

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291559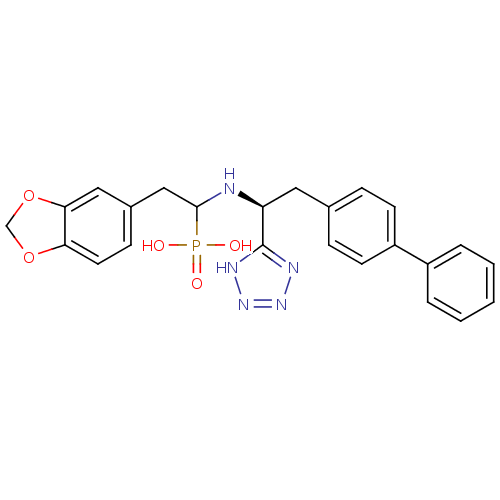 (CHEMBL268473 | {2-Benzo[1,3]dioxol-5-yl-1-[(S)-2-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291555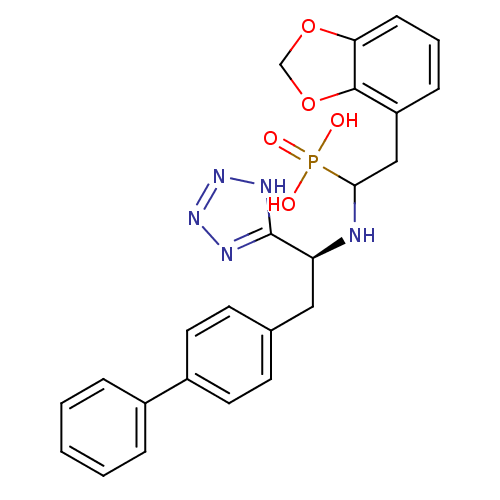 (CHEMBL276690 | {2-Benzo[1,3]dioxol-4-yl-1-[(S)-2-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034841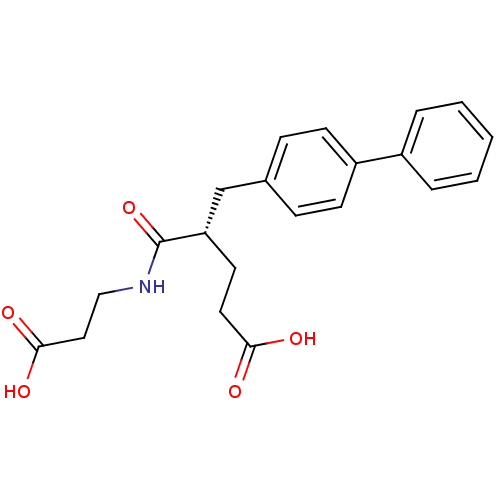 ((S)-5-Biphenyl-4-yl-4-(2-carboxy-ethylcarbamoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034846 ((2R,4R)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291558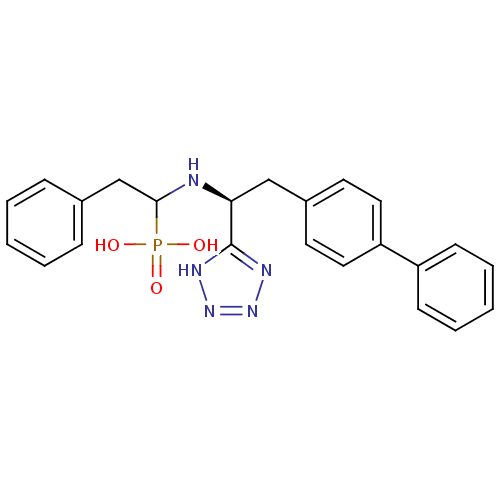 (CHEMBL276872 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034851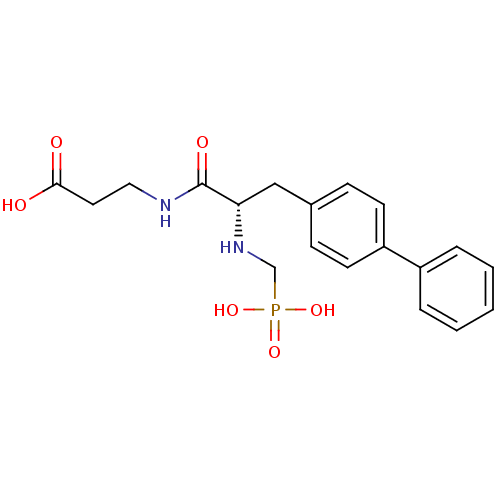 (3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50064106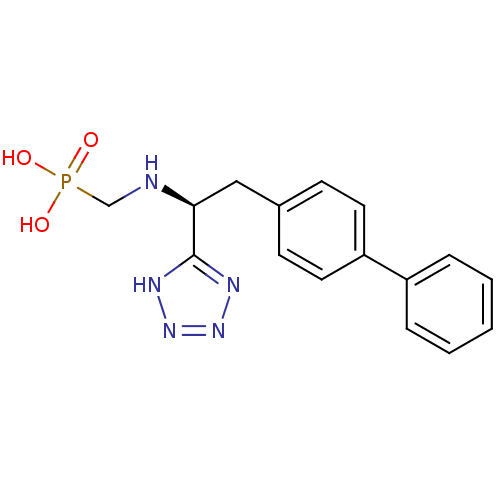 (CGS-26303 | CHEMBL290698 | {[(R)-2-Biphenyl-4-yl-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50017742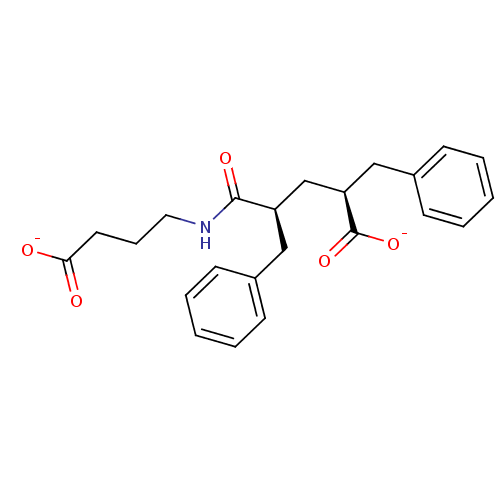 (CHEMBL316180 | disodium 2-benzyl-4-(3-carboxylatop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291557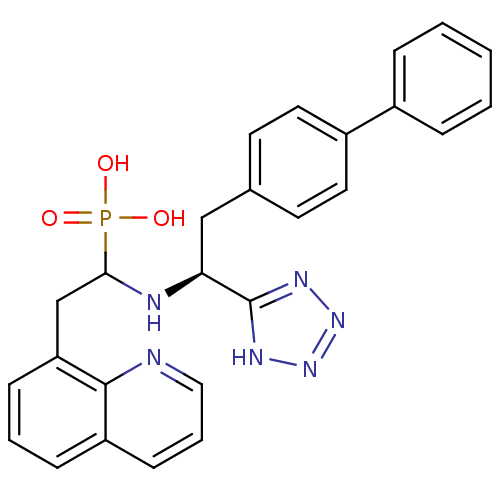 (CHEMBL268761 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetra...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034851 (3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested for inhibition of Neutral endopeptidase by using GAAP as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034851 (3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using ANF(atrial natriuretic factor) as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50038591 (6-(5-Chloro-1-methyl-2-pyridin-3-yl-1H-indol-3-yl)...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of thromboxane synthase (TxS) in human platelets (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021153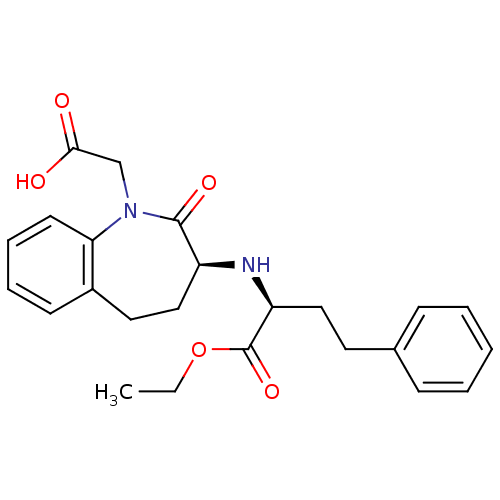 (1H-1-Benzazepine-1-acetic acid, 3-((1-(ethoxycarbo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50017736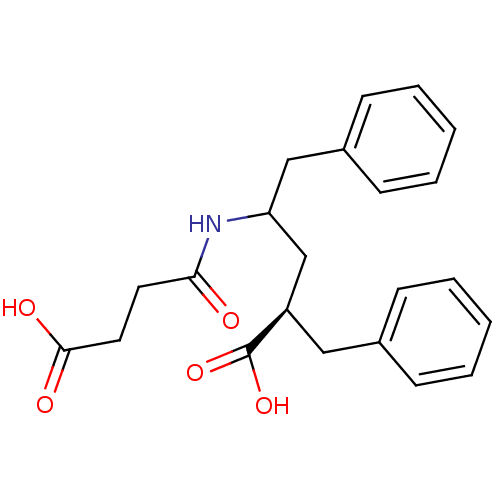 (2-Benzyl-4-(3-carboxy-propionylamino)-5-phenyl-pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291556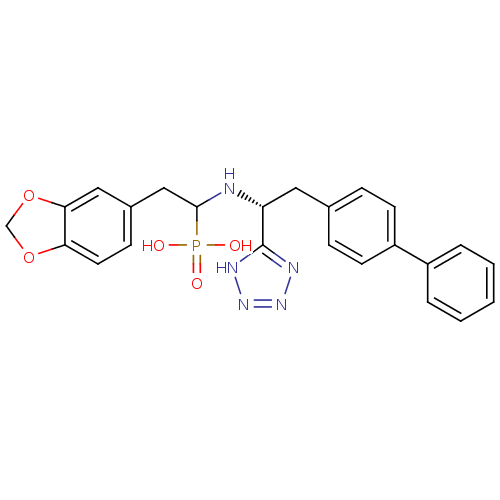 (CHEMBL273489 | {2-Benzo[1,3]dioxol-5-yl-1-[(R)-2-b...