 Found 332 hits with Last Name = 'green' and Initial = 'dw'
Found 332 hits with Last Name = 'green' and Initial = 'dw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037121
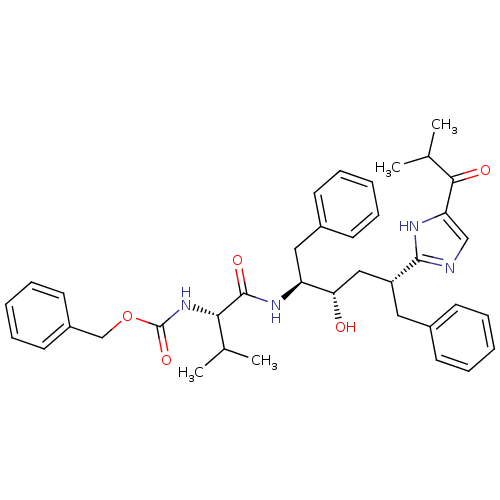
(2-[(1R,3S,4S)-1-BENZYL-4-[N-(BENZYLOXYCARBONYL)-L-...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C(C)C Show InChI InChI=1S/C38H46N4O5/c1-25(2)34(42-38(46)47-24-29-18-12-7-13-19-29)37(45)41-31(21-28-16-10-6-11-17-28)33(43)22-30(20-27-14-8-5-9-15-27)36-39-23-32(40-36)35(44)26(3)4/h5-19,23,25-26,30-31,33-34,43H,20-22,24H2,1-4H3,(H,39,40)(H,41,45)(H,42,46)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037124
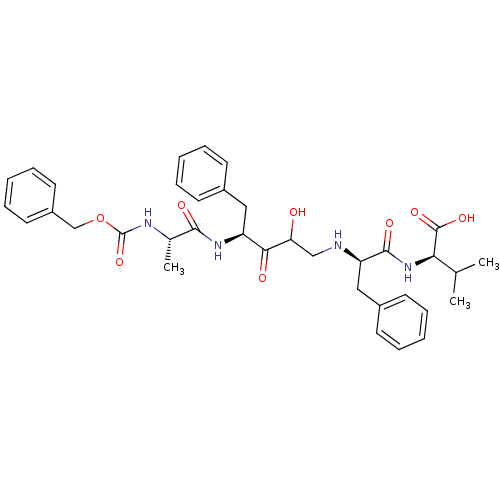
((R)-2-{(R)-2-[(S)-4-((S)-2-Benzyloxycarbonylamino-...)Show SMILES CC(C)[C@@H](NC(=O)[C@@H](Cc1ccccc1)NCC(O)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](C)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C36H44N4O8/c1-23(2)31(35(45)46)40-34(44)29(20-26-15-9-5-10-16-26)37-21-30(41)32(42)28(19-25-13-7-4-8-14-25)39-33(43)24(3)38-36(47)48-22-27-17-11-6-12-18-27/h4-18,23-24,28-31,37,41H,19-22H2,1-3H3,(H,38,47)(H,39,43)(H,40,44)(H,45,46)/t24-,28-,29+,30?,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037122

