

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50465935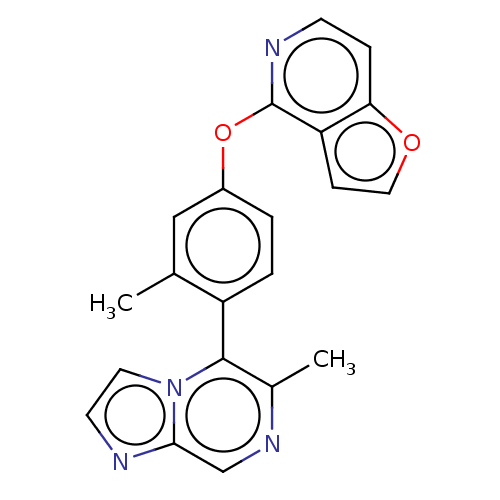 (CHEMBL4277264) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at dopamine D5 receptor (unknown origin) | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465945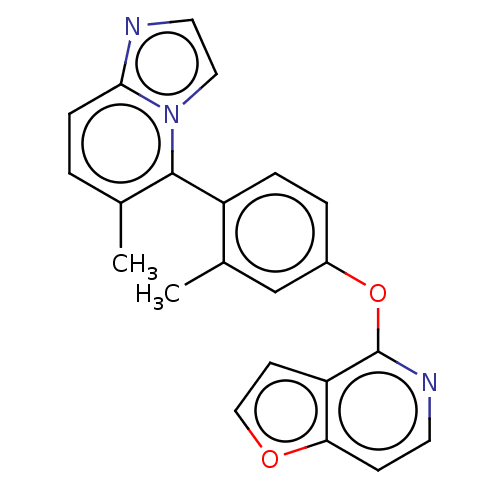 (CHEMBL4279267) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465944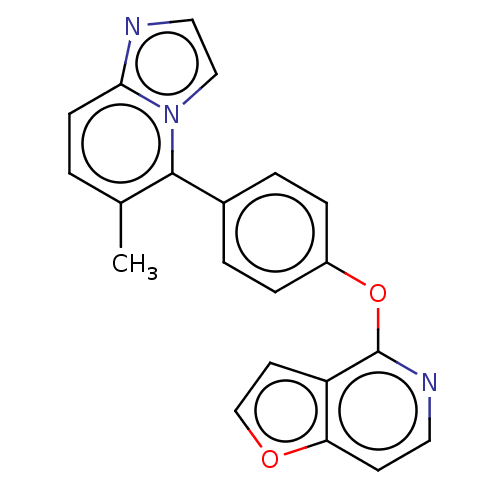 (CHEMBL4286177) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465946 (CHEMBL4294397) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465943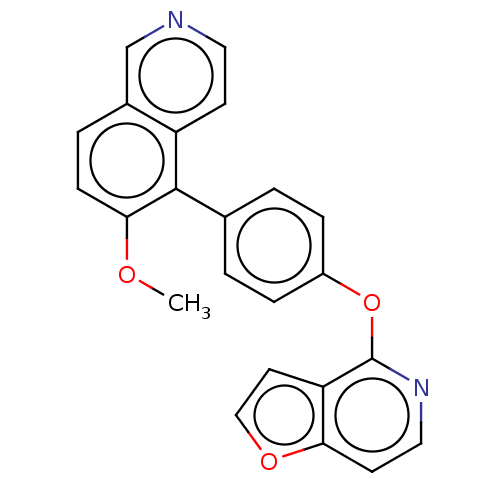 (CHEMBL4286110) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465938 (CHEMBL4294009) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465934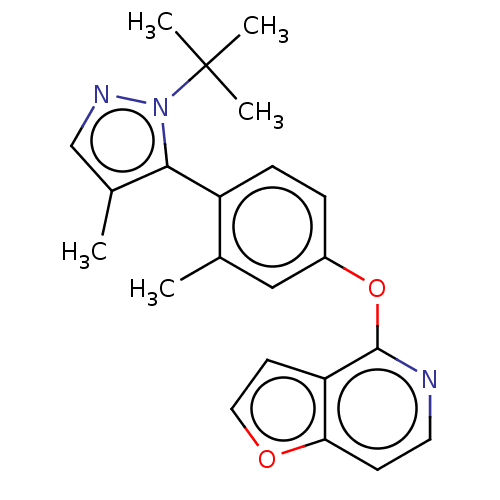 (CHEMBL4293757) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 121 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | 243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465942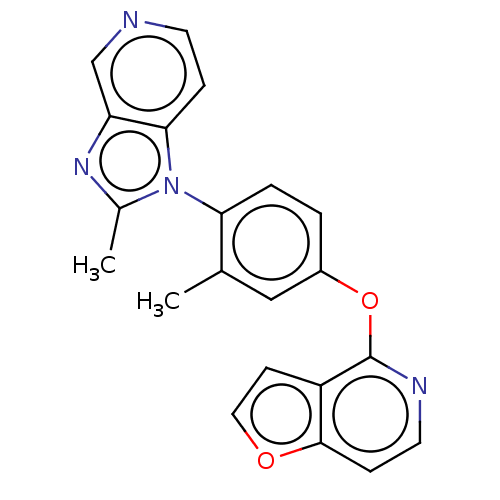 (CHEMBL4278861) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 244 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465936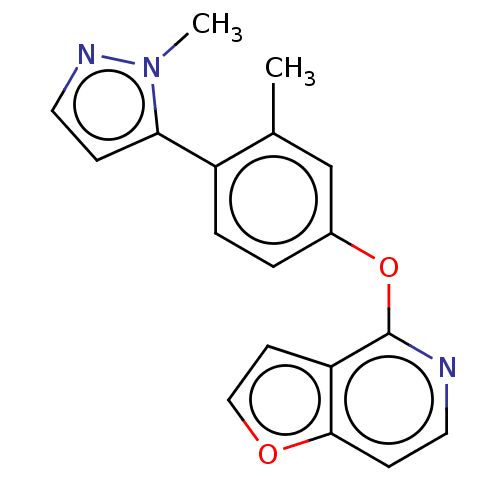 (CHEMBL4293356) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465947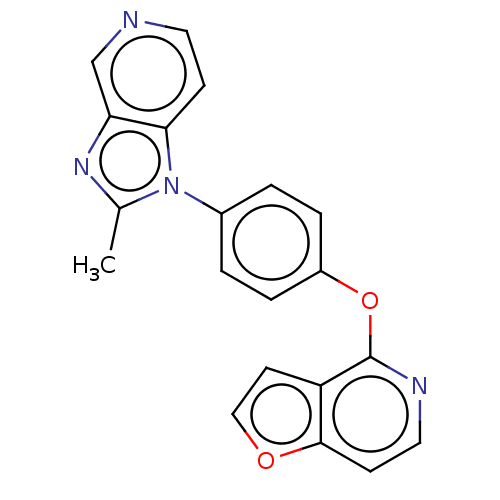 (CHEMBL4289538) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465941 (CHEMBL4283176) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 563 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465939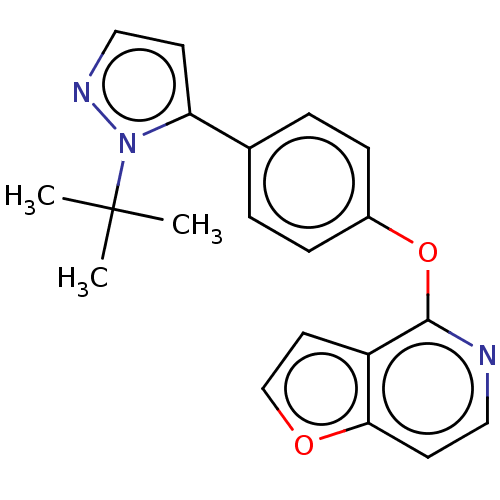 (CHEMBL4285528) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465940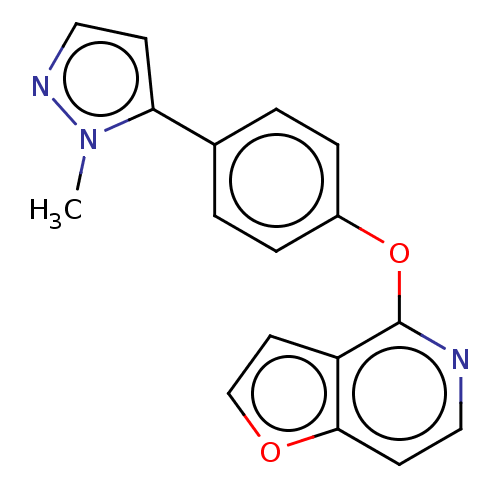 (CHEMBL4282096) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465937 (CHEMBL4287192) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from human dopamine D1 receptor expressed in LTK cell membranes after 30 mins by liquid scintillation counting | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Agonist activity at dopamine D2 receptor (unknown origin) | J Med Chem 61: 11384-11397 (2018) Article DOI: 10.1021/acs.jmedchem.8b01622 BindingDB Entry DOI: 10.7270/Q20G3NT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032162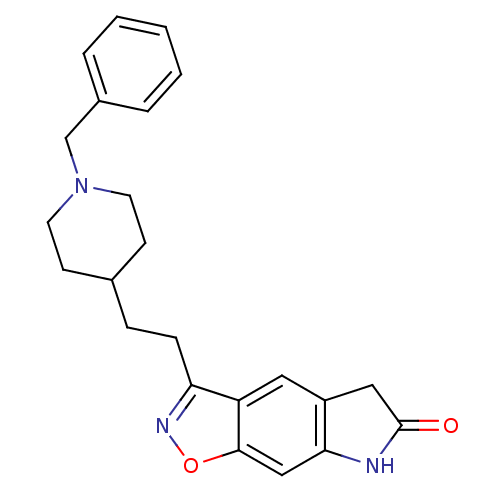 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-5,7-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032161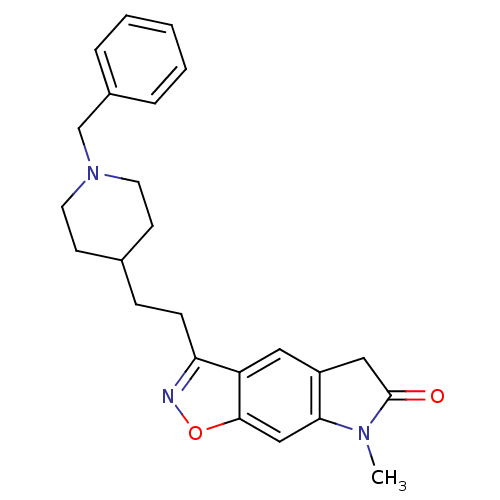 (3-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-7-methyl-5,7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032164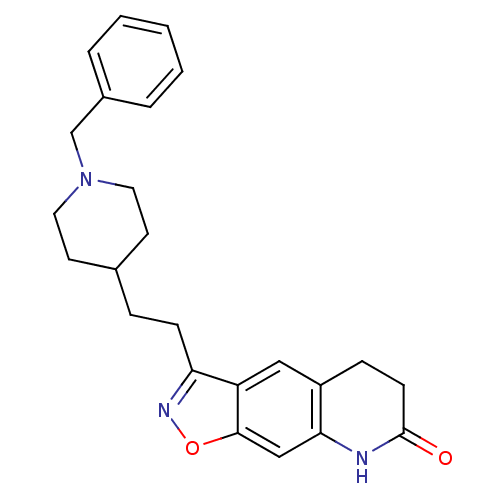 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5,6-dihydroiso...