 Found 13857 hits with Last Name = 'huang' and Initial = 'c'
Found 13857 hits with Last Name = 'huang' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50507816
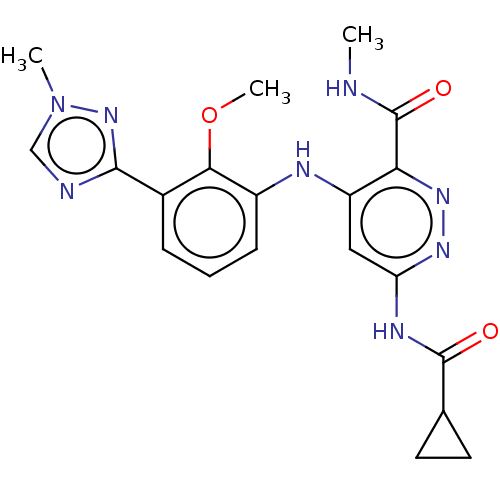
(Bms-986165 | Deucravacitinib)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(NC(=O)C2CC2)cc1Nc1cccc(-c2ncn(C)n2)c1OC Show InChI InChI=1S/C20H22N8O3/c1-21-20(30)16-14(9-15(25-26-16)24-19(29)11-7-8-11)23-13-6-4-5-12(17(13)31-3)18-22-10-28(2)27-18/h4-6,9-11H,7-8H2,1-3H3,(H,21,30)(H2,23,24,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of fluorescein labeled probe binding to His-tagged human TYK2 pseudokinase domain (575-869 residues) by Morrison titration based HTRF assa... |
J Med Chem 62: 8973-8995 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00444
BindingDB Entry DOI: 10.7270/Q2930XJS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PAK 1
(Homo sapiens (Human)) | BDBM50148931
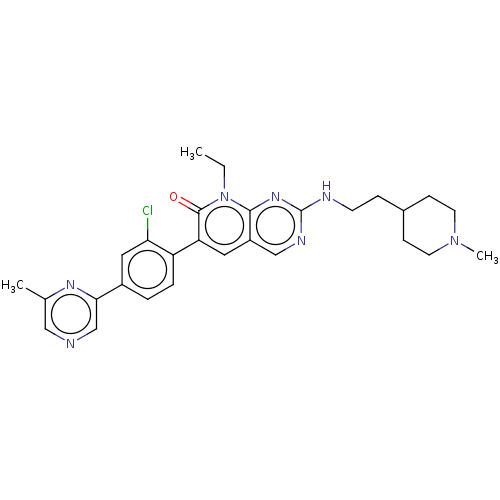
(CHEMBL3770186)Show SMILES CCn1c2nc(NCCC3CCN(C)CC3)ncc2cc(-c2ccc(cc2Cl)-c2cncc(C)n2)c1=O Show InChI InChI=1S/C28H32ClN7O/c1-4-36-26-21(16-32-28(34-26)31-10-7-19-8-11-35(3)12-9-19)13-23(27(36)37)22-6-5-20(14-24(22)29)25-17-30-15-18(2)33-25/h5-6,13-17,19H,4,7-12H2,1-3H3,(H,31,32,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of full length recombinant human N-terminal GST/His6-tagged PAK1 expressed in sf9 insect cells using tetra LRRWSLG as substrate preincubat... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112517
BindingDB Entry DOI: 10.7270/Q2Q243W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394
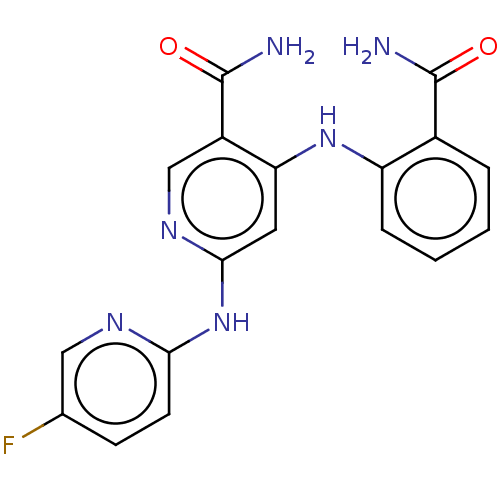
(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50530394

(CHEMBL4561663)Show SMILES NC(=O)c1ccccc1Nc1cc(Nc2ccc(F)cn2)ncc1C(N)=O Show InChI InChI=1S/C18H15FN6O2/c19-10-5-6-15(22-8-10)25-16-7-14(12(9-23-16)18(21)27)24-13-4-2-1-3-11(13)17(20)26/h1-9H,(H2,20,26)(H2,21,27)(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523
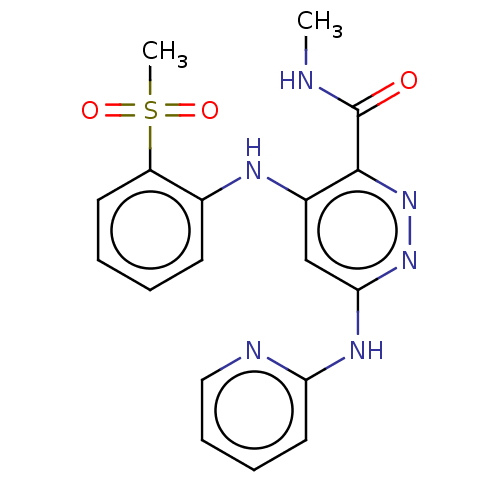
(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Non-receptor tyrosine-protein kinase TYK2
(Homo sapiens (Human)) | BDBM50519523

(CHEMBL4440718)Show SMILES [2H]C([2H])([2H])NC(=O)c1nnc(Nc2ccccn2)cc1Nc1ccccc1S(C)(=O)=O Show InChI InChI=1S/C18H18N6O3S/c1-19-18(25)17-13(21-12-7-3-4-8-14(12)28(2,26)27)11-16(23-24-17)22-15-9-5-6-10-20-15/h3-11H,1-2H3,(H,19,25)(H2,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of TYK2 JH2 (unknown origin) by morrison titration assay |
J Med Chem 62: 8953-8972 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00443
BindingDB Entry DOI: 10.7270/Q2W95DMQ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291847

(7-[3-(Quinolin-2-ylmethoxy)-benzyloxy]-2-(1H-tetra...)Show SMILES O=C1CC(Oc2cc(OCc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C27H21N5O4/c33-24-14-26(27-29-31-32-30-27)36-25-13-21(10-11-22(24)25)34-15-17-4-3-6-20(12-17)35-16-19-9-8-18-5-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291855

(7-[3-(Quinolin-2-ylmethoxy)-phenoxymethyl]-2-(1H-t...)Show SMILES O=C1CC(Oc2cc(COc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C27H21N5O4/c33-24-14-26(27-29-31-32-30-27)36-25-12-17(8-11-22(24)25)15-34-20-5-3-6-21(13-20)35-16-19-10-9-18-4-1-2-7-23(18)28-19/h1-13,26H,14-16H2,(H,29,30,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50550643
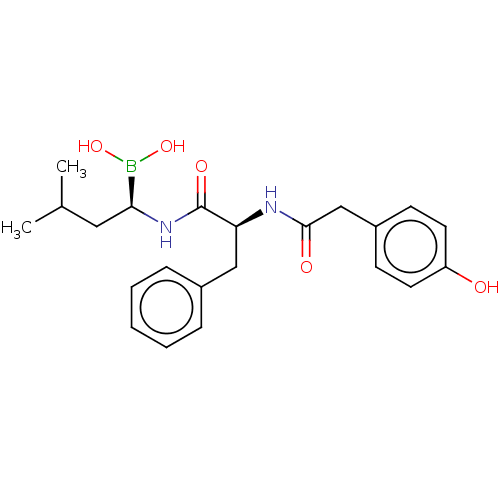
