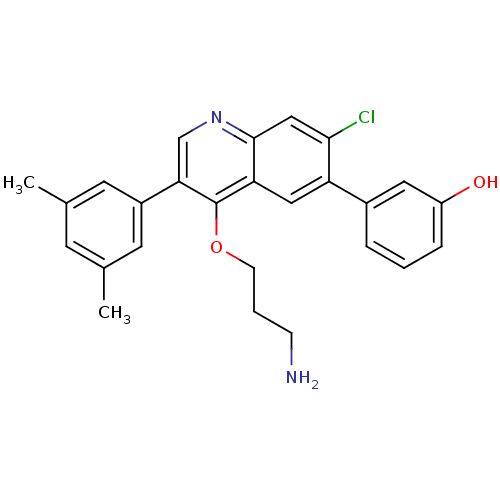 (3-[4-(3-Aminopropoxy)-7-chloro-3-(3,5-dimethylphen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50296345 ((1R,2R,4S,5R,7S)-7-(2-hydroxy-2,2-di-thiophen-2-yl...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0266 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human recombinant M5 receptor expressed in CHO cells after 24 hrs by filter binding assay | J Med Chem 54: 6888-904 (2011) Article DOI: 10.1021/jm200884j BindingDB Entry DOI: 10.7270/Q2CZ37JW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320811 ((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM430 (3-[(2-tert-butyl-4-hydroxy-5-methylphenyl)sulfanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 6.2 | 37 |
Parke-Davis Pharmaceutical Research | Assay Description For determination of IC50 values, HIV-1 protease was added to assay buffer containing inhibitor and the substrate (H-His-Lys-Ala-Arg-Val-Leu- (p-NO2)... | Bioorg Med Chem 7: 2775-800 (1999) Article DOI: 10.1016/s0968-0896(99)00215-1 BindingDB Entry DOI: 10.7270/Q21C1V2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320813 ((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-3- fluorop...