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032163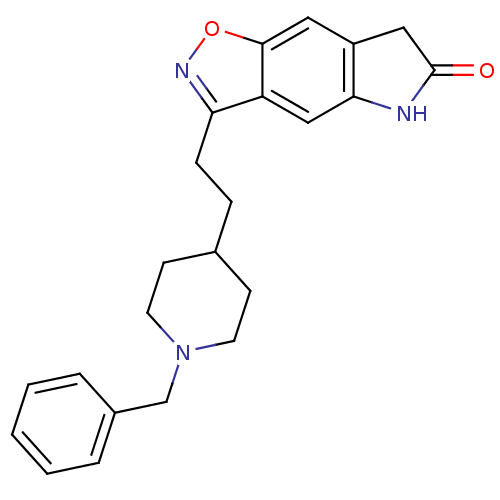 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-isoxazolo[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255776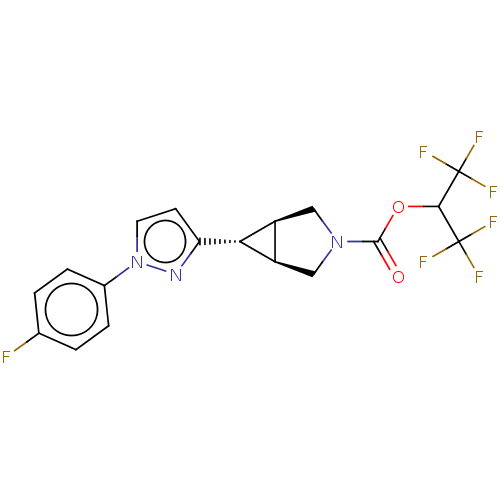 (CHEMBL4075310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032165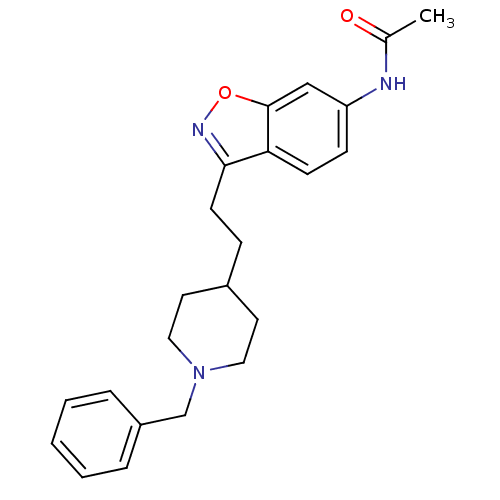 (CHEMBL92736 | CHEMBL94217 | N-{3-[2-(1-Benzyl-pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255775 (CHEMBL4079080) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50032160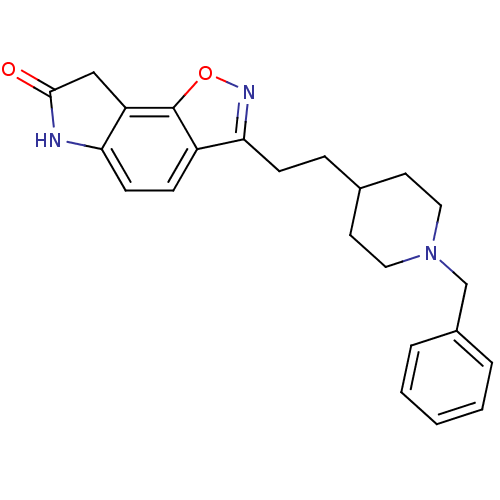 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-6H-isoxazolo[5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM60622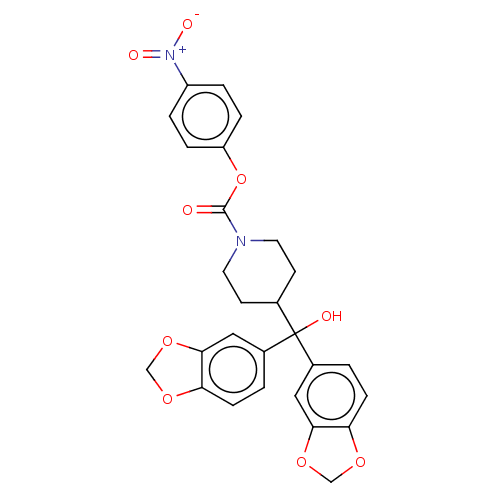 (BDBM50300355 | US11753371, Compound JZL-184 | US91...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255806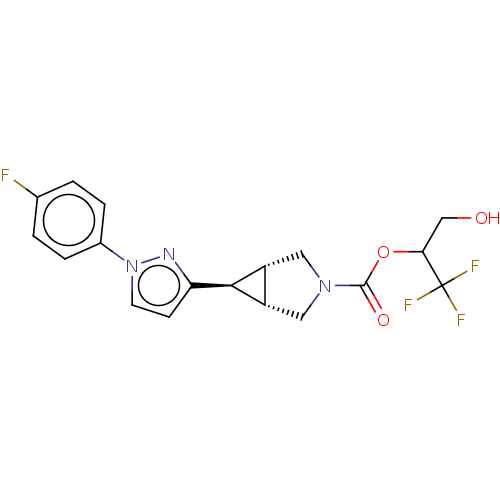 (CHEMBL4097100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Butyrylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50091800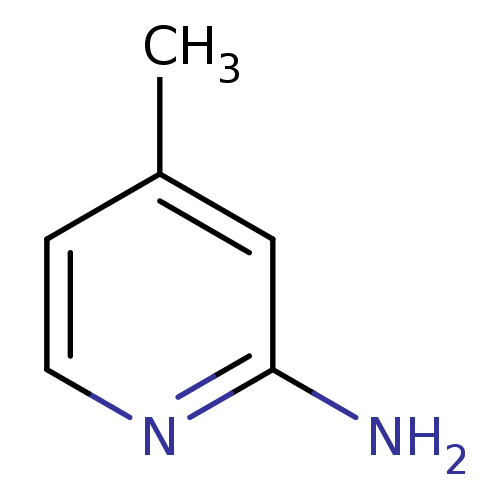 