(CHEMBL4749207)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)Cc1ccc(O)cc1)B(O)O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to human 20S constitutive proteasome beta 5 subunit assessed as equilibrium constant using fluorogenic peptide Ac-WLA-AMC as substra... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01161
BindingDB Entry DOI: 10.7270/Q2K077W3 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50001609
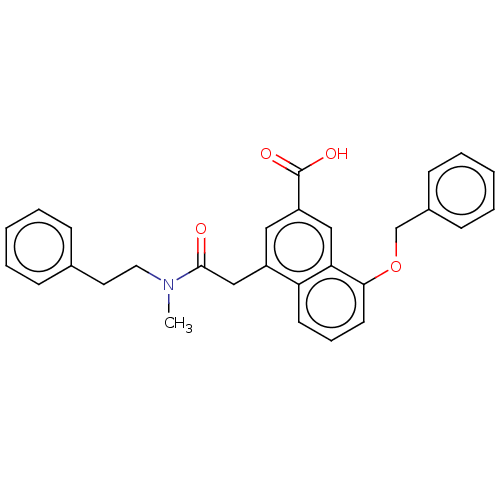
(8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...)Show SMILES CN(CCc1ccccc1)C(=O)Cc1cc(cc2c(OCc3ccccc3)cccc12)C(O)=O Show InChI InChI=1S/C29H27NO4/c1-30(16-15-21-9-4-2-5-10-21)28(31)19-23-17-24(29(32)33)18-26-25(23)13-8-14-27(26)34-20-22-11-6-3-7-12-22/h2-14,17-18H,15-16,19-20H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membrane |
J Med Chem 35: 4253-5 (1992)
BindingDB Entry DOI: 10.7270/Q208648W |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269186
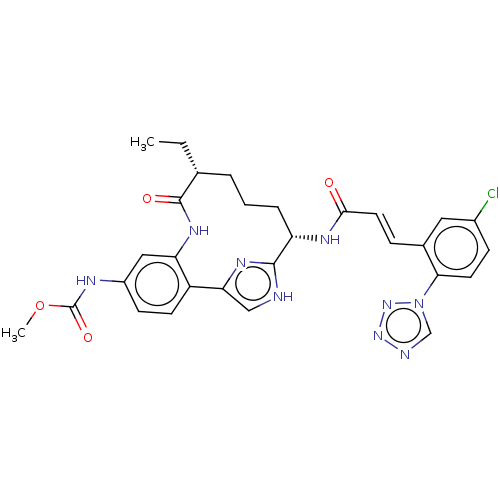
(CHEMBL4089185)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2nc(c[nH]2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C29H30ClN9O4/c1-3-17-5-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h7-17,22H,3-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b12-7+/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514438
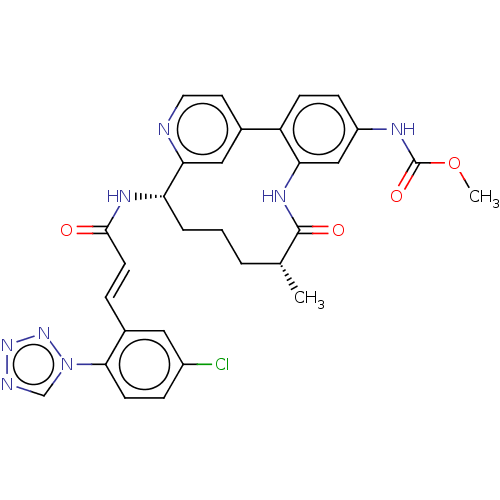
(CHEMBL4439729)Show SMILES OC(=O)C(F)(F)F.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)\C=C\c3cc(Cl)ccc3-n3cnnn3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClN8O4.C2HF3O2/c1-18-4-3-5-24(35-28(40)11-6-20-14-21(31)7-10-27(20)39-17-33-37-38-39)26-15-19(12-13-32-26)23-9-8-22(34-30(42)43-2)16-25(23)36-29(18)41;3-2(4,5)1(6)7/h6-18,24H,3-5H2,1-2H3,(H,34,42)(H,35,40)(H,36,41);(H,6,7)/b11-6+;/t18-,24+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM86492

(CAS_170713-75-4 | NSC_6324645 | Nociceptin)Show SMILES [#6]-[#6](-[#8])-[#6](-[#7]-[#6](=O)-[#6](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6](-[#7])-[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#8])-[#6](=O)-[#7]-[#6](-[#6])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#6](=O)-[#7]-[#6](-[#6]-[#6]-[#6]-[#6]-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C61H100N22O15/c1-34(75-47(87)32-74-59(98)49(36(3)85)83-57(96)44(29-38-18-8-5-9-19-38)77-48(88)31-72-46(86)30-73-53(92)39(64)28-37-16-6-4-7-17-37)51(90)79-43(23-15-27-71-61(68)69)55(94)81-41(21-11-13-25-63)56(95)82-45(33-84)58(97)76-35(2)52(91)80-42(22-14-26-70-60(66)67)54(93)78-40(50(65)89)20-10-12-24-62/h4-9,16-19,34-36,39-45,49,84-85H,10-15,20-33,62-64H2,1-3H3,(H2,65,89)(H,72,86)(H,73,92)(H,74,98)(H,75,87)(H,76,97)(H,77,88)(H,78,93)(H,79,90)(H,80,91)(H,81,94)(H,82,95)(H,83,96)(H4,66,67,70)(H4,68,69,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 310: 783-92 (2004)
Article DOI: 10.1124/jpet.103.063313
BindingDB Entry DOI: 10.7270/Q2J67FHD |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514437
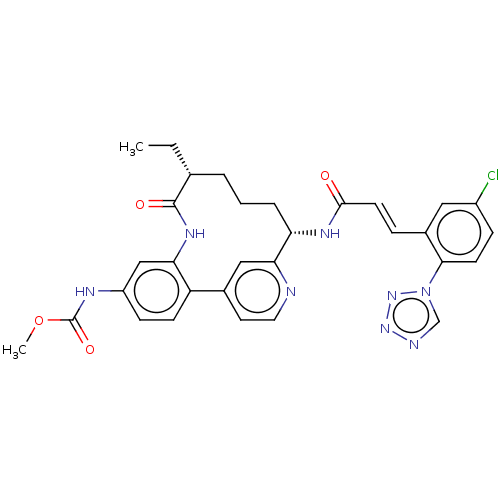
(CHEMBL4444690)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2cc(ccn2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C31H31ClN8O4/c1-3-19-5-4-6-25(36-29(41)12-7-21-15-22(32)8-11-28(21)40-18-34-38-39-40)27-16-20(13-14-33-27)24-10-9-23(35-31(43)44-2)17-26(24)37-30(19)42/h7-19,25H,3-6H2,1-2H3,(H,35,43)(H,36,41)(H,37,42)/b12-7+/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329148