(CHEMBL108016 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-(5...)Show SMILES CC(C)C(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C30H39N3O4/c1-20(2)27(35)25-19-31-28(32-25)23(16-21-12-8-6-9-13-21)18-26(34)24(17-22-14-10-7-11-15-22)33-29(36)37-30(3,4)5/h6-15,19-20,23-24,26,34H,16-18H2,1-5H3,(H,31,32)(H,33,36)/t23-,24+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037126
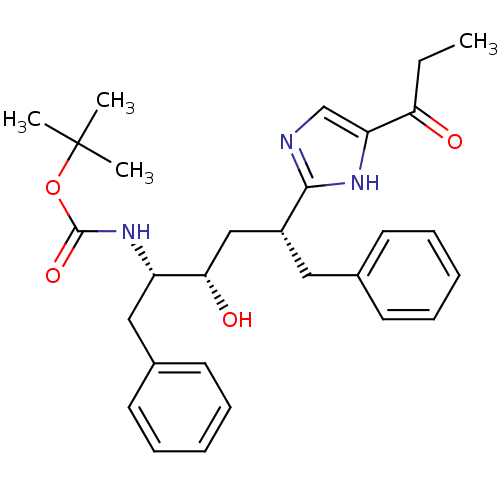
(CHEMBL80098 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-5-phe...)Show SMILES CCC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4/c1-5-25(33)24-19-30-27(31-24)22(16-20-12-8-6-9-13-20)18-26(34)23(17-21-14-10-7-11-15-21)32-28(35)36-29(2,3)4/h6-15,19,22-23,26,34H,5,16-18H2,1-4H3,(H,30,31)(H,32,35)/t22-,23+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037125
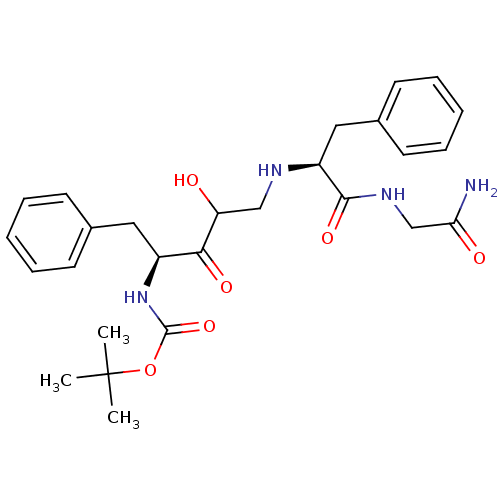
(CHEMBL107849 | {(S)-1-Benzyl-4-[(S)-1-(carbamoylme...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)C(O)CN[C@@H](Cc1ccccc1)C(=O)NCC(N)=O Show InChI InChI=1S/C27H36N4O6/c1-27(2,3)37-26(36)31-20(14-18-10-6-4-7-11-18)24(34)22(32)16-29-21(25(35)30-17-23(28)33)15-19-12-8-5-9-13-19/h4-13,20-22,29,32H,14-17H2,1-3H3,(H2,28,33)(H,30,35)(H,31,36)/t20-,21-,22?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037128
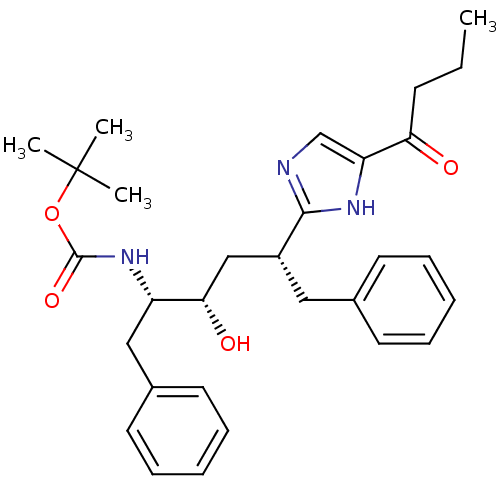
(CHEMBL419921 | [(1S,2S,4R)-1-Benzyl-4-(5-butyryl-1...)Show SMILES CCCC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C30H39N3O4/c1-5-12-26(34)25-20-31-28(32-25)23(17-21-13-8-6-9-14-21)19-27(35)24(18-22-15-10-7-11-16-22)33-29(36)37-30(2,3)4/h6-11,13-16,20,23-24,27,35H,5,12,17-19H2,1-4H3,(H,31,32)(H,33,36)/t23-,24+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037123

(CHEMBL108226 | {(1S,2S,4R)-1-Benzyl-4-[5-(2,2-dime...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C(C)(C)C=C Show InChI InChI=1S/C32H41N3O4/c1-7-32(5,6)28(37)26-21-33-29(34-26)24(18-22-14-10-8-11-15-22)20-27(36)25(19-23-16-12-9-13-17-23)35-30(38)39-31(2,3)4/h7-17,21,24-25,27,36H,1,18-20H2,2-6H3,(H,33,34)(H,35,38)/t24-,25+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037127

(CHEMBL104285 | [(1S,2S,4R)-4-(5-Acetyl-1H-imidazol...)Show SMILES CC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C28H35N3O4/c1-19(32)24-18-29-26(30-24)22(15-20-11-7-5-8-12-20)17-25(33)23(16-21-13-9-6-10-14-21)31-27(34)35-28(2,3)4/h5-14,18,22-23,25,33H,15-17H2,1-4H3,(H,29,30)(H,31,34)/t22-,23+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50032937

(2-{2-[(S)-2-(3-Benzyl-5-{1-[(1-carboxy-ethyl)-meth...)Show SMILES COC(=O)C(NC(=O)C(NC(=O)[C@H](C)N1CCC(=CC(Cc2ccccc2)C1)C(C)N(C)C(C)C(O)=O)C(C)C)C(C)C |c:17| Show InChI InChI=1S/C33H52N4O6/c1-20(2)28(31(39)35-29(21(3)4)33(42)43-9)34-30(38)23(6)37-16-15-27(22(5)36(8)24(7)32(40)41)18-26(19-37)17-25-13-11-10-12-14-25/h10-14,18,20-24,26,28-29H,15-17,19H2,1-9H3,(H,34,38)(H,35,39)(H,40,41)/t22?,23-,24?,26?,28?,29?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibitory activity of the compound was determined against HIV-1 protease |
J Med Chem 38: 3246-52 (1995)
BindingDB Entry DOI: 10.7270/Q2RX9B43 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037120