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320944 ((R)- or (S)-1-(1-(4- (difluoromethyl)-2- fluorophe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320801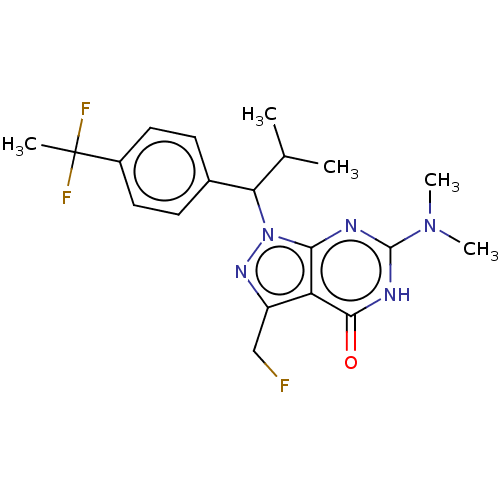 ((R)- or (S)-l-(1-(4-(1,1- Difluoroethyl)phenyl)-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50412728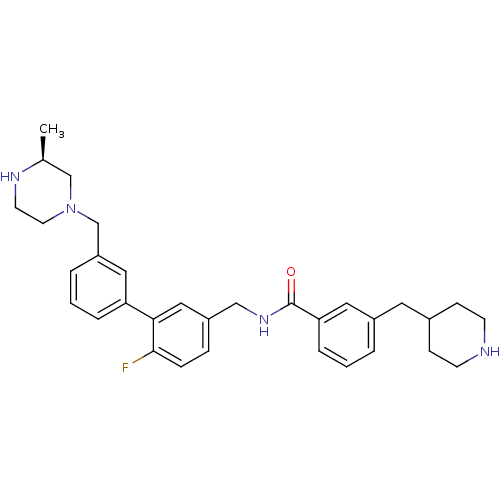 (CHEMBL521523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]N-methyl Scopolamine from human cloned muscarinic M3 receptor expressed in CHO cells by scintillation proximity assay | J Med Chem 51: 5915-8 (2008) Article DOI: 10.1021/jm800935u BindingDB Entry DOI: 10.7270/Q21G0NHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320712 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)-2-fluorop...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341572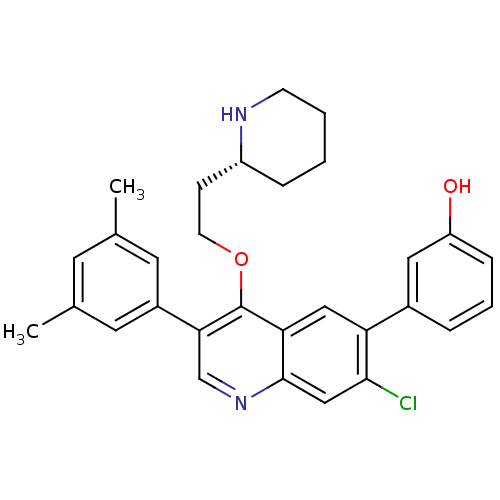 (3-[7-Chloro-3-(3,5-dimethylphenyl)-4-{2-[(2R)-pipe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320799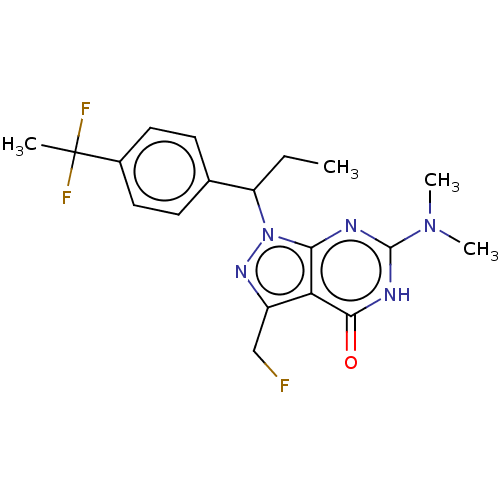 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)phenyl)pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 2 (Homo sapiens (Human)) | BDBM50341574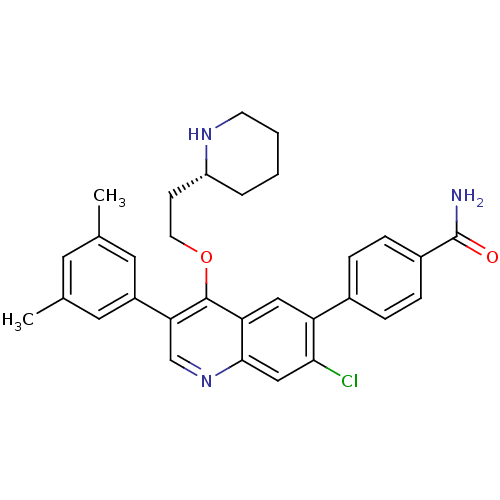 (3-{7-Chloro-3-(3,5-dimethylphenyl)-4-[2-(piperidin...