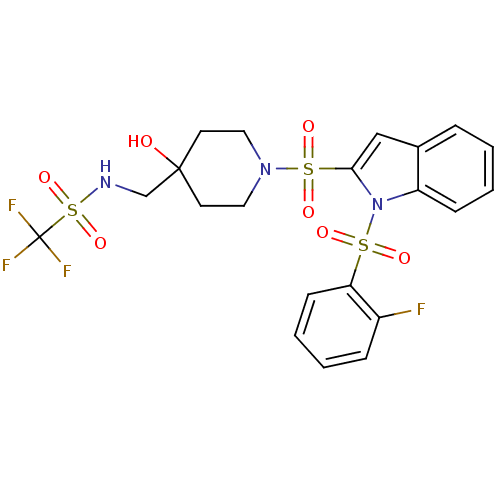
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES OC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H21F4N3O7S3/c22-16-6-2-4-8-18(16)36(30,31)28-17-7-3-1-5-15(17)13-19(28)37(32,33)27-11-9-20(29,10-12-27)14-26-38(34,35)21(23,24)25/h1-8,13,26,29H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1/2
(Homo sapiens (Human)) | BDBM50009075
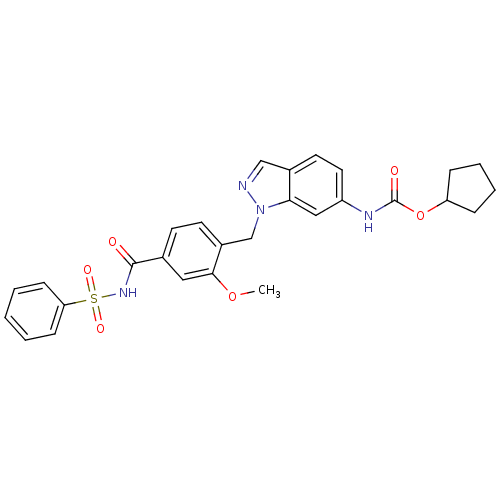
(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research
Curated by ChEMBL
| Assay Description
In vitro binding affinity against cysteinyl leukotriene D4 receptor from guinea pig lung membrane |
J Med Chem 33: 2828-41 (1990)
BindingDB Entry DOI: 10.7270/Q29C6WDM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50156616
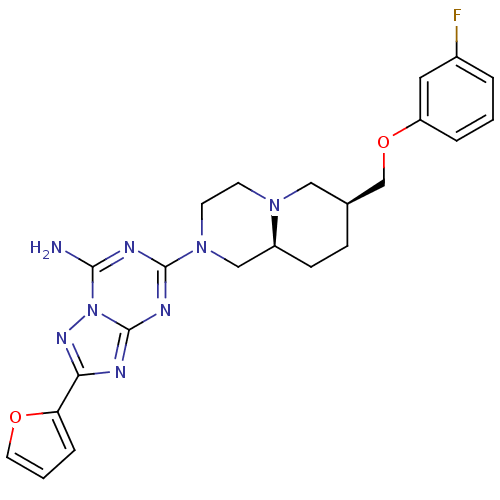
((7RS,9aRS)-5-[7-(3-fluoro-phenoxymethyl)-octahydro...)Show SMILES Nc1nc(nc2nc(nn12)-c1ccco1)N1CCN2C[C@@H](COc3cccc(F)c3)CC[C@H]2C1 |r| Show InChI InChI=1S/C23H25FN8O2/c24-16-3-1-4-18(11-16)34-14-15-6-7-17-13-31(9-8-30(17)12-15)22-27-21(25)32-23(28-22)26-20(29-32)19-5-2-10-33-19/h1-5,10-11,15,17H,6-9,12-14H2,(H2,25,26,27,28,29)/t15-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in rat brain membrane |
J Med Chem 47: 6218-29 (2004)
Article DOI: 10.1021/jm0494321
BindingDB Entry DOI: 10.7270/Q20V8DM9 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213665
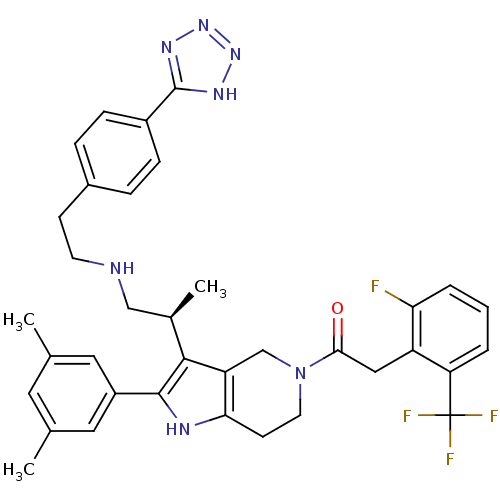
((S)-1-(3-(1-(4-(1H-tetrazol-5-yl)phenethylamino)pr...)Show SMILES C[C@H](CNCCc1ccc(cc1)-c1nnn[nH]1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F |r| Show InChI InChI=1S/C36H37F4N7O/c1-21-15-22(2)17-26(16-21)34-33(23(3)19-41-13-11-24-7-9-25(10-8-24)35-43-45-46-44-35)28-20-47(14-12-31(28)42-34)32(48)18-27-29(36(38,39)40)5-4-6-30(27)37/h4-10,15-17,23,41-42H,11-14,18-20H2,1-3H3,(H,43,44,45,46)/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305990
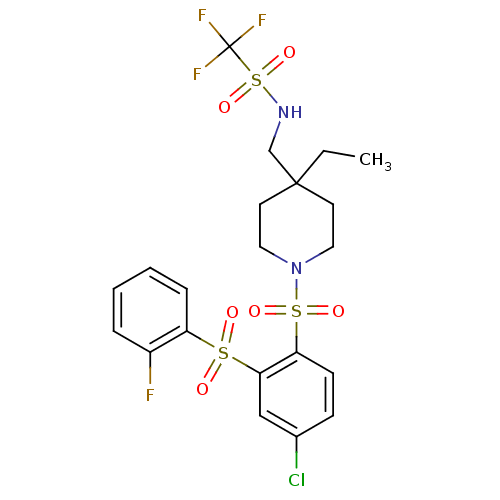
(CHEMBL596388 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H23ClF4N2O6S3/c1-2-20(14-27-37(33,34)21(24,25)26)9-11-28(12-10-20)36(31,32)18-8-7-15(22)13-19(18)35(29,30)17-6-4-3-5-16(17)23/h3-8,13,27H,2,9-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50156595

((7RS,9aRS)-2-furan-2-yl-N5-(2-pyrimidin-2-yloctahy...)Show SMILES Nc1nc(NC[C@@H]2CC[C@@H]3CN(CCN3C2)c2ncccn2)nc2nc(nn12)-c1ccco1 |r| Show InChI InChI=1S/C21H25N11O/c22-18-27-19(28-21-26-17(29-32(18)21)16-3-1-10-33-16)25-11-14-4-5-15-13-31(9-8-30(15)12-14)20-23-6-2-7-24-20/h1-3,6-7,10,14-15H,4-5,8-9,11-13H2,(H3,22,25,26,27,28,29)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in rat brain membrane |
J Med Chem 47: 6218-29 (2004)
Article DOI: 10.1021/jm0494321
BindingDB Entry DOI: 10.7270/Q20V8DM9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50156606

((7RS,9aRS)-2-furan-2-yl-5-[7-(pyridin-3-yloxymethy...)Show SMILES Nc1nc(nc2nc(nn12)-c1ccco1)N1CCN2C[C@@H](COc3cccnc3)CC[C@H]2C1 |r| Show InChI InChI=1S/C22H25N9O2/c23-20-26-21(27-22-25-19(28-31(20)22)18-4-2-10-32-18)30-9-8-29-12-15(5-6-16(29)13-30)14-33-17-3-1-7-24-11-17/h1-4,7,10-11,15-16H,5-6,8-9,12-14H2,(H2,23,25,26,27,28)/t15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in rat brain membrane |
J Med Chem 47: 6218-29 (2004)
Article DOI: 10.1021/jm0494321
BindingDB Entry DOI: 10.7270/Q20V8DM9 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50156605
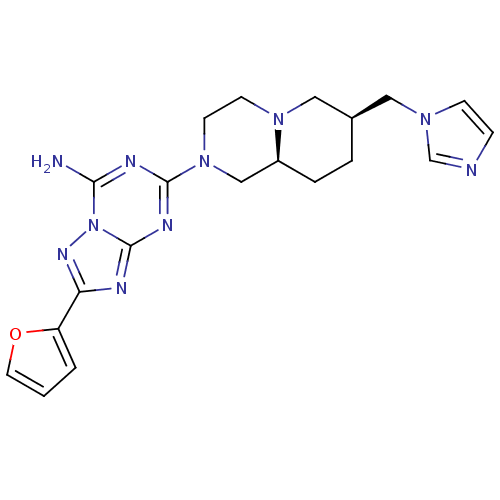