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021184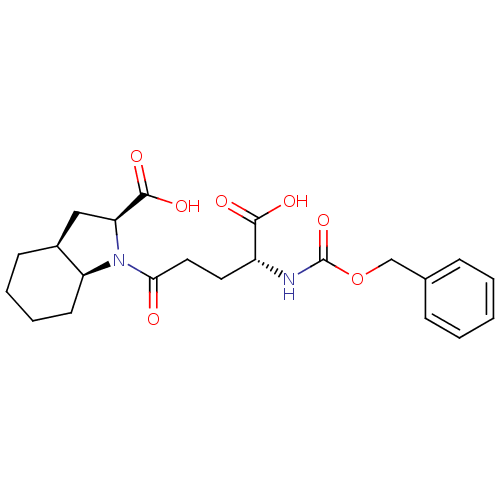 (1-(4-Benzyloxycarbonylamino-4-carboxy-butyryl)-oct...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme from rabbit lung | J Med Chem 28: 1606-11 (1985) BindingDB Entry DOI: 10.7270/Q2TT4RHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038580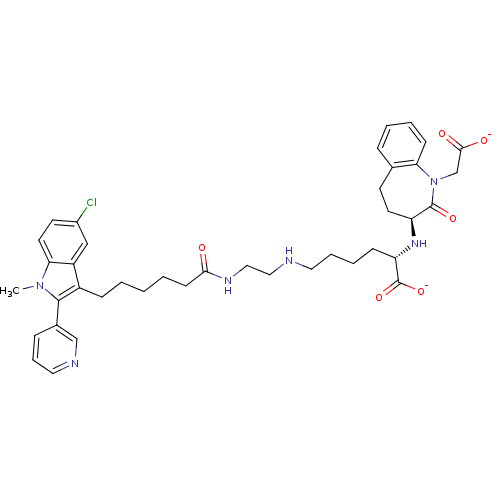 (CHEMBL55763 | Potassium;2-(1-Carboxymethyl-2-oxo-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50038593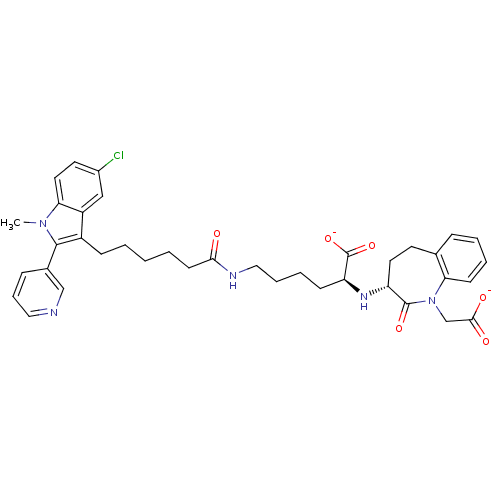 (CHEMBL301130 | Potassium ;2-(1-Carboxymethyl-2-oxo...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of thromboxane synthase (TxS) in human platelets (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017755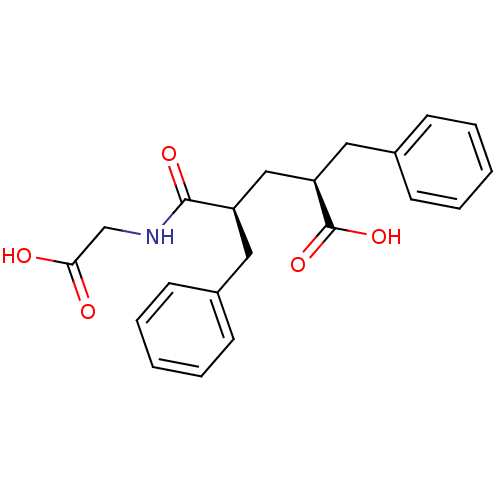 (2-Benzyl-4-(carboxymethyl-carbamoyl)-5-phenyl-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017734 (2-Benzyl-4-(2-benzylsulfanyl-1-carboxy-ethylcarbam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291561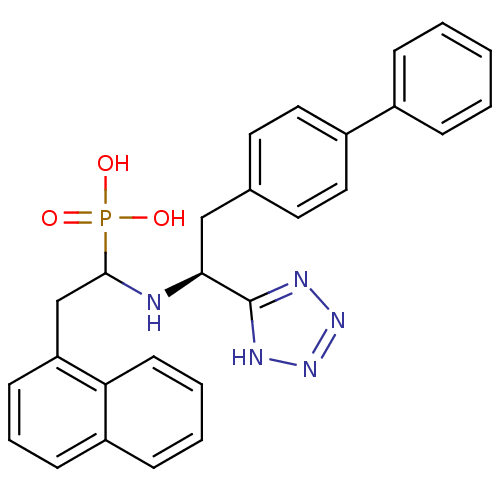 (CHEMBL10251 | {1-[(S)-2-Biphenyl-4-yl-1-(1H-tetraz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase by using GAAP as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017741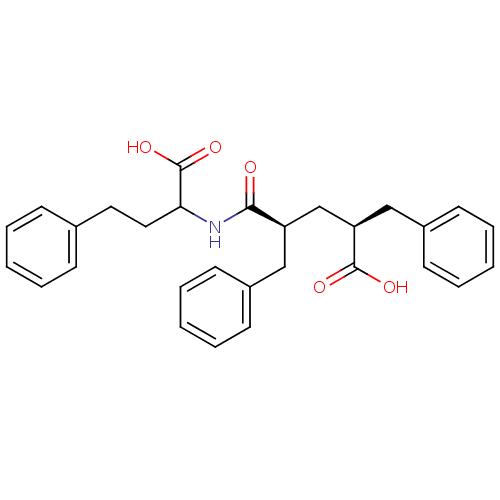 (2-Benzyl-4-(1-carboxy-3-phenyl-propylcarbamoyl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034846 ((2R,4R)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested for inhibition of Neutral endopeptidase by using GAAP as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034842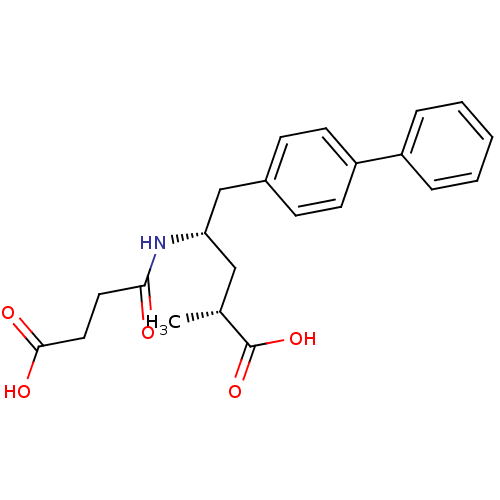 ((2R,4S)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase by using GAAP as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50017733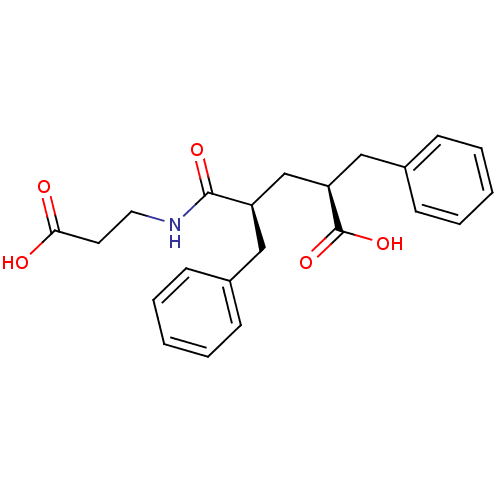 ((2S,4S)-2-Benzyl-4-(2-carboxy-ethylcarbamoyl)-5-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory activity against Enkephalinase from rabbit using method 2 is determined. | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034846 ((2R,4R)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of neutral endopeptidase by using ANF(atrial natriuretic factor) as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017752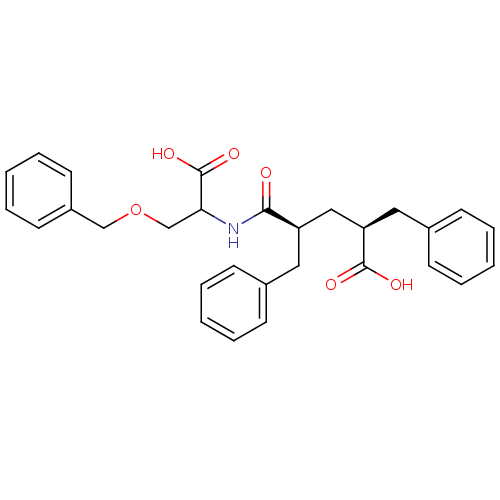 (2-Benzyl-4-(2-benzyloxy-1-carboxy-ethylcarbamoyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038594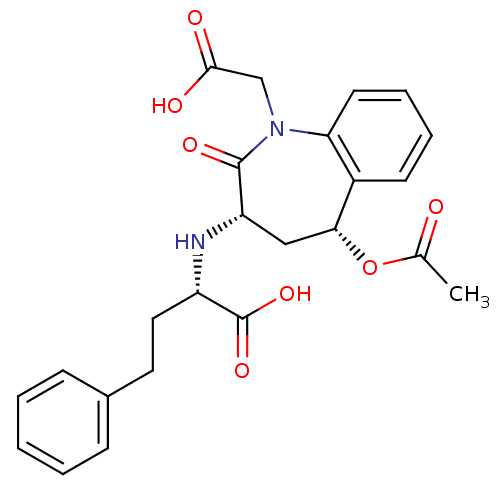 ((S)-2-((3S,5R)-5-Acetoxy-1-carboxymethyl-2-oxo-2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038593 (CHEMBL301130 | Potassium ;2-(1-Carboxymethyl-2-oxo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50159369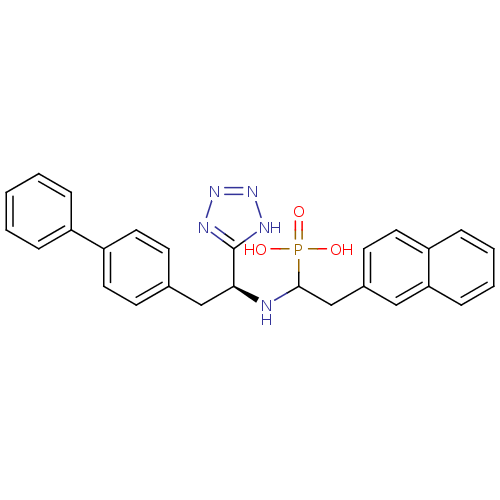 (CGS-31,4447 | CHEMBL415967 | {1-[(S)-2-Biphenyl-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50367235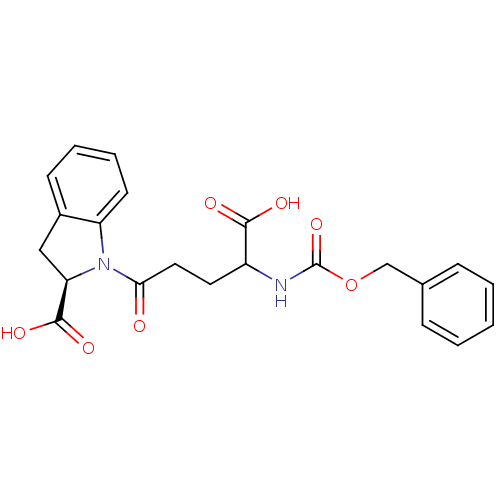 (CHEMBL1169570) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme from rabbit lung | J Med Chem 28: 1606-11 (1985) BindingDB Entry DOI: 10.7270/Q2TT4RHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038598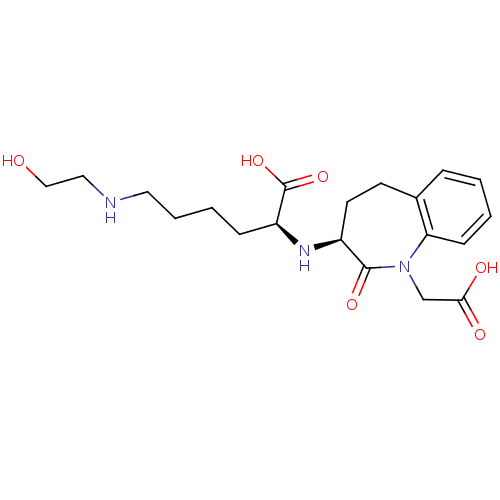 ((S)-2-((S)-1-Carboxymethyl-2-oxo-2,3,4,5-tetrahydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038596 ((S)-6-Amino-2-((R)-1-carboxymethyl-2-oxo-2,3,4,5-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50021176 (1-(4-Benzyloxycarbonylamino-4-carboxy-butyryl)-2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of angiotensin I converting enzyme from rabbit lung | J Med Chem 28: 1606-11 (1985) BindingDB Entry DOI: 10.7270/Q2TT4RHP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50038583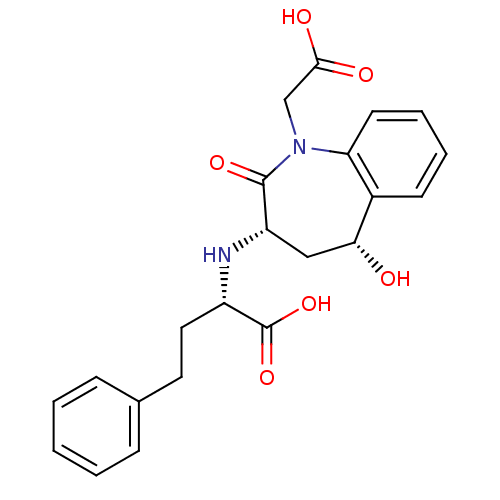 ((S)-2-((3S,5R)-1-Carboxymethyl-5-hydroxy-2-oxo-2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of Angiotensin converting enzyme(ACE) obtained from rabbit lung (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017733 ((2S,4S)-2-Benzyl-4-(2-carboxy-ethylcarbamoyl)-5-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM21641 (2-(2-benzyl-3-sulfanylpropanamido)acetic acid | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using ANF(atrial natriuretic factor) as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50291562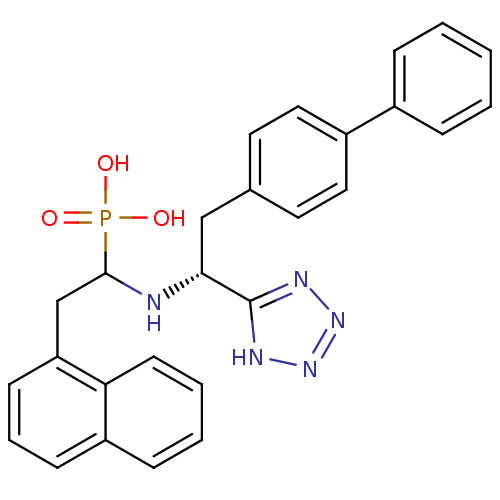 (CHEMBL9963 | {1-[(R)-2-Biphenyl-4-yl-1-(1H-tetrazo...