(CHEMBL104523 | {(1S,2S,4R)-1-Benzyl-2-hydroxy-4-[5...)Show SMILES CC(C)C(O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C30H41N3O4/c1-20(2)27(35)25-19-31-28(32-25)23(16-21-12-8-6-9-13-21)18-26(34)24(17-22-14-10-7-11-15-22)33-29(36)37-30(3,4)5/h6-15,19-20,23-24,26-27,34-35H,16-18H2,1-5H3,(H,31,32)(H,33,36)/t23-,24+,26+,27?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037129
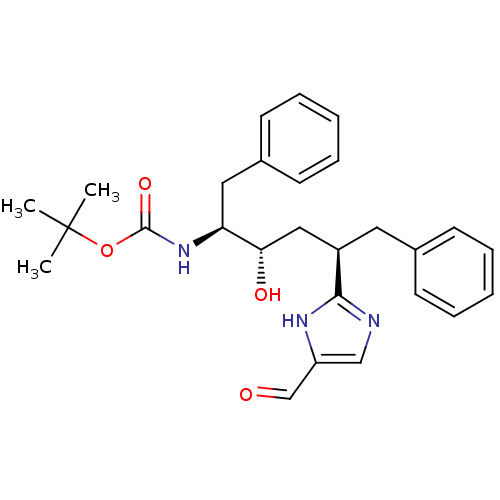
(CHEMBL106776 | [(1S,2S,4R)-1-Benzyl-4-(5-formyl-1H...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc(C=O)[nH]1 Show InChI InChI=1S/C27H33N3O4/c1-27(2,3)34-26(33)30-23(15-20-12-8-5-9-13-20)24(32)16-21(14-19-10-6-4-7-11-19)25-28-17-22(18-31)29-25/h4-13,17-18,21,23-24,32H,14-16H2,1-3H3,(H,28,29)(H,30,33)/t21-,23+,24+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037119
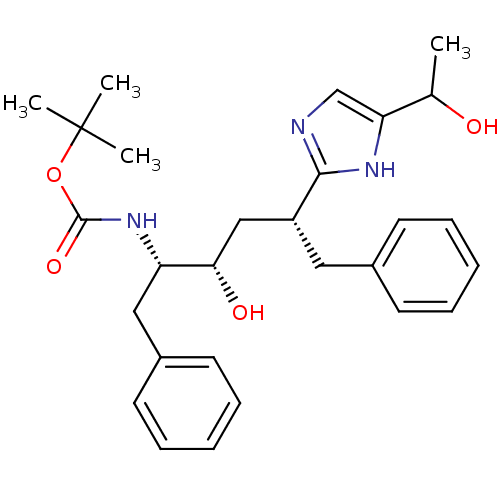
(CHEMBL448480 | {(1S,2S,4R)-1-Benzyl-2-hydroxy-4-[5...)Show SMILES CC(O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C28H37N3O4/c1-19(32)24-18-29-26(30-24)22(15-20-11-7-5-8-12-20)17-25(33)23(16-21-13-9-6-10-14-21)31-27(34)35-28(2,3)4/h5-14,18-19,22-23,25,32-33H,15-17H2,1-4H3,(H,29,30)(H,31,34)/t19?,22-,23+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Apparent inhibition constant against recombinant HIV-1 protease |
J Med Chem 37: 3100-7 (1994)
BindingDB Entry DOI: 10.7270/Q2T72GG9 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50032938

(2-{2-[(S)-2-(3-Benzyl-5-{1-[(1-carboxy-ethyl)-meth...)Show SMILES COC(=O)C(NC(=O)C(NC(=O)[C@H](C)N1CCC(=CC(Cc2ccccc2)C1=O)C(C)N(C)C(C)C(O)=O)C(C)C)C(C)C |c:17| Show InChI InChI=1S/C33H50N4O7/c1-19(2)27(30(39)35-28(20(3)4)33(43)44-9)34-29(38)22(6)37-16-15-25(21(5)36(8)23(7)32(41)42)18-26(31(37)40)17-24-13-11-10-12-14-24/h10-14,18-23,26-28H,15-17H2,1-9H3,(H,34,38)(H,35,39)(H,41,42)/t21?,22-,23?,26?,27?,28?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Competitive inhibitory activity of the compound was determined against HIV-1 protease |
J Med Chem 38: 3246-52 (1995)
BindingDB Entry DOI: 10.7270/Q2RX9B43 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375120

(CHEMBL403040)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(CC[C@@H]2CCCN2C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-22-13-11-20(12-14-22)27(39)19-38-26-10-2-9-25(33)29(26)37(31(38)34)18-15-23-7-4-17-36(23)30(40)24-8-1-5-21-6-3-16-35-28(21)24/h1-3,5-6,8-14,16,23,34H,4,7,15,17-19H2/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50142256