((7RS,9aRS)-2-furan-2-yl-5-(7-imidazol-1-ylmethyloc...)Show SMILES Nc1nc(nc2nc(nn12)-c1ccco1)N1CCN2C[C@@H](Cn3ccnc3)CC[C@H]2C1 |r| Show InChI InChI=1S/C20H24N10O/c21-18-24-19(25-20-23-17(26-30(18)20)16-2-1-9-31-16)29-8-7-28-11-14(3-4-15(28)12-29)10-27-6-5-22-13-27/h1-2,5-6,9,13-15H,3-4,7-8,10-12H2,(H2,21,23,24,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biogen Idec Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]ZM241385 from adenosine A2A receptor in rat brain membrane |
J Med Chem 47: 6218-29 (2004)
Article DOI: 10.1021/jm0494321
BindingDB Entry DOI: 10.7270/Q20V8DM9 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009075

(CHEMBL22033 | ICI 198615 | ICI-198615 | [1-(4-Benz...)Show SMILES COc1cc(ccc1Cn1ncc2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C28H28N4O6S/c1-37-26-15-19(27(33)31-39(35,36)24-9-3-2-4-10-24)11-12-21(26)18-32-25-16-22(14-13-20(25)17-29-32)30-28(34)38-23-7-5-6-8-23/h2-4,9-17,23H,5-8,18H2,1H3,(H,30,34)(H,31,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213671
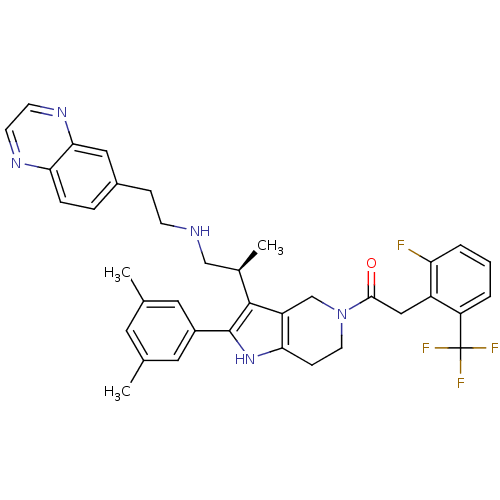
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(quinoxalin-...)Show SMILES C[C@H](CNCCc1ccc2nccnc2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C37H37F4N5O/c1-22-15-23(2)17-26(16-22)36-35(24(3)20-42-11-9-25-7-8-32-33(18-25)44-13-12-43-32)28-21-46(14-10-31(28)45-36)34(47)19-27-29(37(39,40)41)5-4-6-30(27)38/h4-8,12-13,15-18,24,42,45H,9-11,14,19-21H2,1-3H3/t24-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50291848
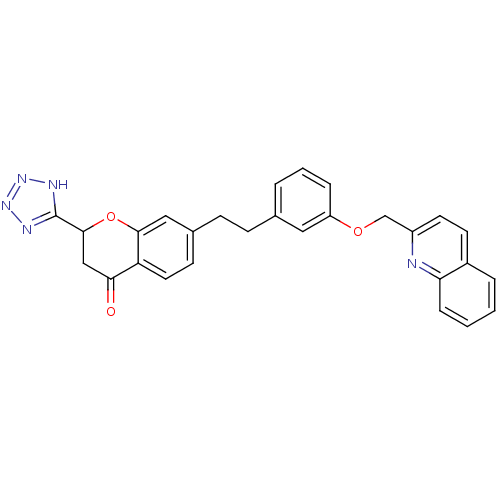
(7-{2-[3-(Quinolin-2-ylmethoxy)-phenyl]-ethyl}-2-(1...)Show SMILES O=C1CC(Oc2cc(CCc3cccc(OCc4ccc5ccccc5n4)c3)ccc12)c1nnn[nH]1 Show InChI InChI=1S/C28H23N5O3/c34-25-16-27(28-30-32-33-31-28)36-26-15-19(10-13-23(25)26)9-8-18-4-3-6-22(14-18)35-17-21-12-11-20-5-1-2-7-24(20)29-21/h1-7,10-15,27H,8-9,16-17H2,(H,30,31,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against LTD4 receptor in guinea pig lung membranes. |
J Med Chem 34: 1704-7 (1991)
BindingDB Entry DOI: 10.7270/Q2FJ2FR5 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50120625

(CHEMBL3618330)Show SMILES CNC1CCN(C1)c1ccc(cn1)-n1cnn2cc(cc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H21ClN6O/c1-24-18-8-9-27(13-18)21-7-6-19(11-25-21)28-14-26-29-12-16(10-20(29)22(28)30)15-2-4-17(23)5-3-15/h2-7,10-12,14,18,24H,8-9,13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis |
Bioorg Med Chem Lett 25: 4412-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.018
BindingDB Entry DOI: 10.7270/Q2JD4ZMW |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50241083
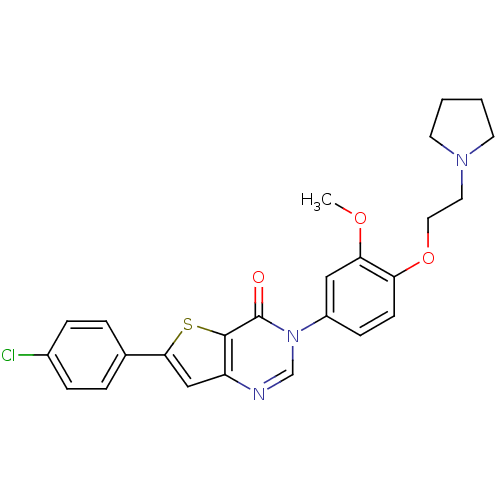
(6-(4-chlorophenyl)-3-(3-methoxy-4-(2-(pyrrolidin-1...)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnc2cc(sc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H24ClN3O3S/c1-31-22-14-19(8-9-21(22)32-13-12-28-10-2-3-11-28)29-16-27-20-15-23(33-24(20)25(29)30)17-4-6-18(26)7-5-17/h4-9,14-16H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at rat MCHR1 |
Bioorg Med Chem Lett 25: 2793-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.008
BindingDB Entry DOI: 10.7270/Q29C7053 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329147
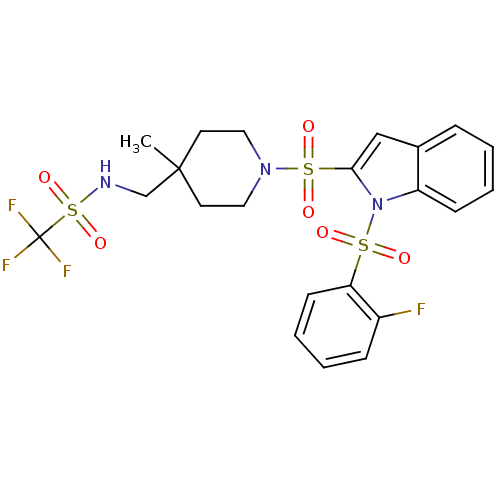