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human inducible nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50255777 (CHEMBL4061795) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human FAAH using fluorogenic arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Acetylcholinesterase from human erythrocytes | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50141086 (6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255777 (CHEMBL4061795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255805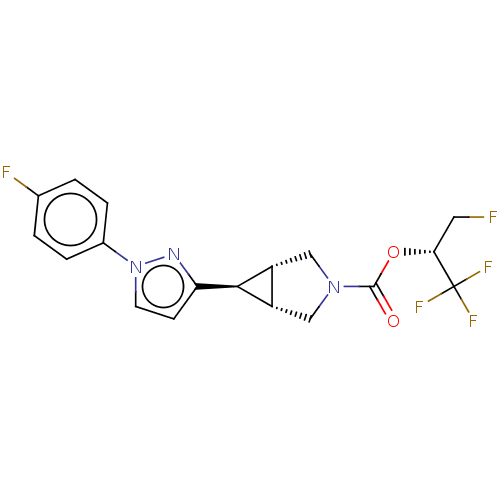 (CHEMBL4086931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50141082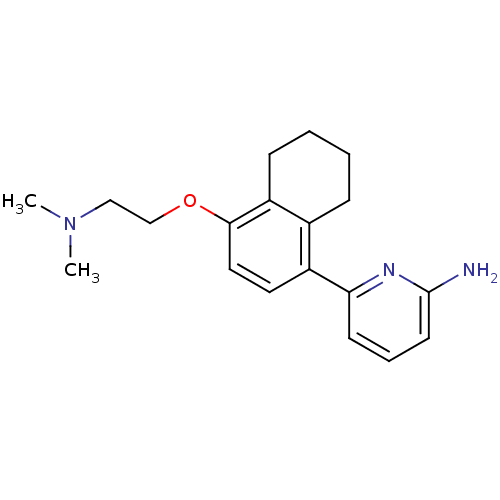 (6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50091800 (2-amino-4-methylpyridine | 4-METHYLPYRIDIN-2-AMINE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50255786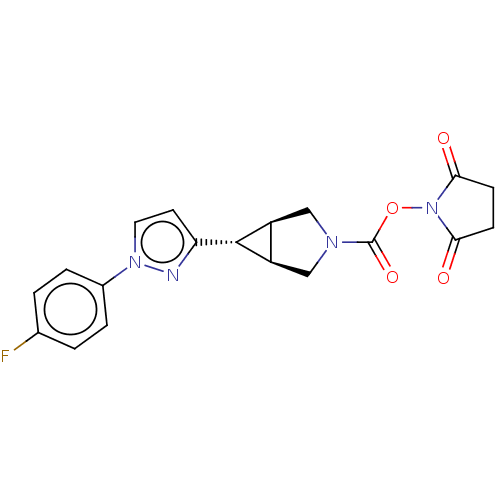 (CHEMBL4076777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Worldwide Research and Development , 610 Main Street , Cambridge , Massachusetts 02139 , United States. Curated by ChEMBL | Assay Description Inhibition of recombinant human MAGL using fluorogenic-7HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 