(4-(4-tert-Butyl-benzenesulfonyl)-1-(3H-imidazol-4-...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CC(CCc2ccccc2)N(Cc2cnc[nH]2)c2ccccc2C1 Show InChI InChI=1S/C31H36N4O2S/c1-31(2,3)26-14-17-29(18-15-26)38(36,37)34-20-25-11-7-8-12-30(25)35(21-27-19-32-23-33-27)28(22-34)16-13-24-9-5-4-6-10-24/h4-12,14-15,17-19,23,28H,13,16,20-22H2,1-3H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375119
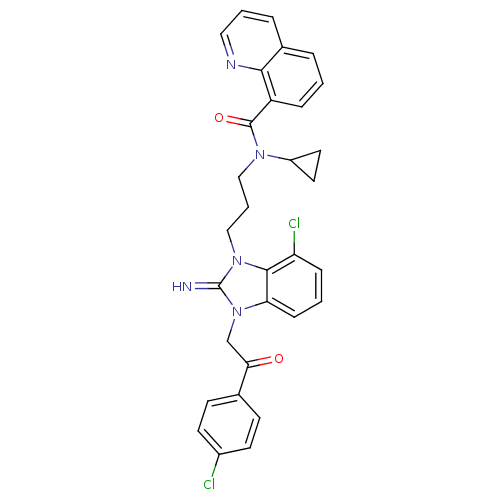
(CHEMBL401868)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(CCCN(C2CC2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-22-12-10-20(11-13-22)27(39)19-38-26-9-2-8-25(33)29(26)37(31(38)34)18-4-17-36(23-14-15-23)30(40)24-7-1-5-21-6-3-16-35-28(21)24/h1-3,5-13,16,23,34H,4,14-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375121
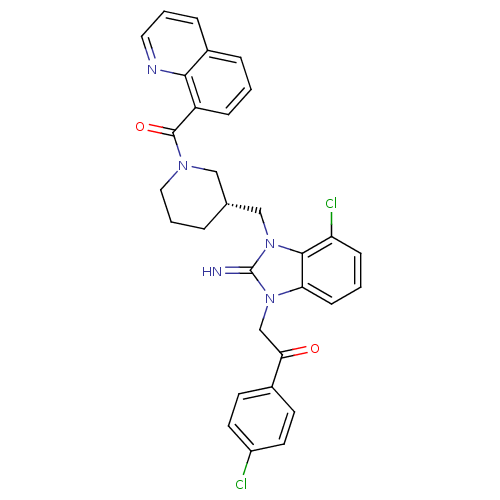
(CHEMBL257038)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375116
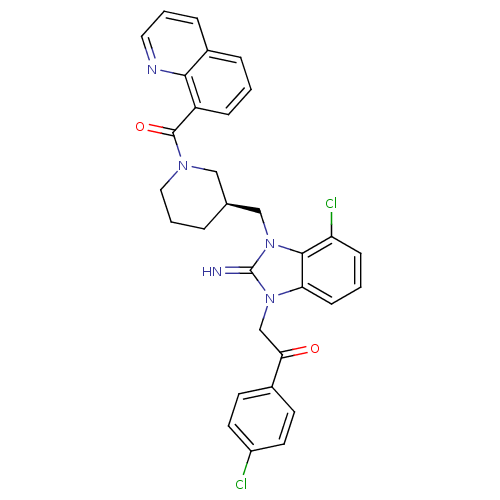
(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375124
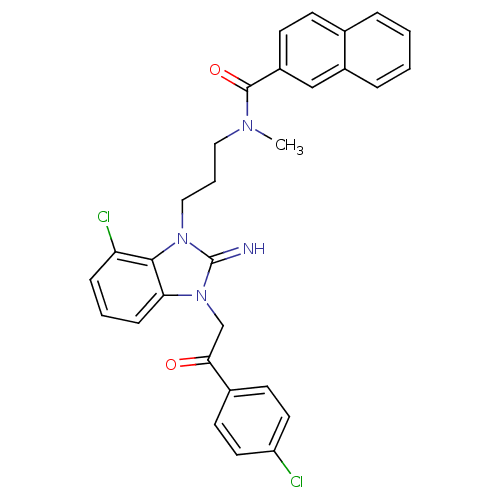
(CHEMBL256457)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H26Cl2N4O2/c1-34(29(38)23-11-10-20-6-2-3-7-22(20)18-23)16-5-17-35-28-25(32)8-4-9-26(28)36(30(35)33)19-27(37)21-12-14-24(31)15-13-21/h2-4,6-15,18,33H,5,16-17,19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375116

(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375116

(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375121

(CHEMBL257038)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375119

(CHEMBL401868)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(CCCN(C2CC2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-22-12-10-20(11-13-22)27(39)19-38-26-9-2-8-25(33)29(26)37(31(38)34)18-4-17-36(23-14-15-23)30(40)24-7-1-5-21-6-3-16-35-28(21)24/h1-3,5-13,16,23,34H,4,14-15,17-19H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375123
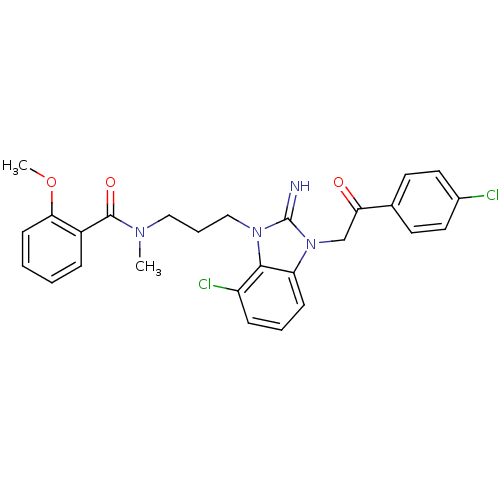
(CHEMBL272558)Show SMILES COc1ccccc1C(=O)N(C)CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N Show InChI InChI=1S/C27H26Cl2N4O3/c1-31(26(35)20-7-3-4-10-24(20)36-2)15-6-16-32-25-21(29)8-5-9-22(25)33(27(32)30)17-23(34)18-11-13-19(28)14-12-18/h3-5,7-14,30H,6,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375122
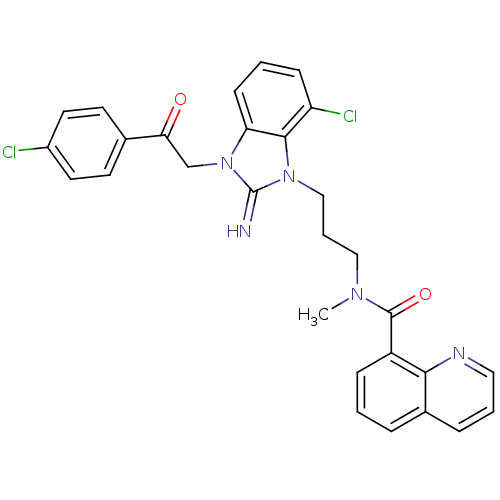
(CHEMBL427833)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1cccc2cccnc12 Show InChI InChI=1S/C29H25Cl2N5O2/c1-34(28(38)22-8-2-6-20-7-4-15-33-26(20)22)16-5-17-35-27-23(31)9-3-10-24(27)36(29(35)32)18-25(37)19-11-13-21(30)14-12-19/h2-4,6-15,32H,5,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375117
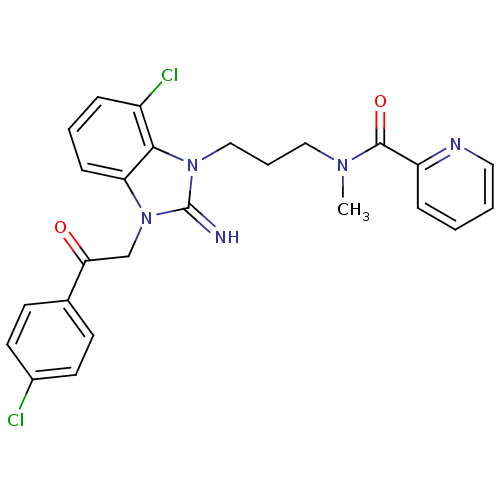
(CHEMBL255798)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1ccccn1 Show InChI InChI=1S/C25H23Cl2N5O2/c1-30(24(34)20-7-2-3-13-29-20)14-5-15-31-23-19(27)6-4-8-21(23)32(25(31)28)16-22(33)17-9-11-18(26)12-10-17/h2-4,6-13,28H,5,14-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375122

(CHEMBL427833)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1cccc2cccnc12 Show InChI InChI=1S/C29H25Cl2N5O2/c1-34(28(38)22-8-2-6-20-7-4-15-33-26(20)22)16-5-17-35-27-23(31)9-3-10-24(27)36(29(35)32)18-25(37)19-11-13-21(30)14-12-19/h2-4,6-15,32H,5,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375116

(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375120

(CHEMBL403040)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(CC[C@@H]2CCCN2C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-22-13-11-20(12-14-22)27(39)19-38-26-10-2-9-25(33)29(26)37(31(38)34)18-15-23-7-4-17-36(23)30(40)24-8-1-5-21-6-3-16-35-28(21)24/h1-3,5-6,8-14,16,23,34H,4,7,15,17-19H2/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375111
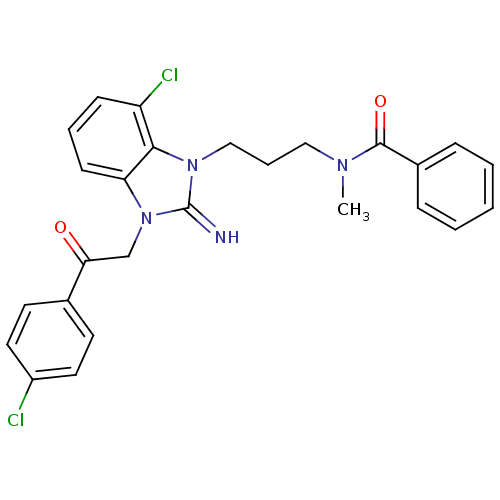
(CHEMBL409499)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1ccccc1 Show InChI InChI=1S/C26H24Cl2N4O2/c1-30(25(34)19-7-3-2-4-8-19)15-6-16-31-24-21(28)9-5-10-22(24)32(26(31)29)17-23(33)18-11-13-20(27)14-12-18/h2-5,7-14,29H,6,15-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50142274

(4-(3,4-Dichloro-benzenesulfonyl)-1-(5-methyl-3H-im...)Show SMILES Cc1nc[nH]c1CN1C(CCc2ccccc2)CN(Cc2ccccc12)S(=O)(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C28H28Cl2N4O2S/c1-20-27(32-19-31-20)18-34-23(12-11-21-7-3-2-4-8-21)17-33(16-22-9-5-6-10-28(22)34)37(35,36)24-13-14-25(29)26(30)15-24/h2-10,13-15,19,23H,11-12,16-18H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP hydrolase |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50371837
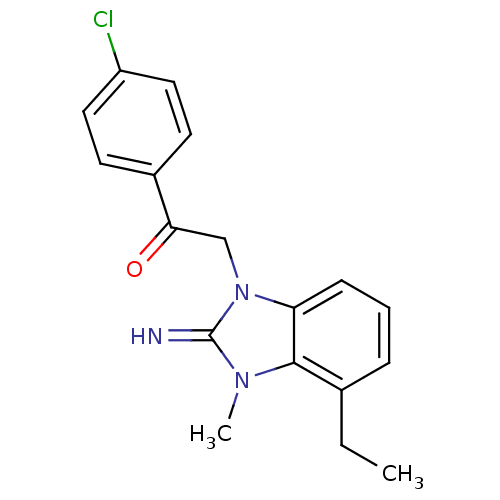
(CHEMBL257663)Show InChI InChI=1S/C18H18ClN3O/c1-3-12-5-4-6-15-17(12)21(2)18(20)22(15)11-16(23)13-7-9-14(19)10-8-13/h4-10,20H,3,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50371837

(CHEMBL257663)Show InChI InChI=1S/C18H18ClN3O/c1-3-12-5-4-6-15-17(12)21(2)18(20)22(15)11-16(23)13-7-9-14(19)10-8-13/h4-10,20H,3,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125]CXCL10 from human CXCR3 expressed in CHO cells |
Bioorg Med Chem Lett 18: 1573-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.074
BindingDB Entry DOI: 10.7270/Q2QZ2BTF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404297
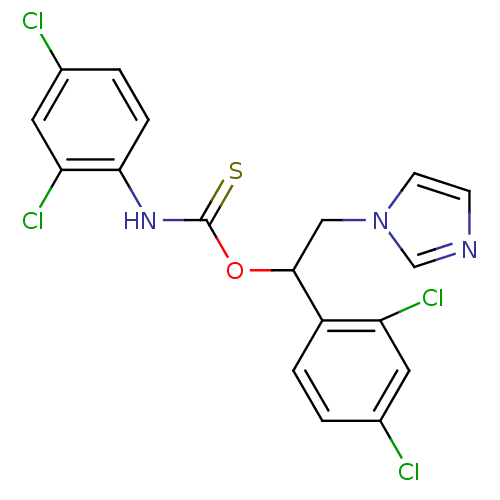
(CHEMBL269080)Show SMILES Clc1ccc(NC(=S)OC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H13Cl4N3OS/c19-11-1-3-13(14(21)7-11)17(9-25-6-5-23-10-25)26-18(27)24-16-4-2-12(20)8-15(16)22/h1-8,10,17H,9H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375117

(CHEMBL255798)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1ccccn1 Show InChI InChI=1S/C25H23Cl2N5O2/c1-30(24(34)20-7-2-3-13-29-20)14-5-15-31-23-19(27)6-4-8-21(23)32(25(31)28)16-22(33)17-9-11-18(26)12-10-17/h2-4,6-13,28H,5,14-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288
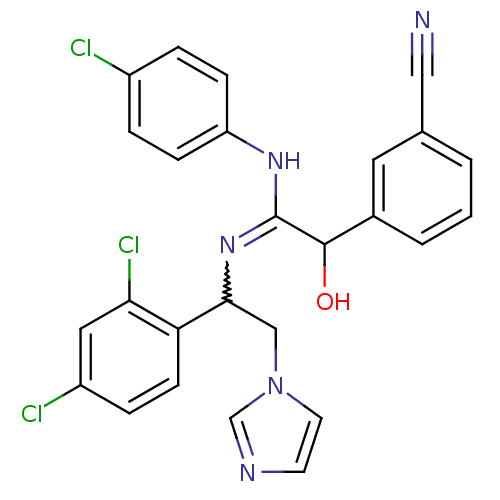
(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404288