(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES CC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-21(15-27-38(34,35)22(24,25)26)10-12-28(13-11-21)37(32,33)20-14-16-6-2-4-8-18(16)29(20)36(30,31)19-9-5-3-7-17(19)23/h2-9,14,27H,10-13,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329151

(1,1,1-trifluoro-N-((1-(1-(2-fluorophenylsulfonyl)-...)Show SMILES Fc1ccccc1S(=O)(=O)n1c(cc2ccccc12)S(=O)(=O)N1CCC(CNS(=O)(=O)C(F)(F)F)CC1 Show InChI InChI=1S/C21H21F4N3O6S3/c22-17-6-2-4-8-19(17)35(29,30)28-18-7-3-1-5-16(18)13-20(28)36(31,32)27-11-9-15(10-12-27)14-26-37(33,34)21(23,24)25/h1-8,13,15,26H,9-12,14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50305989
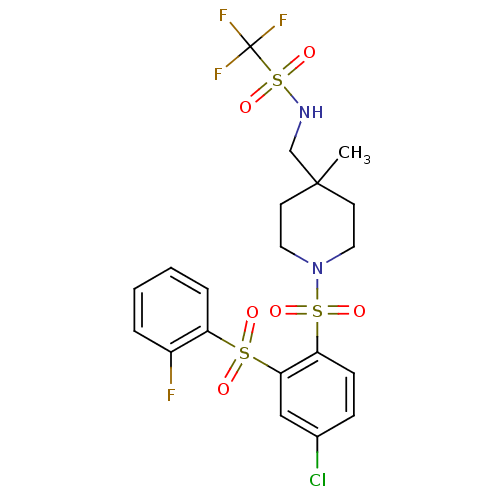
(CHEMBL596387 | N-((1-(4-chloro-2-(2-fluorophenylsu...)Show SMILES CC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1ccc(Cl)cc1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C20H21ClF4N2O6S3/c1-19(13-26-36(32,33)20(23,24)25)8-10-27(11-9-19)35(30,31)17-7-6-14(21)12-18(17)34(28,29)16-5-3-2-4-15(16)22/h2-7,12,26H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor |
Bioorg Med Chem Lett 20: 608-11 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.084
BindingDB Entry DOI: 10.7270/Q2SB45V0 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269195
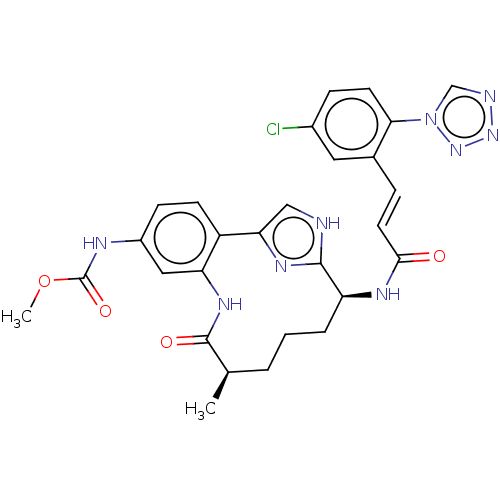
(CHEMBL4101766)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H28ClN9O4/c1-16-4-3-5-21(33-25(39)11-6-17-12-18(29)7-10-24(17)38-15-31-36-37-38)26-30-14-23(34-26)20-9-8-19(32-28(41)42-2)13-22(20)35-27(16)40/h6-16,21H,3-5H2,1-2H3,(H,30,34)(H,32,41)(H,33,39)(H,35,40)/b11-6+/t16-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213664
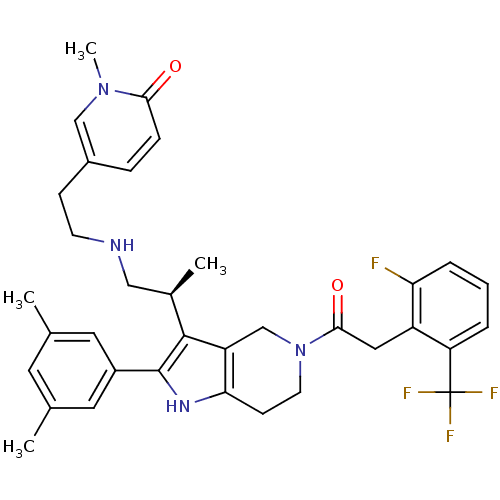
((S)-5-(2-(2-(2-(3,5-dimethylphenyl)-5-(2-(2-fluoro...)Show SMILES C[C@H](CNCCc1ccc(=O)n(C)c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H38F4N4O2/c1-21-14-22(2)16-25(15-21)34-33(23(3)18-40-12-10-24-8-9-31(44)42(4)19-24)27-20-43(13-11-30(27)41-34)32(45)17-26-28(35(37,38)39)6-5-7-29(26)36/h5-9,14-16,19,23,40-41H,10-13,17-18,20H2,1-4H3/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(Homo sapiens (Human)) | BDBM14775
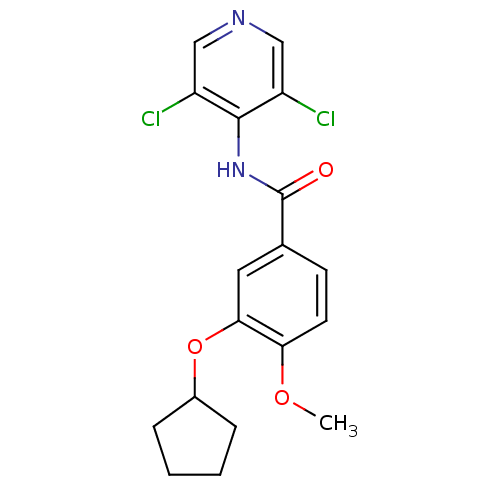
(3-(cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-...)Show InChI InChI=1S/C18H18Cl2N2O3/c1-24-15-7-6-11(8-16(15)25-12-4-2-3-5-12)18(23)22-17-13(19)9-21-10-14(17)20/h6-10,12H,2-5H2,1H3,(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rh£ne-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Binding affinity against PDE4 was determined using [3H]- rolipram in guinea pig brain membrane binding assay |
Bioorg Med Chem Lett 8: 1867-72 (1998)
BindingDB Entry DOI: 10.7270/Q2CJ8GPB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329150

(1,1,1-trifluoro-N-(1-(1-(1-(2-fluorophenylsulfonyl...)Show SMILES CC(NS(=O)(=O)C(F)(F)F)C1CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C22H23F4N3O6S3/c1-15(27-38(34,35)22(24,25)26)16-10-12-28(13-11-16)37(32,33)21-14-17-6-2-4-8-19(17)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,14-16,27H,10-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329129
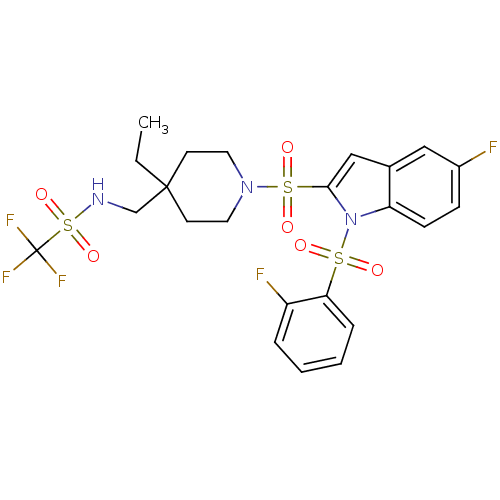
(CHEMBL1271093 | N-((4-ethyl-1-(5-fluoro-1-(2-fluor...)Show SMILES CCC1(CNS(=O)(=O)C(F)(F)F)CCN(CC1)S(=O)(=O)c1cc2cc(F)ccc2n1S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C23H24F5N3O6S3/c1-2-22(15-29-40(36,37)23(26,27)28)9-11-30(12-10-22)39(34,35)21-14-16-13-17(24)7-8-19(16)31(21)38(32,33)20-6-4-3-5-18(20)25/h3-8,13-14,29H,2,9-12,15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329130
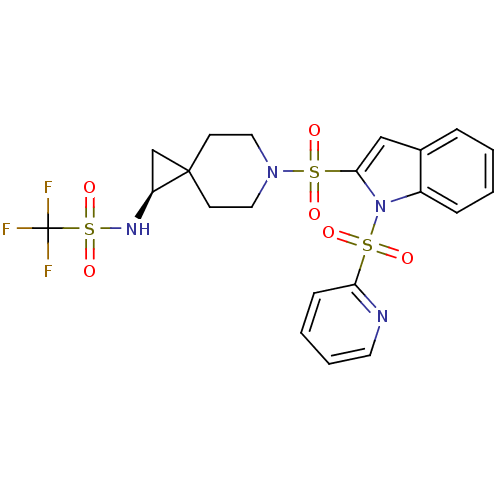
((S)-1,1,1-trifluoro-N-(6-(1-(pyridin-2-ylsulfonyl)...)Show SMILES FC(F)(F)S(=O)(=O)N[C@H]1CC11CCN(CC1)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccn1 |r| Show InChI InChI=1S/C21H21F3N4O6S3/c22-21(23,24)37(33,34)26-17-14-20(17)8-11-27(12-9-20)36(31,32)19-13-15-5-1-2-6-16(15)28(19)35(29,30)18-7-3-4-10-25-18/h1-7,10,13,17,26H,8-9,11-12,14H2/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50120625

(CHEMBL3618330)Show SMILES CNC1CCN(C1)c1ccc(cn1)-n1cnn2cc(cc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H21ClN6O/c1-24-18-8-9-27(13-18)21-7-6-19(11-25-21)28-14-26-29-12-16(10-20(29)22(28)30)15-2-4-17(23)5-3-15/h2-7,10-12,14,18,24H,8-9,13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis |
Bioorg Med Chem Lett 25: 4412-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.018
BindingDB Entry DOI: 10.7270/Q2JD4ZMW |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50120624
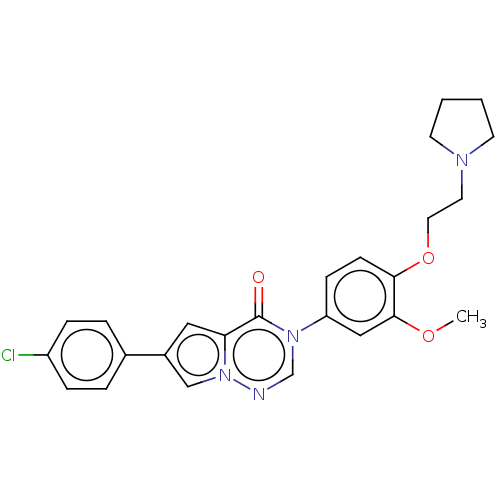
(CHEMBL3618324)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnn2cc(cc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H25ClN4O3/c1-32-24-15-21(8-9-23(24)33-13-12-28-10-2-3-11-28)29-17-27-30-16-19(14-22(30)25(29)31)18-4-6-20(26)7-5-18/h4-9,14-17H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis |
Bioorg Med Chem Lett 25: 4412-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.018
BindingDB Entry DOI: 10.7270/Q2JD4ZMW |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50166441
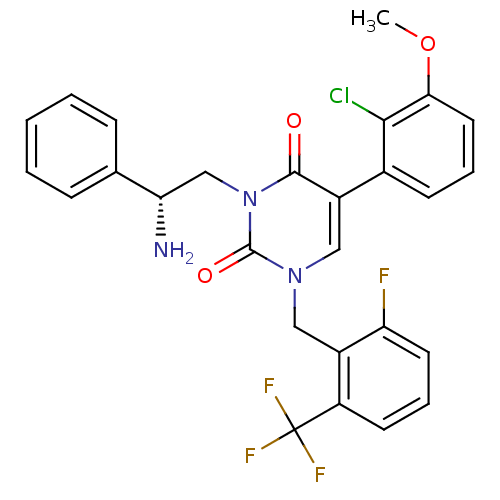
((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1Cl)-c1cn(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r| Show InChI InChI=1S/C27H22ClF4N3O3/c1-38-23-12-5-9-17(24(23)28)18-13-34(14-19-20(27(30,31)32)10-6-11-21(19)29)26(37)35(25(18)36)15-22(33)16-7-3-2-4-8-16/h2-13,22H,14-15,33H2,1H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand |
Bioorg Med Chem Lett 15: 2519-22 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.057
BindingDB Entry DOI: 10.7270/Q2M044ZX |
More data for this
Ligand-Target Pair | |
Growth hormone secretagogue receptor type 1
(Ovis aries) | BDBM50222885
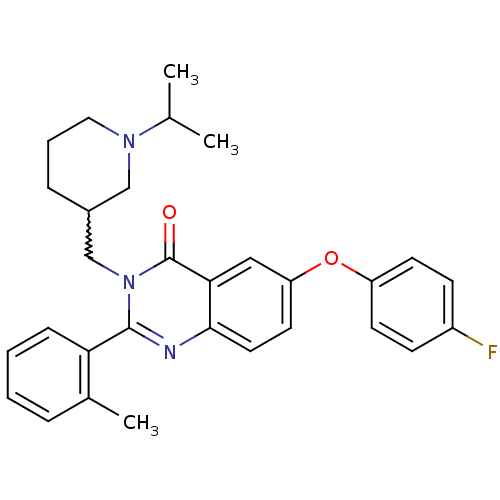
(6-(4-fluorophenoxy)-3-[(1-isopropylpiperidin-3-yl)...)Show SMILES CC(C)N1CCCC(Cn2c(nc3ccc(Oc4ccc(F)cc4)cc3c2=O)-c2ccccc2C)C1 |w:7.7| Show InChI InChI=1S/C30H32FN3O2/c1-20(2)33-16-6-8-22(18-33)19-34-29(26-9-5-4-7-21(26)3)32-28-15-14-25(17-27(28)30(34)35)36-24-12-10-23(31)11-13-24/h4-5,7,9-15,17,20,22H,6,8,16,18-19H2,1-3H3 | PDB
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Pharmaceuticals Corporation
Curated by ChEMBL
| Assay Description
Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay |
J Med Chem 50: 5202-16 (2007)
Article DOI: 10.1021/jm070071+
BindingDB Entry DOI: 10.7270/Q2WH2QT2 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50213658
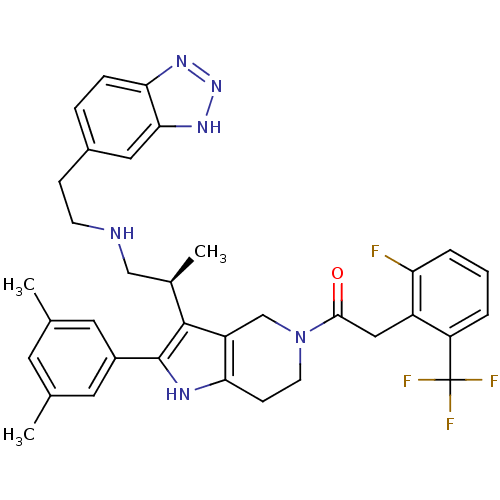
((S)-1-(3-(1-(2-(1H-benzo[d][1,2,3]triazol-5-yl)eth...)Show SMILES C[C@H](CNCCc1ccc2nn[nH]c2c1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C35H36F4N6O/c1-20-13-21(2)15-24(14-20)34-33(22(3)18-40-11-9-23-7-8-30-31(16-23)43-44-42-30)26-19-45(12-10-29(26)41-34)32(46)17-25-27(35(37,38)39)5-4-6-28(25)36/h4-8,13-16,22,40-41H,9-12,17-19H2,1-3H3,(H,42,43,44)/t22-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50120635
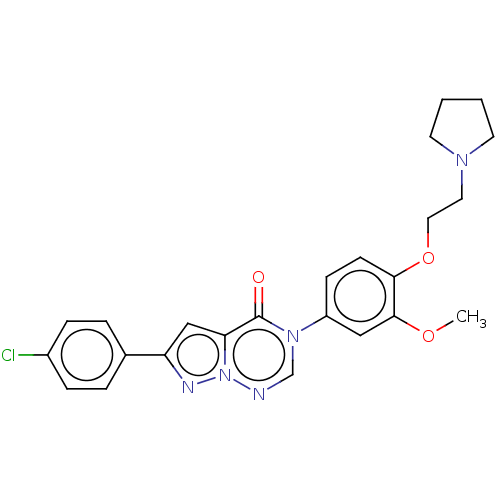