61: 3008-3026 (2018) Article DOI: 10.1021/acs.jmedchem.8b00070 BindingDB Entry DOI: 10.7270/Q2S46VD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50141081 (6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50141086 (6-[7-(2-Dimethylamino-ethoxy)-indan-4-yl]-pyridin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50150885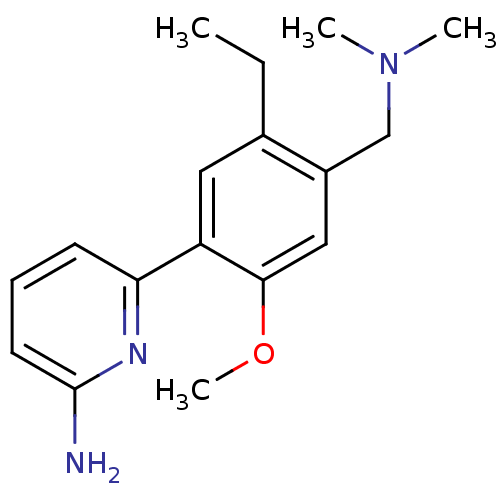 (6-(4-Dimethylaminomethyl-5-ethyl-2-methoxy-phenyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase in human | Bioorg Med Chem Lett 14: 4511-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.043 BindingDB Entry DOI: 10.7270/Q28S4QP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50150887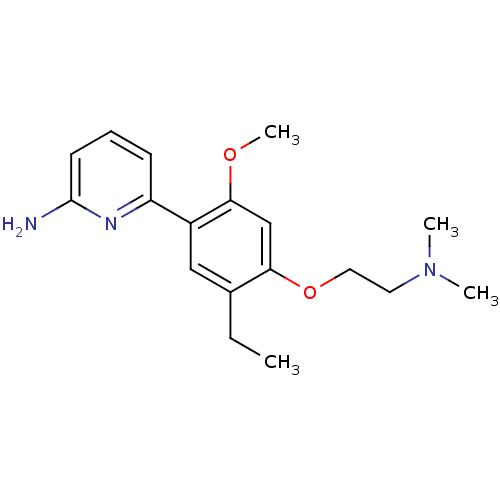 (6-[4-(2-Dimethylamino-ethoxy)-5-ethyl-2-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase in human | Bioorg Med Chem Lett 14: 4511-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.043 BindingDB Entry DOI: 10.7270/Q28S4QP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50141089 (6-[7-(2-Pyrrolidin-1-yl-ethoxy)-indan-4-yl]-pyridi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of rat neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50141081 (6-[4-(1-Methyl-pyrrolidin-3-yloxy)-naphthalen-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50141082 (6-[4-(2-Dimethylamino-ethoxy)-5,6,7,8-tetrahydro-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibition of human neuronal nitric oxide synthase | J Med Chem 47: 1575-86 (2004) Article DOI: 10.1021/jm030519g BindingDB Entry DOI: 10.7270/Q2J102MT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description In vitro inhibition of Butyrylcholinesterase from human serum | J Med Chem 38: 2802-8 (1995) BindingDB Entry DOI: 10.7270/Q2Q52NN0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50150884 (6-[4-(3-Dimethylamino-propyl)-5-ethyl-2-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase in human | Bioorg Med Chem Lett 14: 4511-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.043 BindingDB Entry DOI: 10.7270/Q28S4QP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50150890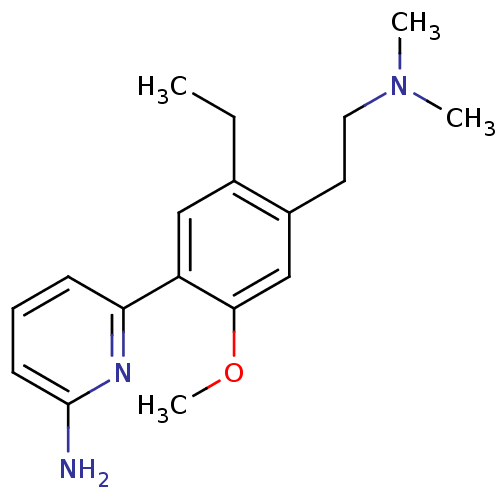 (6-[4-(2-Dimethylamino-ethyl)-5-ethyl-2-methoxy-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against neuronal nitric oxide synthase in human | Bioorg Med Chem Lett 14: 4511-4 (2004) Article DOI: 10.1016/j.bmcl.2004.06.043 BindingDB Entry DOI: 10.7270/Q28S4QP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |