

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50291312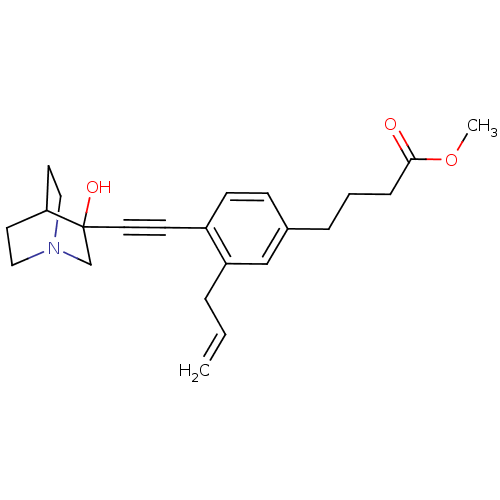 (4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50075719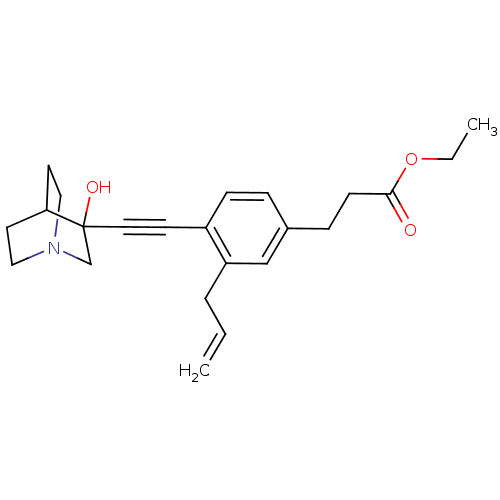 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291315 (5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291311 (6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291316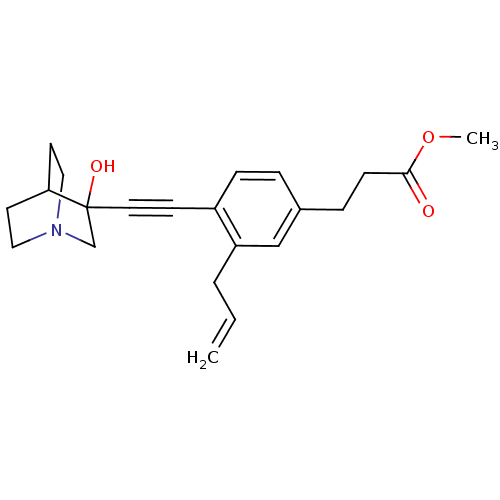 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Homo sapiens (Human)) | BDBM50075719 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291317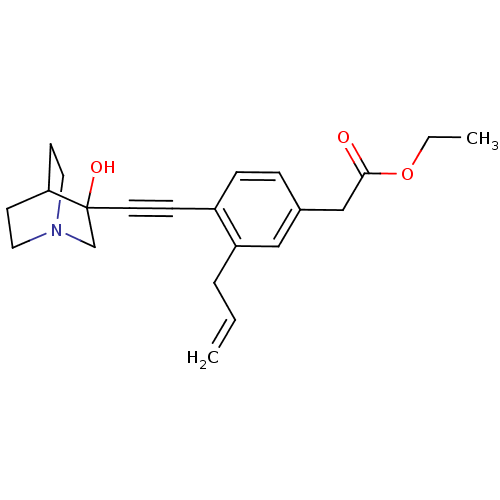 (CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291318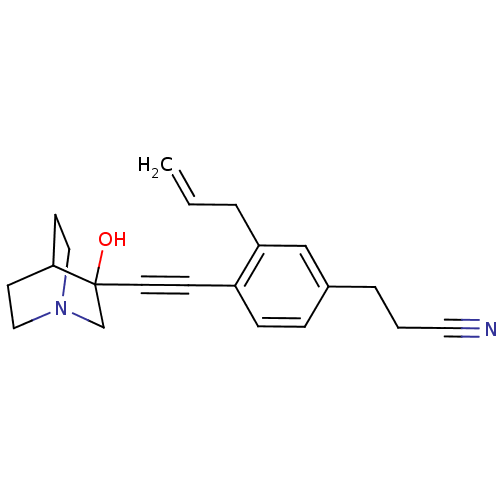 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291313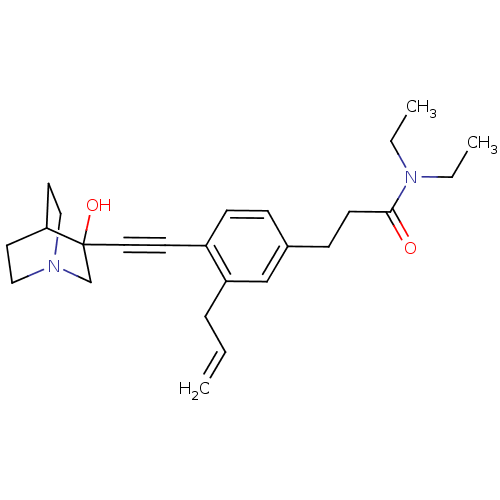 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291319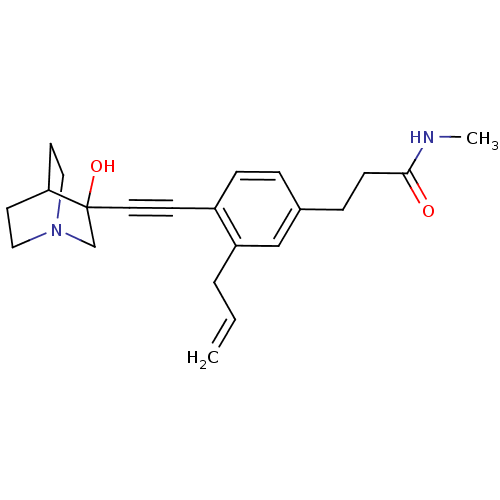 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50291314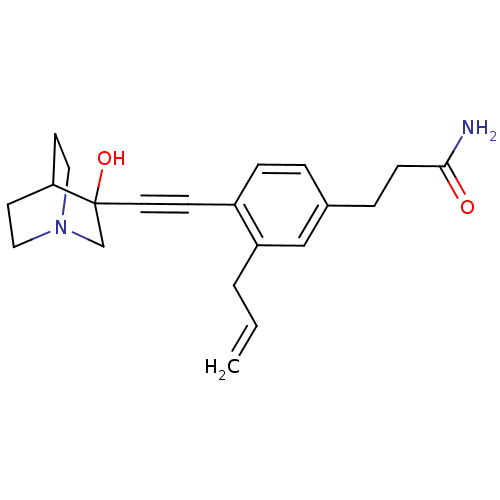 (3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) | Bioorg Med Chem Lett 7: 597-600 (1997) Article DOI: 10.1016/S0960-894X(97)00053-X BindingDB Entry DOI: 