(CHEMBL3618325)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnn2nc(cc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H24ClN5O3/c1-32-23-14-19(8-9-22(23)33-13-12-28-10-2-3-11-28)29-16-26-30-21(24(29)31)15-20(27-30)17-4-6-18(25)7-5-17/h4-9,14-16H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis |
Bioorg Med Chem Lett 25: 4412-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.018
BindingDB Entry DOI: 10.7270/Q2JD4ZMW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Bos taurus) | BDBM50071484
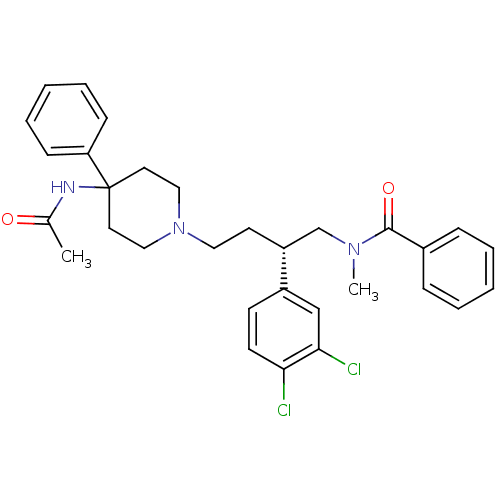
(CHEMBL308148 | N-[(R)-4-(4-Acetylamino-4-phenyl-pi...)Show SMILES CN(C[C@@H](CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. John's University
Curated by ChEMBL
| Assay Description
Binding affinity against bovine Tachykinin receptor 2 |
Bioorg Med Chem Lett 14: 4779-82 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.053
BindingDB Entry DOI: 10.7270/Q2VH5PKB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50329144
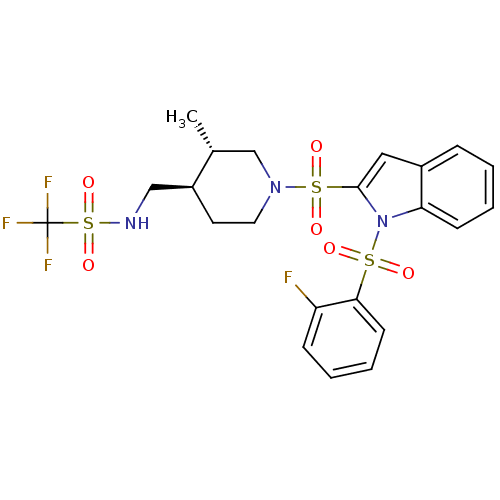
(1,1,1-trifluoro-N-(((3S,4R)-1-(1-(2-fluorophenylsu...)Show SMILES C[C@@H]1CN(CC[C@H]1CNS(=O)(=O)C(F)(F)F)S(=O)(=O)c1cc2ccccc2n1S(=O)(=O)c1ccccc1F |r| Show InChI InChI=1S/C22H23F4N3O6S3/c1-15-14-28(11-10-17(15)13-27-38(34,35)22(24,25)26)37(32,33)21-12-16-6-2-4-8-19(16)29(21)36(30,31)20-9-5-3-7-18(20)23/h2-9,12,15,17,27H,10-11,13-14H2,1H3/t15-,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of beta-arrestin binding to recombinant cannabinoid CB2 receptor |
Bioorg Med Chem Lett 20: 6785-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.126
BindingDB Entry DOI: 10.7270/Q26Q1XGJ |
More data for this
Ligand-Target Pair | |
Corticotropin-releasing factor receptor 1
(Homo sapiens (Human)) | BDBM50132965
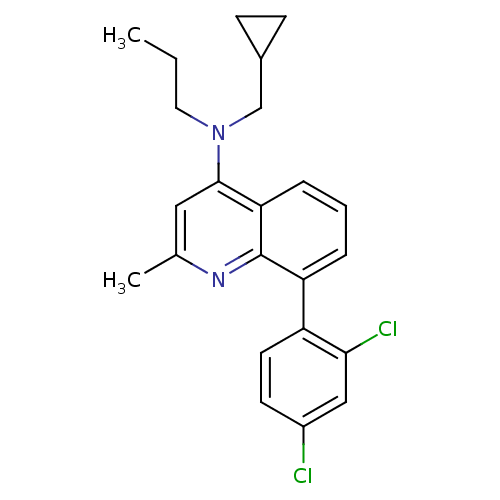
(CHEMBL115389 | Cyclopropylmethyl-[8-(2,4-dichloro-...)Show SMILES CCCN(CC1CC1)c1cc(C)nc2c(cccc12)-c1ccc(Cl)cc1Cl Show InChI InChI=1S/C23H24Cl2N2/c1-3-11-27(14-16-7-8-16)22-12-15(2)26-23-19(5-4-6-20(22)23)18-10-9-17(24)13-21(18)25/h4-6,9-10,12-13,16H,3,7-8,11,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Corticotropin releasing factor receptor 1 expressed in CHO cells using [125I]-o-CRF as the radioligand |
Bioorg Med Chem Lett 13: 3375-9 (2003)
BindingDB Entry DOI: 10.7270/Q2WM1CS4 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50261243
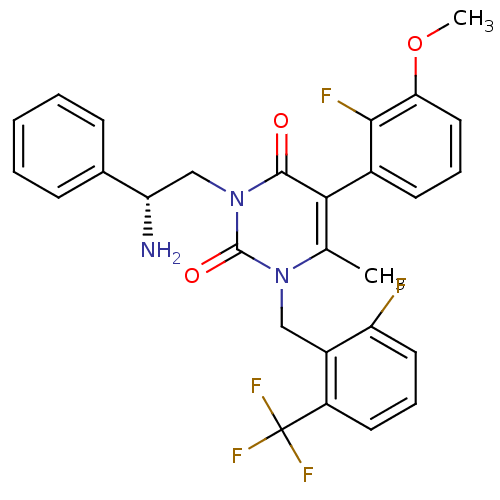
((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...)Show SMILES COc1cccc(c1F)-c1c(C)n(Cc2c(F)cccc2C(F)(F)F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r,wD:29.31,(2.33,6.39,;1,5.62,;1.01,4.08,;2.35,3.31,;2.35,1.77,;1.02,.99,;-.31,1.77,;-.32,3.3,;-1.66,4.06,;-1.64,1,;-1.64,-.54,;-.31,-1.32,;-2.97,-1.31,;-2.97,-2.85,;-1.64,-3.62,;-.32,-2.85,;-1.66,-2.08,;1.02,-3.61,;1.02,-5.16,;-.31,-5.93,;-1.65,-5.16,;-2.98,-5.92,;-4.32,-6.68,;-2.22,-7.26,;-3.74,-4.59,;-4.3,-.54,;-5.63,-1.32,;-4.3,1,;-5.64,1.76,;-6.97,.99,;-6.96,-.55,;-8.3,1.75,;-8.3,3.29,;-9.64,4.06,;-10.97,3.28,;-10.96,1.74,;-9.62,.98,;-2.97,1.77,;-2.97,3.31,)| Show InChI InChI=1S/C28H24F5N3O3/c1-16-24(18-10-6-13-23(39-2)25(18)30)26(37)36(15-22(34)17-8-4-3-5-9-17)27(38)35(16)14-19-20(28(31,32)33)11-7-12-21(19)29/h3-13,22H,14-15,34H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 51: 7478-85 (2009)
Article DOI: 10.1021/jm8006454