(CHEMBL269369)Show SMILES OC(C(Nc1ccc(Cl)cc1)=NC(Cn1ccnc1)c1ccc(Cl)cc1Cl)c1cccc(c1)C#N |w:11.12| Show InChI InChI=1S/C26H20Cl3N5O/c27-19-4-7-21(8-5-19)32-26(25(35)18-3-1-2-17(12-18)14-30)33-24(15-34-11-10-31-16-34)22-9-6-20(28)13-23(22)29/h1-13,16,24-25,35H,15H2,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0 ATP hydrolase |
Bioorg Med Chem Lett 14: 1027-30 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.077
BindingDB Entry DOI: 10.7270/Q2VX0HQ9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50142256

(4-(4-tert-Butyl-benzenesulfonyl)-1-(3H-imidazol-4-...)Show SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)N1CC(CCc2ccccc2)N(Cc2cnc[nH]2)c2ccccc2C1 Show InChI InChI=1S/C31H36N4O2S/c1-31(2,3)26-14-17-29(18-15-26)38(36,37)34-20-25-11-7-8-12-30(25)35(21-27-19-32-23-33-27)28(22-34)16-13-24-9-5-4-6-10-24/h4-12,14-15,17-19,23,28H,13,16,20-22H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against ccytochrome P450 2C9 |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50375120

(CHEMBL403040)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(CC[C@@H]2CCCN2C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-22-13-11-20(12-14-22)27(39)19-38-26-10-2-9-25(33)29(26)37(31(38)34)18-15-23-7-4-17-36(23)30(40)24-8-1-5-21-6-3-16-35-28(21)24/h1-3,5-6,8-14,16,23,34H,4,7,15,17-19H2/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from mouse CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375124

(CHEMBL256457)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C30H26Cl2N4O2/c1-34(29(38)23-11-10-20-6-2-3-7-22(20)18-23)16-5-17-35-28-25(32)8-4-9-26(28)36(30(35)33)19-27(37)21-12-14-24(31)15-13-21/h2-4,6-15,18,33H,5,16-17,19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375123

(CHEMBL272558)Show SMILES COc1ccccc1C(=O)N(C)CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N Show InChI InChI=1S/C27H26Cl2N4O3/c1-31(26(35)20-7-3-4-10-24(20)36-2)15-6-16-32-25-21(29)8-5-9-22(25)33(27(32)30)17-23(34)18-11-13-19(28)14-12-18/h3-5,7-14,30H,6,15-17H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50375122

(CHEMBL427833)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)c1cccc2cccnc12 Show InChI InChI=1S/C29H25Cl2N5O2/c1-34(28(38)22-8-2-6-20-7-4-15-33-26(20)22)16-5-17-35-27-23(31)9-3-10-24(27)36(29(35)32)18-25(37)19-11-13-21(30)14-12-19/h2-4,6-15,32H,5,16-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from mouse CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50375121

(CHEMBL257038)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from mouse CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50375126
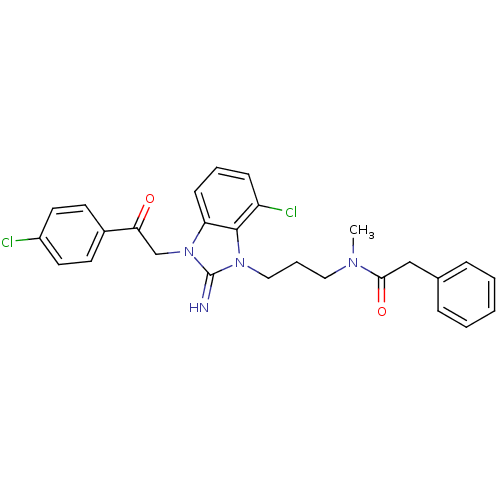
(CHEMBL255583)Show SMILES CN(CCCn1c2c(Cl)cccc2n(CC(=O)c2ccc(Cl)cc2)c1=N)C(=O)Cc1ccccc1 Show InChI InChI=1S/C27H26Cl2N4O2/c1-31(25(35)17-19-7-3-2-4-8-19)15-6-16-32-26-22(29)9-5-10-23(26)33(27(32)30)18-24(34)20-11-13-21(28)14-12-20/h2-5,7-14,30H,6,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cell membrane assessed as inhibition of calcium flux by FLIPR |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50375116

(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from mouse CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50371837