10.7270/Q2C24WFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50201701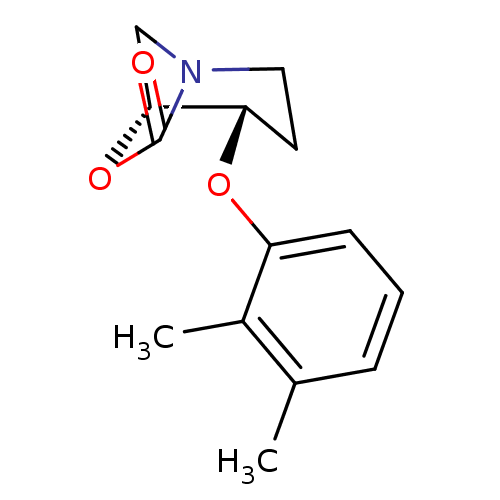 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50201705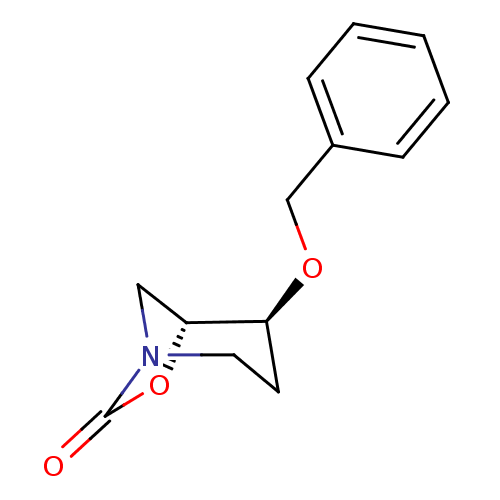 ((1R,2S)-2-(benzyloxy)-7-oxa-5-aza-bicyclo[3.2.1]oc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of EGFR (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50201700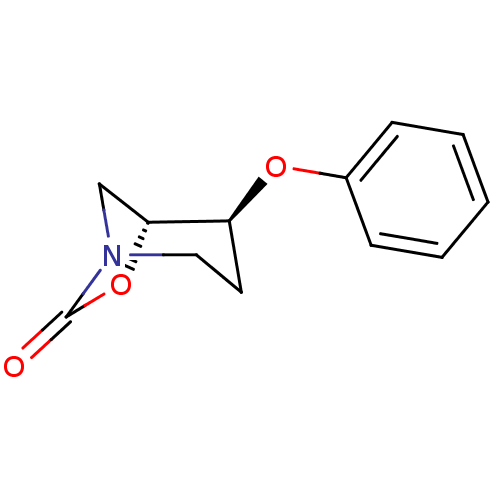 ((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50047126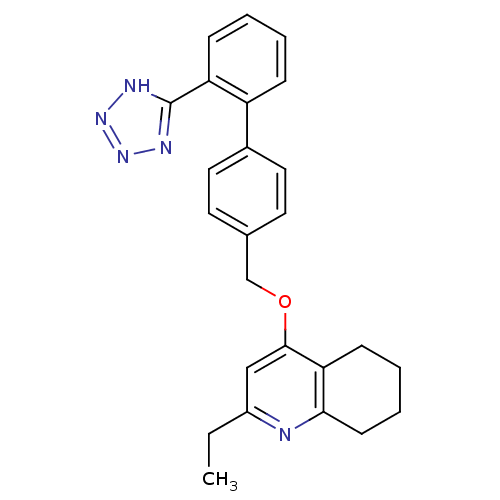 (2-Ethyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283596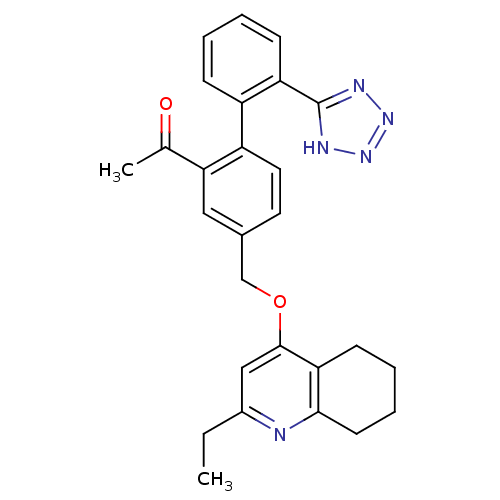 (1-[4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxym...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283591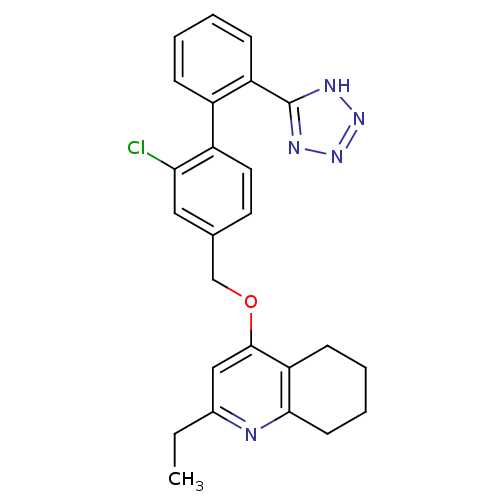 (4-[2-Chloro-2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50201699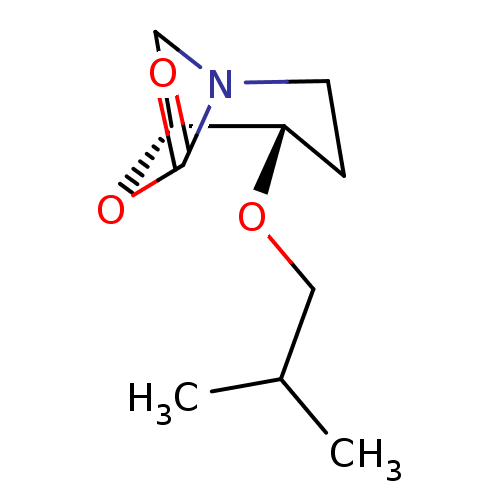 ((1R,2S)-2-isobutoxy-7-oxa-5-aza-bicyclo[3.2.1]octa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283597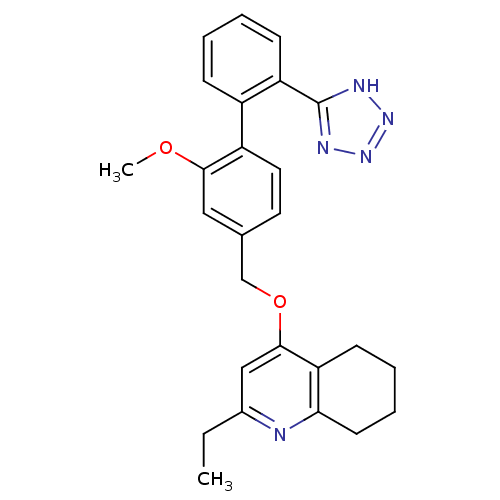 (2-Ethyl-4-[2-methoxy-2'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283587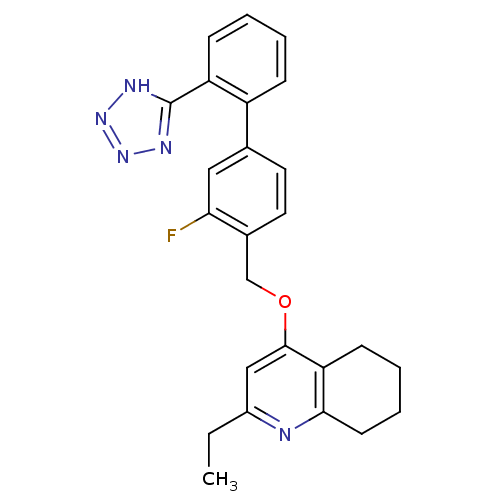 (2-Ethyl-4-[3-fluoro-2'-(2H-tetrazol-5-yl)-biphenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM4567 (4-anilinoquinazoline deriv. 2 | BMC163482 Compound...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HER4 (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HER4 (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50201701 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-dependent protein kinase 1 (Plasmodium Falciparum) | BDBM36336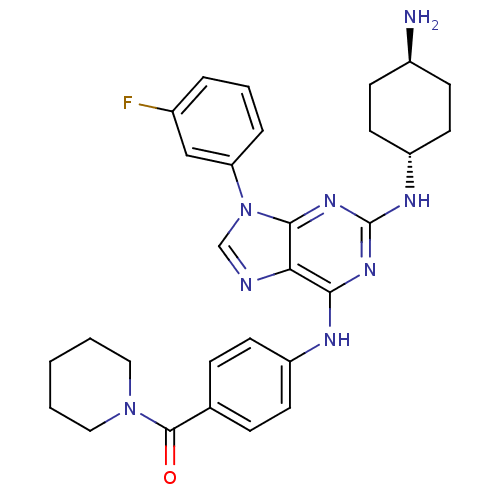 (CID24762166 | Purfalcamine, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.5 | 24 |
The Scripps Research Institute | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Nat Chem Biol 4: 347-56 (2008) Article DOI: 10.1038/nchembio.87 BindingDB Entry DOI: 10.7270/Q2TB1573 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50201700 ((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin S | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50201701 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin C | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50009714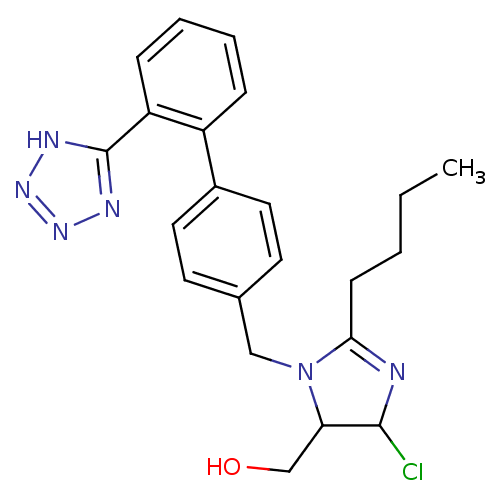 (CHEMBL191 | {2-Butyl-5-chloro-3-[2'-(2H-tetrazol-5...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283593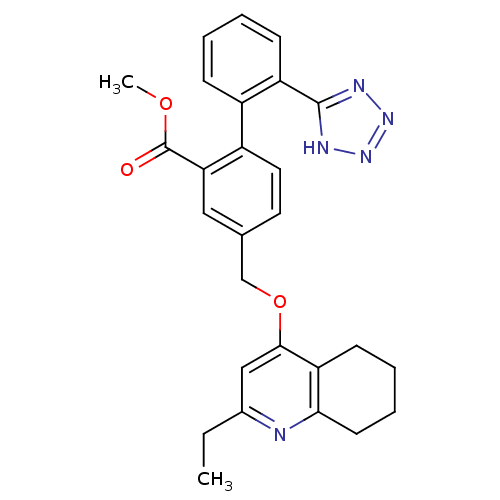 (4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50201701 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM4567 (4-anilinoquinazoline deriv. 