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of I125-somatostatin-14 from human SST2 expressed in CHO cells after 3 hrs by scintillation counting | J Med Chem 54: 2351-8 (2011) Article DOI: 10.1021/jm101501b BindingDB Entry DOI: 10.7270/Q2668DH8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17428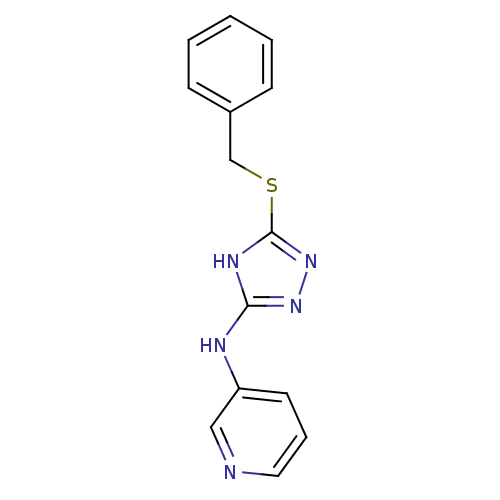 (1,2,4-Triazole Compound, 86 | N-[5-(benzylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM17355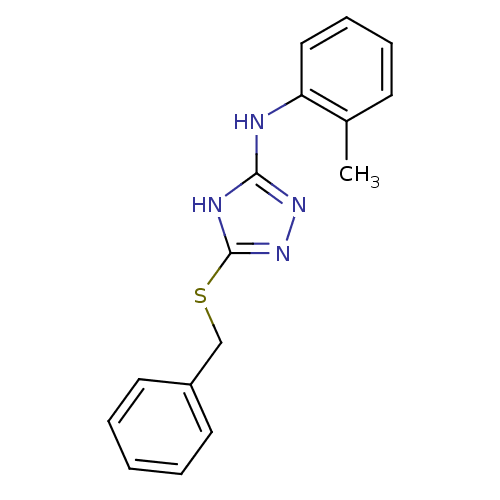 (1,2,4-Triazole Compound, 13 | 5-(benzylsulfanyl)-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
GSK | Assay Description MetAP2 activity was monitored by measuring the initial velocity of turnover of the artificial substrate Met-AMC. Assays were performed in 96-well mi... | J Med Chem 50: 3777-85 (2007) Article DOI: 10.1021/jm061182w BindingDB Entry DOI: 10.7270/Q2B856D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM357210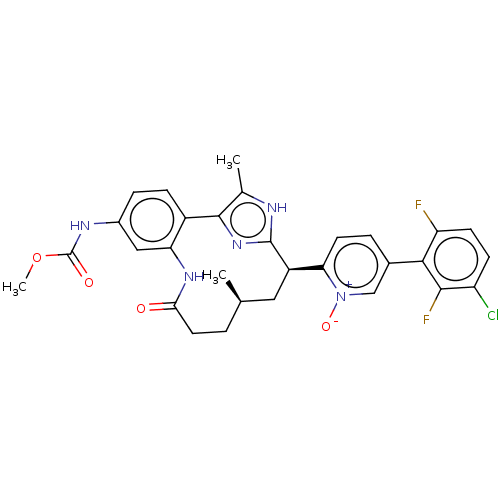 (US10214512, Example 151-a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10214512 (2019) BindingDB Entry DOI: 10.7270/Q2668GGN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320726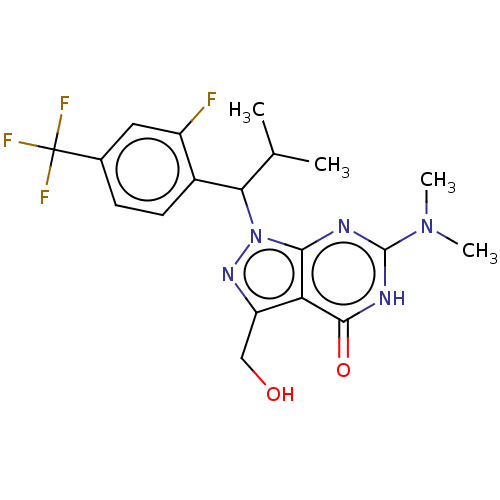 ((R)- or (S)-6-(Dimethylamino)- 1-(1-(2-fluoro-4- (...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320732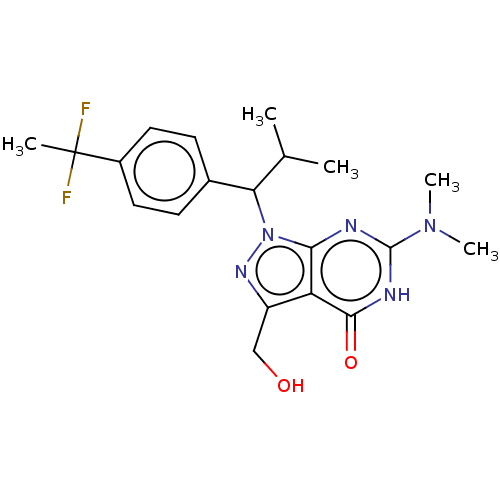 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)phenyl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320795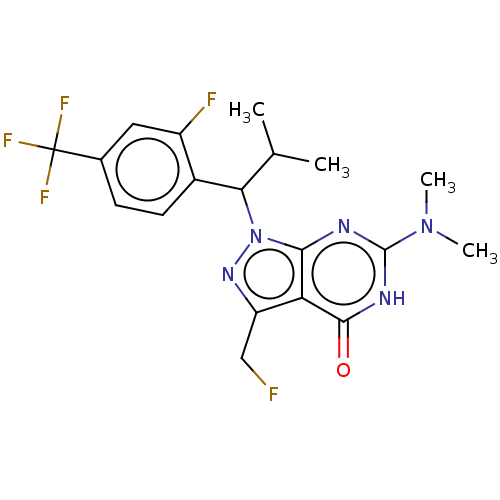 ((R)- or (S)-6-(Dimethylamino)-1-(1-(2- fluoro-4-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545960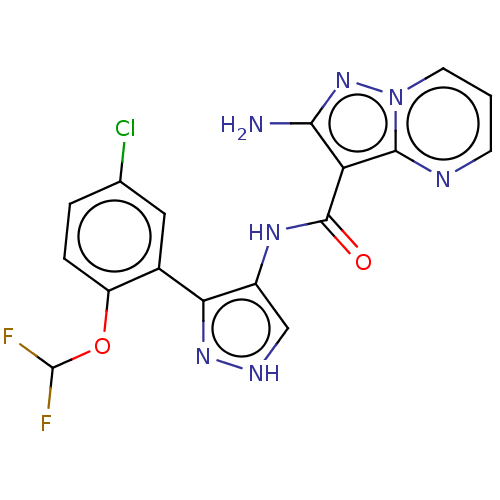 (CHEMBL4740778 | US11649241, Example 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320797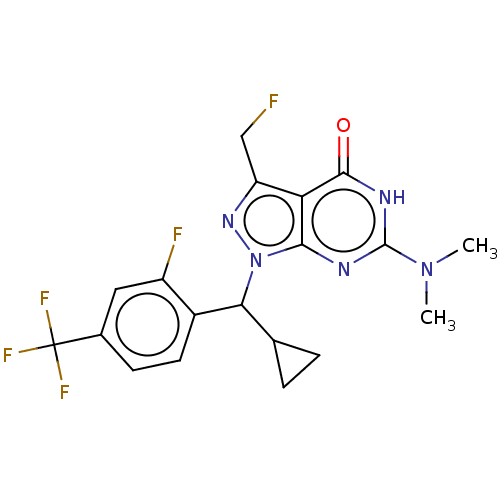 ((R)- or (S)-1-(Cyclopropyl(2-fluoro-4- (trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphodiesterase (Macaca mulatta (Rhesus macaque)) | BDBM320801 ((R)- or (S)-l-(1-(4-(1,1- Difluoroethyl)phenyl)-2-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320670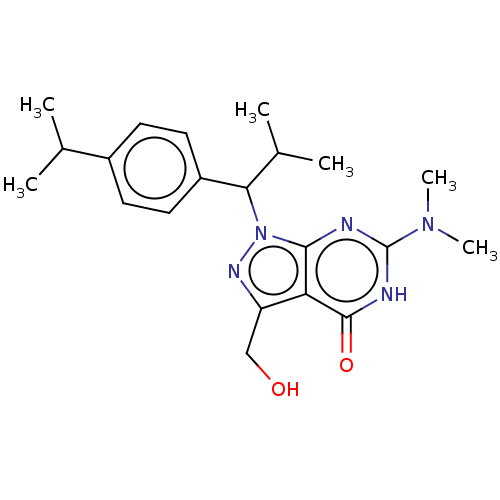 ((R)- or (S)-6-(Dimethylamino)-3- (hydroxymethyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320817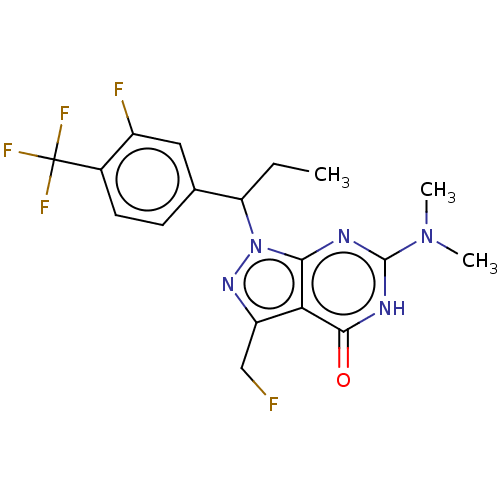 ((R)- or (S)-6-(Dimethylamino)-1-(1-(3- fluoro-4-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320809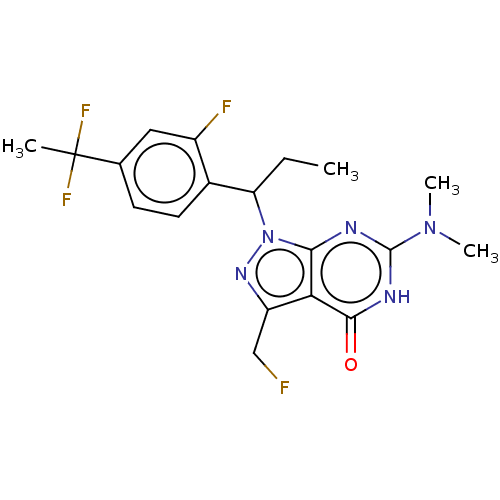 ((R)- or (S)-1-(1-(4-(1,1-Difluoroethyl)-2- fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545980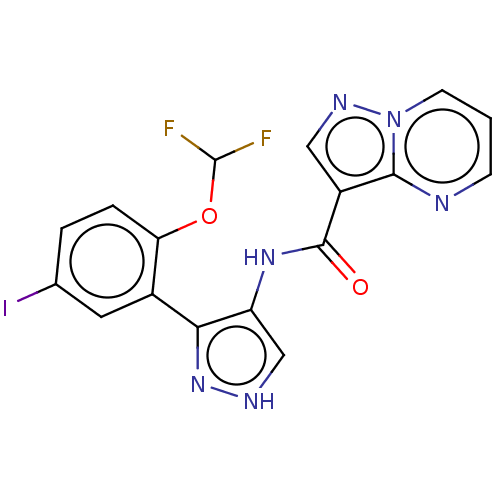 (CHEMBL4764019) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320815 ((R)- or (S)-6-(Dimethylamino)-1-(1-(3- fluoro-4-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane | Bioorg Med Chem Lett 19: 1682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.099 BindingDB Entry DOI: 10.7270/Q2HD7VJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320770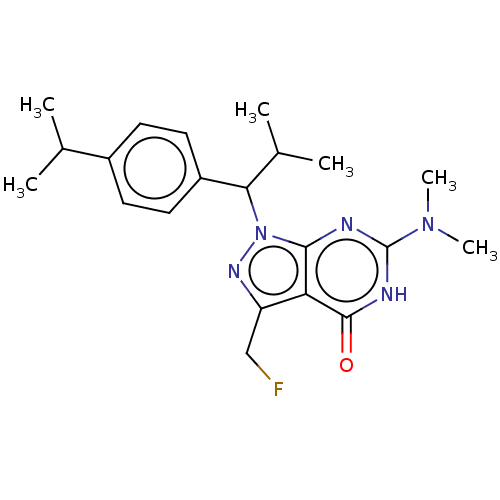 ((R)- or (S)-6-(Dimethylamino)-3- (fluoromethyl)-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320728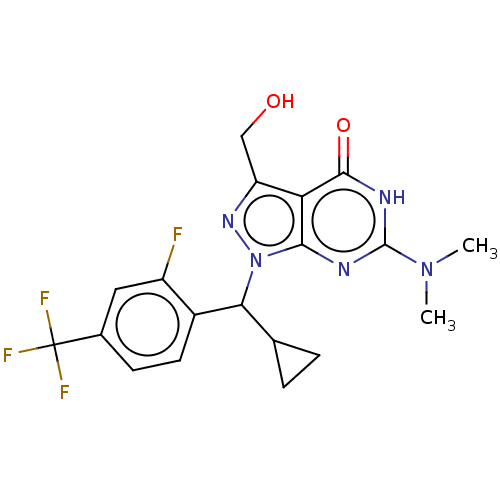 ((R)- or (S)-1-(Cyclopropyl(2- fluoro-4- (trifluoro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50545979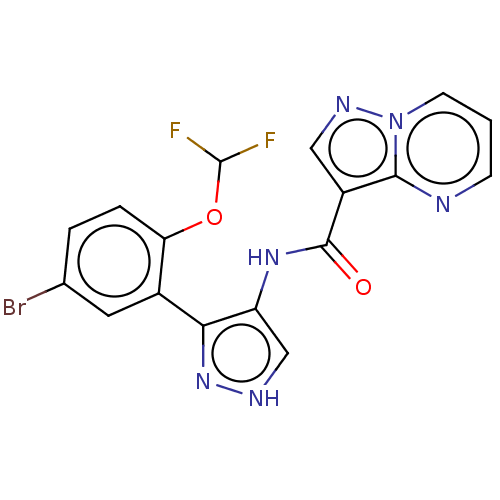 (CHEMBL4742159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human JAK2 (812 to 1132 residues) expressed in insect cells using Y1-B as