 Found 4574 hits with Last Name = 'ong' and Initial = 'e'
Found 4574 hits with Last Name = 'ong' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D3 receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202775
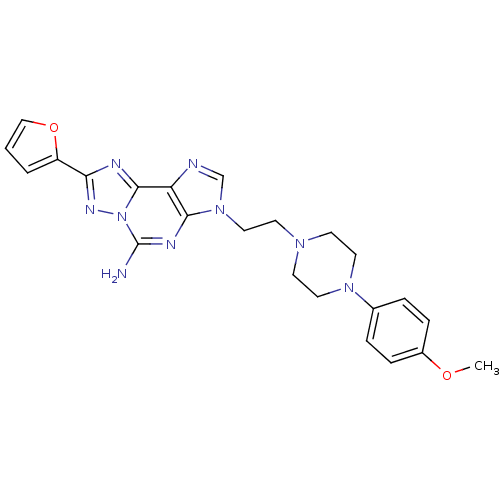
(8-(furan-2-yl)-3-(2-(4-(4-methoxyphenyl)piperazin-...)Show SMILES COc1ccc(cc1)N1CCN(CCn2cnc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C23H25N9O2/c1-33-17-6-4-16(5-7-17)30-11-8-29(9-12-30)10-13-31-15-25-19-21(31)27-23(24)32-22(19)26-20(28-32)18-3-2-14-34-18/h2-7,14-15H,8-13H2,1H3,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108043
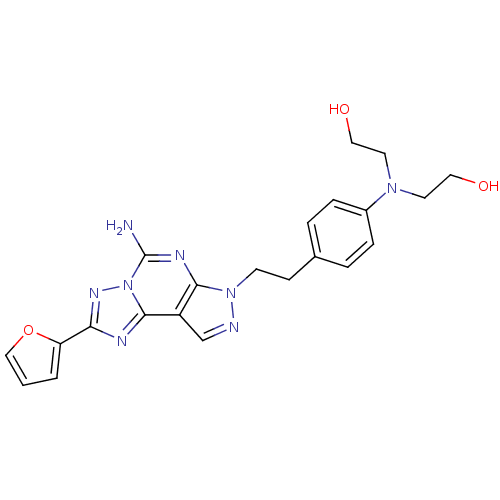
(2,2'-(4-(2-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3...)Show SMILES Nc1nc2n(CCc3ccc(cc3)N(CCO)CCO)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H24N8O3/c23-22-26-20-17(21-25-19(27-30(21)22)18-2-1-13-33-18)14-24-29(20)8-7-15-3-5-16(6-4-15)28(9-11-31)10-12-32/h1-6,13-14,31-32H,7-12H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells. |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108020
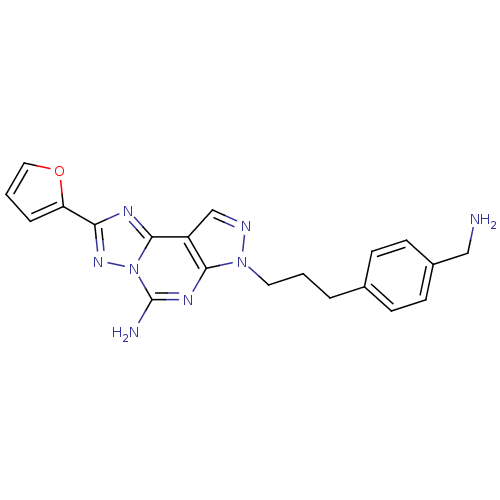
(7-(3-(4-(aminomethyl)phenyl)propyl)-2-(furan-2-yl)...)Show SMILES NCc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C20H20N8O/c21-11-14-7-5-13(6-8-14)3-1-9-27-18-15(12-23-27)19-24-17(16-4-2-10-29-16)26-28(19)20(22)25-18/h2,4-8,10,12H,1,3,9,11,21H2,(H2,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells. |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108019
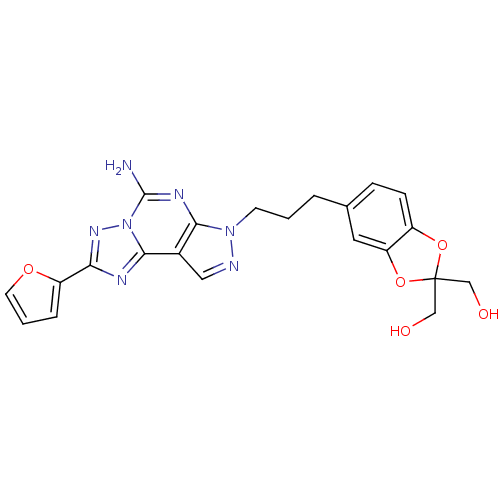
((5-(3-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1...)Show SMILES Nc1nc2n(CCCc3ccc4OC(CO)(CO)Oc4c3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21N7O5/c23-21-26-19-14(20-25-18(27-29(20)21)16-4-2-8-32-16)10-24-28(19)7-1-3-13-5-6-15-17(9-13)34-22(11-30,12-31)33-15/h2,4-6,8-10,30-31H,1,3,7,11-12H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells; ranges from 0.16-0.21 |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108018