2 | BMC163482 Compound...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HER2 (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50201700 ((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin L | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283590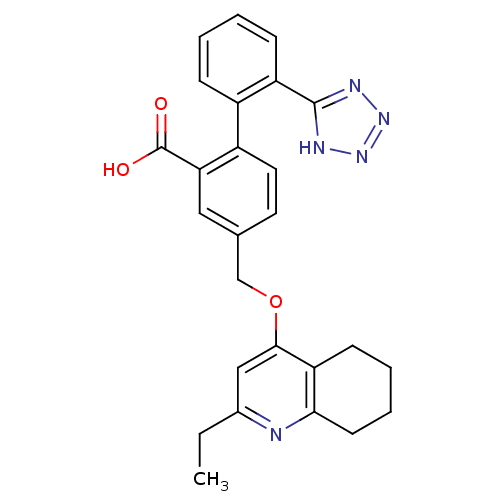 (4-(2-Ethyl-5,6,7,8-tetrahydro-quinolin-4-yloxymeth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to AT1 receptor in guinea pig adrenal membrane preparation | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcium-dependent protein kinase 1 (Plasmodium Falciparum) | BDBM36340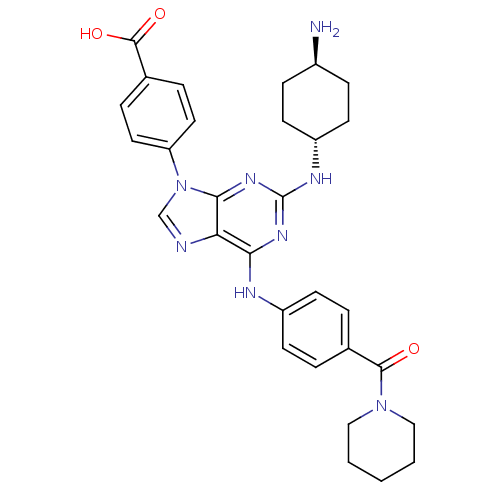 (4-{2-trans-(4-Amino-cyclohexylamino)-6-[4-(piperid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.5 | 24 |
The Scripps Research Institute | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Nat Chem Biol 4: 347-56 (2008) Article DOI: 10.1038/nchembio.87 BindingDB Entry DOI: 10.7270/Q2TB1573 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Blk (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283589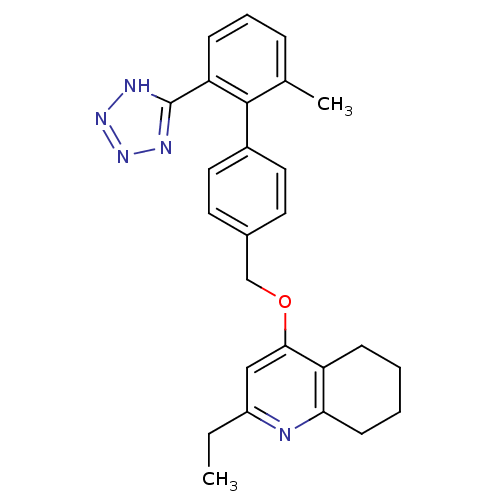 (2-Ethyl-4-[2'-methyl-6'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Homo