substrate incubated for 30 mins by microfluidic m... | Citation and Details Article DOI: 10.1016/j.bmcl.2019.04.008 BindingDB Entry DOI: 10.7270/Q269776T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4 (Rattus norvegicus (Rat)) | BDBM50062641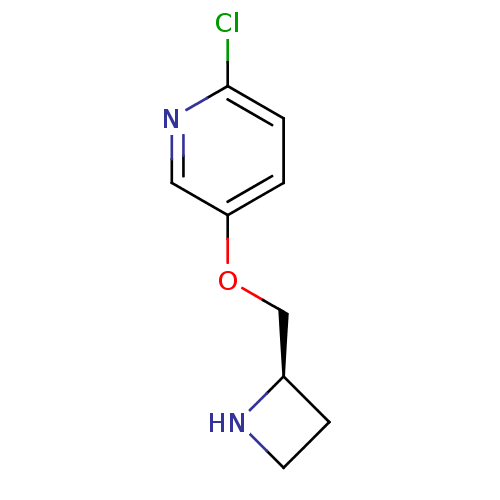 (5-((R)-1-Azetidin-2-ylmethoxy)-2-chloro-pyridine |...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from alpha4beta2 nAChR in rat brain membrane | Bioorg Med Chem Lett 19: 1682-5 (2009) Article DOI: 10.1016/j.bmcl.2009.01.099 BindingDB Entry DOI: 10.7270/Q2HD7VJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320793 ((R)- or (S)-6-(Dimethylamino)-1-(1-(2- fluoro-4-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM320789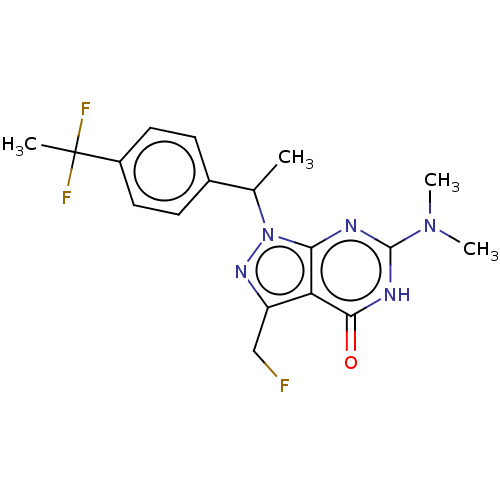 ((R)- or (S)-1-(1-(4-(1,1- Difluoroethyl)phenyl)eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Performed by Lab B. The activity of the compounds in accordance with the present invention as PDE2 inhibitors may be readily determined using a fluor... | US Patent US10174037 (2019) BindingDB Entry DOI: 10.7270/Q29K4DB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77059 total ) | Next | Last >> |