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase 24.11(NEP) | Bioorg Med Chem Lett 7: 1059-1064 (1997) Article DOI: 10.1016/S0960-894X(97)00159-5 BindingDB Entry DOI: 10.7270/Q2WH2QGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50038586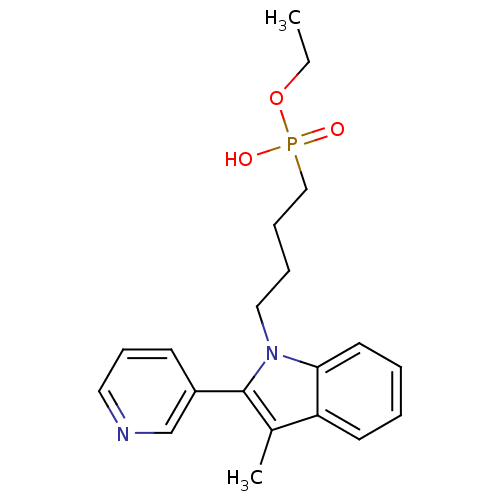 (CHEMBL544493 | [4-(3-Methyl-2-pyridin-3-yl-indol-1...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Tested for 50% inhibition of thromboxane synthase (TxS) in human platelets (in vitro) | J Med Chem 37: 1823-32 (1994) BindingDB Entry DOI: 10.7270/Q280537C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017743 (2-Benzyl-4-(3-carboxy-propionylamino)-5-phenyl-pen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50017733 ((2S,4S)-2-Benzyl-4-(2-carboxy-ethylcarbamoyl)-5-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50017738 (CHEMBL316172 | N-(4-Hydroxy-3-methoxy-benzyl)-2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 164 total ) | Next | Last >> |