sapiens (Human)) | BDBM50201700 ((1R,2S)-2-phenoxy-7-oxa-5-aza-bicyclo[3.2.1]octan-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin C | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50201701 (CHEMBL390474 | cis-4-(2,3-dimethylphenoxy)-6-oxa-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin K | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50201698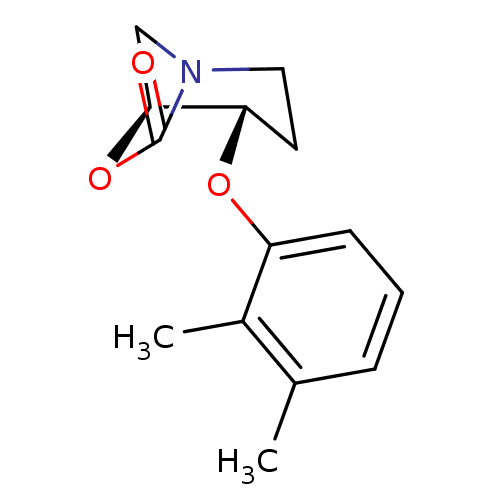 (CHEMBL230473 | rac-trans4-(2,3-dimethylphenoxy)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human recombinant cathepsin B | Bioorg Med Chem Lett 17: 1254-9 (2007) Article DOI: 10.1016/j.bmcl.2006.12.014 BindingDB Entry DOI: 10.7270/Q2QN66DT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of HER2 (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Blk (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Blk (unknown origin) | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283592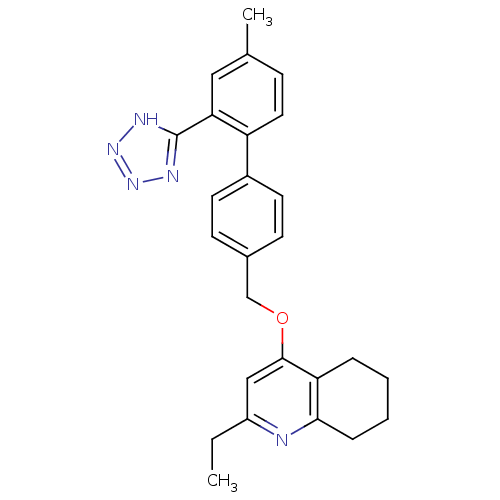 (2-Ethyl-4-[4'-methyl-2'-(2H-tetrazol-5-yl)-bipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM4779 (CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of Bmx (unknown origin) expressed in mouse BAF3 cells assessed as cytotoxicity | Bioorg