(CHEMBL257663)Show InChI InChI=1S/C18H18ClN3O/c1-3-12-5-4-6-15-17(12)21(2)18(20)22(15)11-16(23)13-7-9-14(19)10-8-13/h4-10,20H,3,11H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Antagonist activity at human CXCR3 expressed in CHO cells by FLIPR-based calcium mobilization assay |
Bioorg Med Chem Lett 18: 1573-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.074
BindingDB Entry DOI: 10.7270/Q2QZ2BTF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50142258
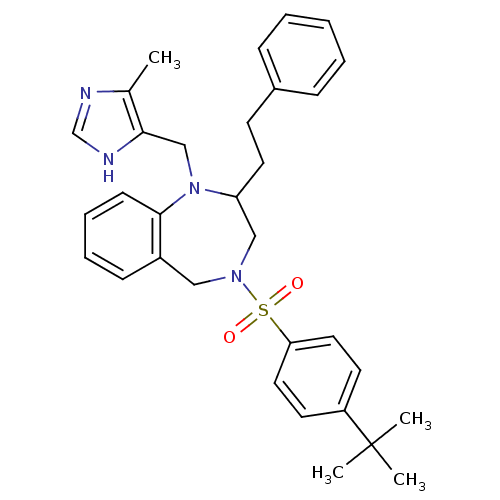
(4-(4-tert-Butyl-benzenesulfonyl)-1-(5-methyl-3H-im...)Show SMILES Cc1nc[nH]c1CN1C(CCc2ccccc2)CN(Cc2ccccc12)S(=O)(=O)c1ccc(cc1)C(C)(C)C Show InChI InChI=1S/C32H38N4O2S/c1-24-30(34-23-33-24)22-36-28(17-14-25-10-6-5-7-11-25)21-35(20-26-12-8-9-13-31(26)36)39(37,38)29-18-15-27(16-19-29)32(2,3)4/h5-13,15-16,18-19,23,28H,14,17,20-22H2,1-4H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP hydrolase |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404319
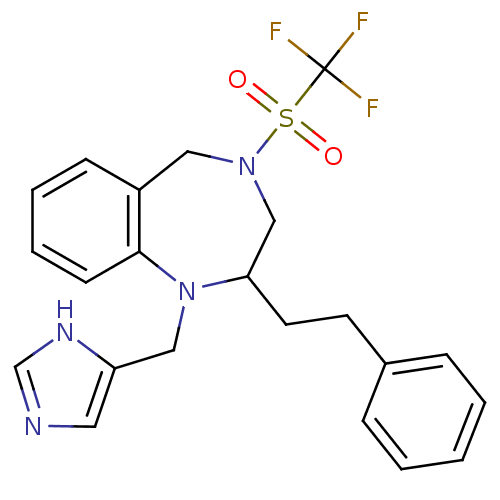
(CHEMBL266556)Show SMILES FC(F)(F)S(=O)(=O)N1CC(CCc2ccccc2)N(Cc2cnc[nH]2)c2ccccc2C1 Show InChI InChI=1S/C22H23F3N4O2S/c23-22(24,25)32(30,31)28-13-18-8-4-5-9-21(18)29(14-19-12-26-16-27-19)20(15-28)11-10-17-6-2-1-3-7-17/h1-9,12,16,20H,10-11,13-15H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP synthase |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
ATP synthase subunit beta,/delta,/epsilon,/gamma, mitochondrial
(Bos taurus) | BDBM50404307

(CHEMBL8366)Show SMILES COc1ccc(cc1)S(=O)(=O)N1CC(CCc2ccccc2)N(Cc2cnc[nH]2)c2ccccc2C1 Show InChI InChI=1S/C28H30N4O3S/c1-35-26-13-15-27(16-14-26)36(33,34)31-18-23-9-5-6-10-28(23)32(19-24-17-29-21-30-24)25(20-31)12-11-22-7-3-2-4-8-22/h2-10,13-17,21,25H,11-12,18-20H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against bovine mitochondrial F1F0-ATP hydrolase |
Bioorg Med Chem Lett 14: 1031-4 (2004)
Article DOI: 10.1016/j.bmcl.2003.11.052
BindingDB Entry DOI: 10.7270/Q2D50MCF |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Mus musculus) | BDBM50375116

(CHEMBL403290)Show SMILES Clc1ccc(cc1)C(=O)Cn1c2cccc(Cl)c2n(C[C@H]2CCCN(C2)C(=O)c2cccc3cccnc23)c1=N Show InChI InChI=1S/C31H27Cl2N5O2/c32-23-13-11-21(12-14-23)27(39)19-37-26-10-2-9-25(33)29(26)38(31(37)34)18-20-5-4-16-36(17-20)30(40)24-8-1-6-22-7-3-15-35-28(22)24/h1-3,6-15,20,34H,4-5,16-19H2/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Bioresearch Center
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from mouse CXCR3 expressed in CHO cell membrane |
Bioorg Med Chem Lett 18: 2414-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.049
BindingDB Entry DOI: 10.7270/Q2BP03NC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data