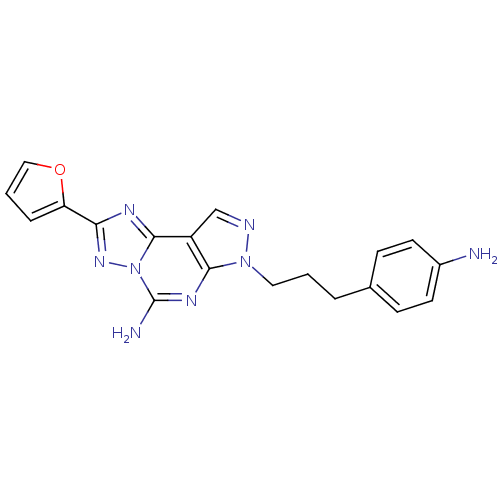
(7-(3-(4-aminophenyl)propyl)-2-(furan-2-yl)-7H-pyra...)Show SMILES Nc1ccc(CCCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C19H18N8O/c20-13-7-5-12(6-8-13)3-1-9-26-17-14(11-22-26)18-23-16(15-4-2-10-28-15)25-27(18)19(21)24-17/h2,4-8,10-11H,1,3,9,20H2,(H2,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells. |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202777
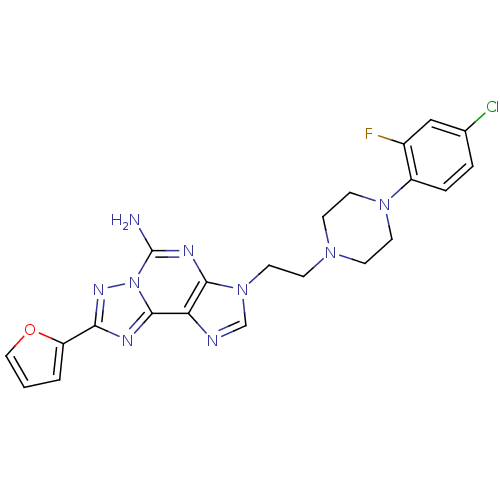
(3-(2-(4-(4-chloro-2-fluorophenyl)piperazin-1-yl)et...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(Cl)cc3F)cnc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21ClFN9O/c23-14-3-4-16(15(24)12-14)31-8-5-30(6-9-31)7-10-32-13-26-18-20(32)28-22(25)33-21(18)27-19(29-33)17-2-1-11-34-17/h1-4,11-13H,5-10H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505
(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(RAT) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 4
(RAT) | BDBM82505

(CAS_121881 | NSC_121881 | SB204070)Show InChI InChI=1S/C19H27ClN2O4/c1-2-3-6-22-7-4-13(5-8-22)12-26-19(23)14-11-15(20)16(21)18-17(14)24-9-10-25-18/h11,13H,2-10,12,21H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience
Curated by PDSP Ki Database
| |
Neuropharmacology 36: 671-9 (1997)
Article DOI: 10.1016/s0028-3908(97)00039-7
BindingDB Entry DOI: 10.7270/Q2RN36D0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202788
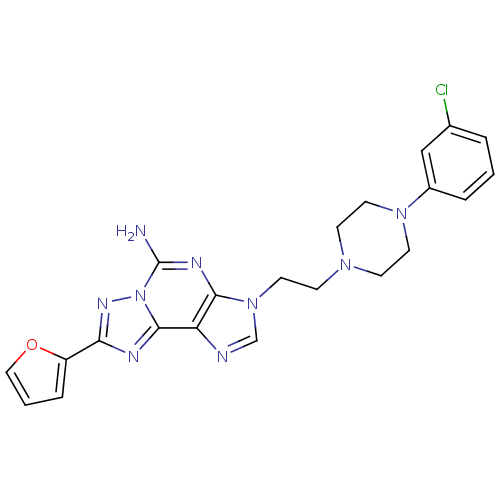
(3-(2-(4-(3-chlorophenyl)piperazin-1-yl)ethyl)-8-(f...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3cccc(Cl)c3)cnc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22ClN9O/c23-15-3-1-4-16(13-15)30-9-6-29(7-10-30)8-11-31-14-25-18-20(31)27-22(24)32-21(18)26-19(28-32)17-5-2-12-33-17/h1-5,12-14H,6-11H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202991