Med Chem Lett 18: 5916-9 (2008) Article DOI: 10.1016/j.bmcl.2008.07.062 BindingDB Entry DOI: 10.7270/Q2M0457X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283595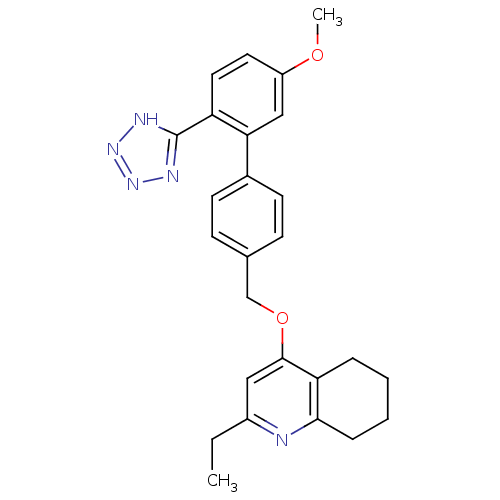 (2-Ethyl-4-[5'-methoxy-2'-(2H-tetrazol-5-yl)-biphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196094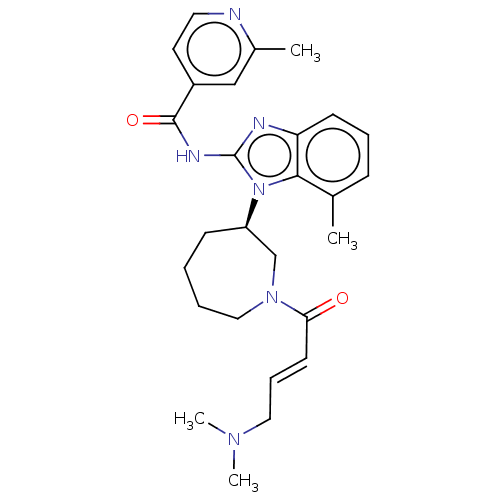 (CHEMBL3960167) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcium-dependent protein kinase 1 (Plasmodium Falciparum) | BDBM36339 (3-{2-trans-(4-Amino-cyclohexylamino)-6-[4-(piperid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.5 | 24 |
The Scripps Research Institute | Assay Description The test compounds and enzyme were mixed in buffer, and the substrate and [gamma-33P] ATP/ATP were added to the mixture to initiate the reaction. Aft... | Nat Chem Biol 4: 347-56 (2008) Article DOI: 10.1038/nchembio.87 BindingDB Entry DOI: 10.7270/Q2TB1573 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50196093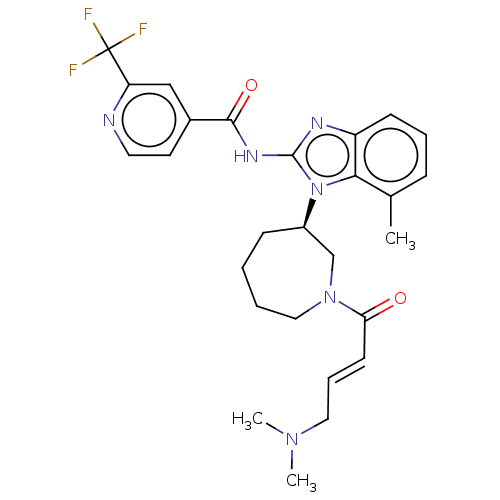 (CHEMBL3939913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50029668 (AZD-9291 | Osimertinib | US10085983, Compound AZD-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of wild type EGFR phosphorylation in