BindingDB Entry DOI: 10.7270/Q2S46RT8 |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50120624

(CHEMBL3618324)Show SMILES COc1cc(ccc1OCCN1CCCC1)-n1cnn2cc(cc2c1=O)-c1ccc(Cl)cc1 Show InChI InChI=1S/C25H25ClN4O3/c1-32-24-15-21(8-9-23(24)33-13-12-28-10-2-3-11-28)29-17-27-30-16-19(14-22(30)25(29)31)18-4-6-20(26)7-5-18/h4-9,14-17H,2-3,10-13H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis |
Bioorg Med Chem Lett 25: 4412-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.018
BindingDB Entry DOI: 10.7270/Q2JD4ZMW |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
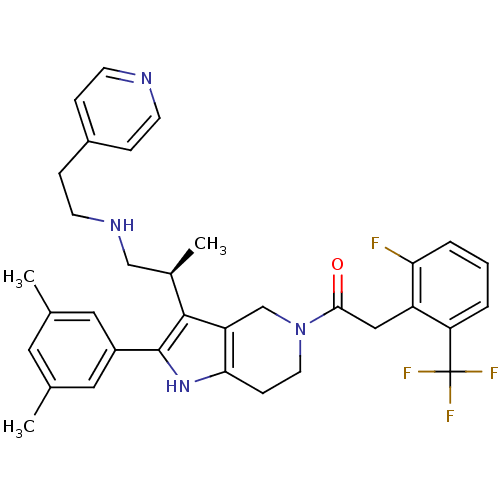
(Rattus norvegicus) | BDBM50213659
((S)-1-(2-(3,5-dimethylphenyl)-3-(1-(2-(pyridin-4-y...)Show SMILES C[C@H](CNCCc1ccncc1)c1c2CN(CCc2[nH]c1-c1cc(C)cc(C)c1)C(=O)Cc1c(F)cccc1C(F)(F)F Show InChI InChI=1S/C34H36F4N4O/c1-21-15-22(2)17-25(16-21)33-32(23(3)19-40-13-9-24-7-11-39-12-8-24)27-20-42(14-10-30(27)41-33)31(43)18-26-28(34(36,37)38)5-4-6-29(26)35/h4-8,11-12,15-17,23,40-41H,9-10,13-14,18-20H2,1-3H3/t23-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-Tyr5, DLeu6, NMeLeu7, Pro-N-Et-GnRH from cloned rat GnRHR |
Bioorg Med Chem Lett 17: 3845-50 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.009
BindingDB Entry DOI: 10.7270/Q2JD4WHQ |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50162007
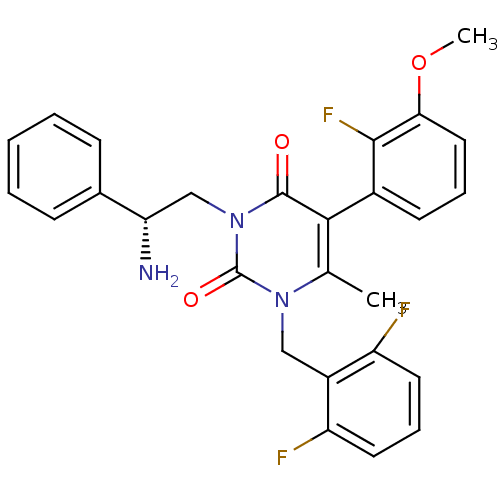
((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...)Show SMILES COc1cccc(c1F)-c1c(C)n(Cc2c(F)cccc2F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r,wD:26.28,(6.65,3.5,;5.31,2.74,;3.98,3.51,;3.98,5.05,;2.64,5.83,;1.31,5.06,;1.31,3.52,;2.64,2.75,;2.64,1.21,;-.02,2.76,;-.02,1.22,;1.3,.46,;-1.35,.46,;-1.35,-1.08,;-.01,-1.85,;1.31,-1.08,;2.64,-.3,;2.64,-1.85,;2.65,-3.39,;1.31,-4.16,;-.02,-3.39,;-1.36,-4.16,;-2.68,1.22,;-4.01,.45,;-2.68,2.76,;-4.01,3.53,;-5.34,2.75,;-5.34,1.21,;-6.68,3.52,;-6.68,5.06,;-8.02,5.83,;-9.36,5.04,;-9.35,3.51,;-8.01,2.75,;-1.35,3.54,;-1.35,5.08,)| Show InChI InChI=1S/C27H24F3N3O3/c1-16-24(18-10-6-13-23(36-2)25(18)30)26(34)33(15-22(31)17-8-4-3-5-9-17)27(35)32(16)14-19-20(28)11-7-12-21(19)29/h3-13,22H,14-15,31H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt-]GnRH from human GnRH receptor expressed in HEK293 cells by liquid scintillation counting |
J Med Chem 51: 7478-85 (2009)
Article DOI: 10.1021/jm8006454
BindingDB Entry DOI: 10.7270/Q2S46RT8 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50162007

((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...)Show SMILES COc1cccc(c1F)-c1c(C)n(Cc2c(F)cccc2F)c(=O)n(C[C@H](N)c2ccccc2)c1=O |r,wD:26.28,(6.65,3.5,;5.31,2.74,;3.98,3.51,;3.98,5.05,;2.64,5.83,;1.31,5.06,;1.31,3.52,;2.64,2.75,;2.64,1.21,;-.02,2.76,;-.02,1.22,;1.3,.46,;-1.35,.46,;-1.35,-1.08,;-.01,-1.85,;1.31,-1.08,;2.64,-.3,;2.64,-1.85,;2.65,-3.39,;1.31,-4.16,;-.02,-3.39,;-1.36,-4.16,;-2.68,1.22,;-4.01,.45,;-2.68,2.76,;-4.01,3.53,;-5.34,2.75,;-5.34,1.21,;-6.68,3.52,;-6.68,5.06,;-8.02,5.83,;-9.36,5.04,;-9.35,3.51,;-8.01,2.75,;-1.35,3.54,;-1.35,5.08,)| Show InChI InChI=1S/C27H24F3N3O3/c1-16-24(18-10-6-13-23(36-2)25(18)30)26(34)33(15-22(31)17-8-4-3-5-9-17)27(35)32(16)14-19-20(28)11-7-12-21(19)29/h3-13,22H,14-15,31H2,1-2H3/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand |
Bioorg Med Chem Lett 15: 2519-22 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.057
BindingDB Entry DOI: 10.7270/Q2M044ZX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data