human HaCaT cells incubated for 3 hrs by ELISA method | J Med Chem 59: 6671-89 (2016) Article DOI: 10.1021/acs.jmedchem.5b01985 BindingDB Entry DOI: 10.7270/Q2RF5X00 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Type-1 angiotensin II receptor (Cavia porcellus) | BDBM50283588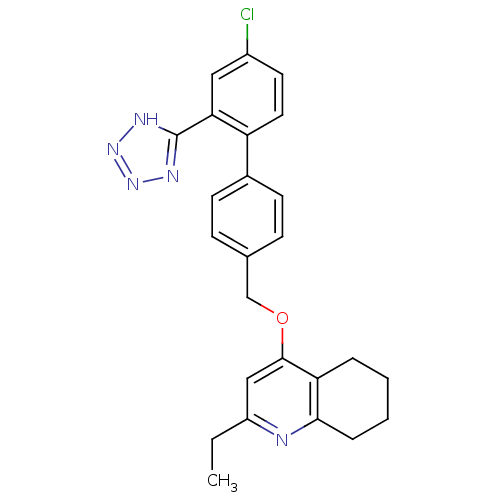 (4-[4'-Chloro-2'-(2H-tetrazol-5-yl)-biphenyl-4-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of specific binding of [I-125]AII to Angiotensin II receptor, type 1 in guinea pig adrenal membrane prepa... | Bioorg Med Chem Lett 4: 2615-2620 (1994) Article DOI: 10.1016/S0960-894X(01)80295-X BindingDB Entry DOI: 10.7270/Q22J6BTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1/Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50005340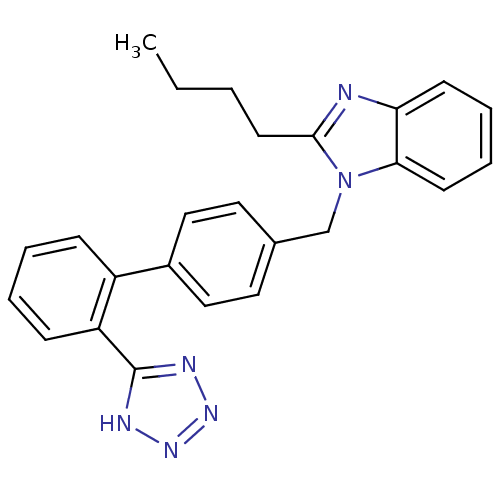 (2-Butyl-1-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
ICI Pharmaceuticals Curated by ChEMBL | Assay Description Concentration required for 50% displacement of the specifically bound [3-[125I]-iodotyrosyl]-angiotensin II from angiotensin II receptor in the membr... | J Med Chem 35: 877-85 (1992) BindingDB Entry DOI: 10.7270/Q23R0W37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 261 total ) | Next | Last >> |