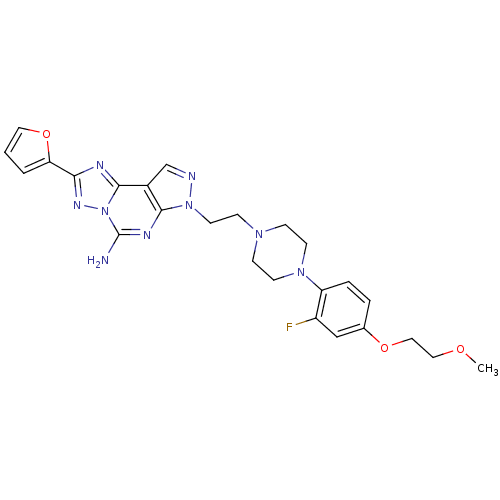
(7-(2-{4-[2-fluoro-4-(2-methoxy-ethoxy)-phenyl]-pip...)Show SMILES COCCOc1ccc(N2CCN(CCn3ncc4c3nc(N)n3nc(nc43)-c3ccco3)CC2)c(F)c1 Show InChI InChI=1S/C25H28FN9O3/c1-36-13-14-37-17-4-5-20(19(26)15-17)33-9-6-32(7-10-33)8-11-34-23-18(16-28-34)24-29-22(21-3-2-12-38-21)31-35(24)25(27)30-23/h2-5,12,15-16H,6-11,13-14H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 7-10 (1999)
Article DOI: 10.1007/pl00005326
BindingDB Entry DOI: 10.7270/Q24F1P91 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202771
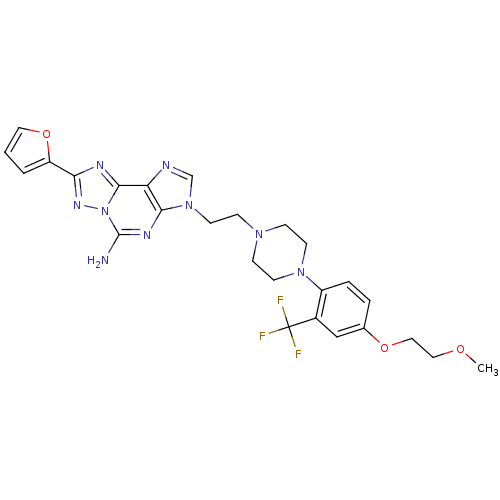
(8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)-2-(tri...)Show SMILES COCCOc1ccc(N2CCN(CCn3cnc4c3nc(N)n3nc(nc43)-c3ccco3)CC2)c(c1)C(F)(F)F Show InChI InChI=1S/C26H28F3N9O3/c1-39-13-14-40-17-4-5-19(18(15-17)26(27,28)29)36-9-6-35(7-10-36)8-11-37-16-31-21-23(37)33-25(30)38-24(21)32-22(34-38)20-3-2-12-41-20/h2-5,12,15-16H,6-11,13-14H2,1H3,(H2,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108035

(CHEMBL22200 | {4-[2-(5-Amino-2-furan-2-yl-pyrazolo...)Show SMILES CCOC(=O)COc1ccc(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)cc1 Show InChI InChI=1S/C22H21N7O4/c1-2-31-18(30)13-33-15-7-5-14(6-8-15)9-10-28-20-16(12-24-28)21-25-19(17-4-3-11-32-17)27-29(21)22(23)26-20/h3-8,11-12H,2,9-10,13H2,1H3,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells. |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50382312
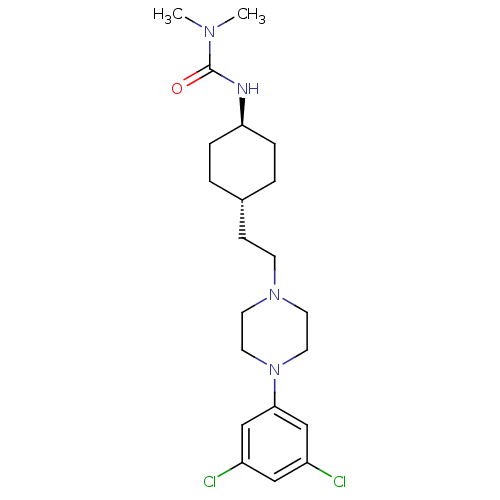
(CHEMBL2024677)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)cc(Cl)c2)CC1 |r,wU:6.5,wD:9.9,(6.35,-23.87,;7.68,-24.65,;7.68,-26.19,;9.02,-23.88,;9.02,-22.34,;10.35,-24.65,;11.68,-23.88,;11.68,-22.34,;13.01,-21.56,;14.34,-22.34,;15.68,-21.57,;17.01,-22.35,;18.34,-21.58,;19.67,-22.36,;21,-21.6,;21.01,-20.06,;19.68,-19.28,;18.34,-20.04,;22.35,-19.3,;23.68,-20.08,;25.02,-19.32,;26.35,-20.1,;25.03,-17.78,;23.69,-17,;23.7,-15.46,;22.36,-17.76,;14.34,-23.88,;13.01,-24.64,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-19-5-3-16(4-6-19)7-8-26-9-11-27(12-10-26)20-14-17(22)13-18(23)15-20/h13-16,19H,3-12H2,1-2H3,(H,24,28)/t16-,19- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202994
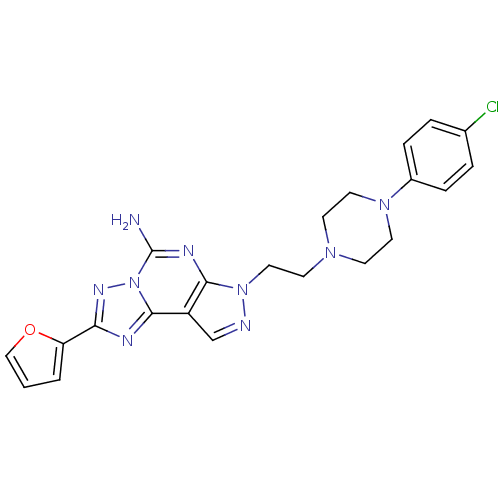
(7-{2-[4-(4-chloro-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(Cl)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22ClN9O/c23-15-3-5-16(6-4-15)30-10-7-29(8-11-30)9-12-31-20-17(14-25-31)21-26-19(18-2-1-13-33-18)28-32(21)22(24)27-20/h1-6,13-14H,7-12H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Thromboxane A2 receptor
(Homo sapiens (Human)) | BDBM50161746

((R)-3-(3-(4-fluorophenylsulfonamido)-1,2,3,4-tetra...)Show SMILES OC(=O)CCn1c2CC[C@H](Cc2c2ccccc12)NS(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C21H21FN2O4S/c22-14-5-8-16(9-6-14)29(27,28)23-15-7-10-20-18(13-15)17-3-1-2-4-19(17)24(20)12-11-21(25)26/h1-6,8-9,15,23H,7,10-13H2,(H,25,26)/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Binding affinity to thromboxane receptor |
Bioorg Med Chem Lett 21: 288-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.015
BindingDB Entry DOI: 10.7270/Q2959HTH |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108027
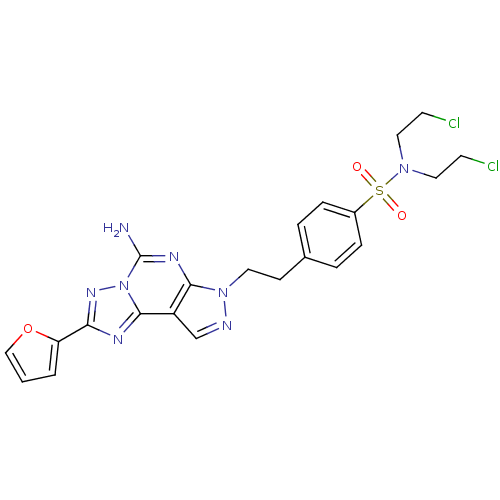
(4-(2-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,...)Show SMILES Nc1nc2n(CCc3ccc(cc3)S(=O)(=O)N(CCCl)CCCl)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22Cl2N8O3S/c23-8-11-30(12-9-24)36(33,34)16-5-3-15(4-6-16)7-10-31-20-17(14-26-31)21-27-19(18-2-1-13-35-18)29-32(21)22(25)28-20/h1-6,13-14H,7-12H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of specific [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells. |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50048466
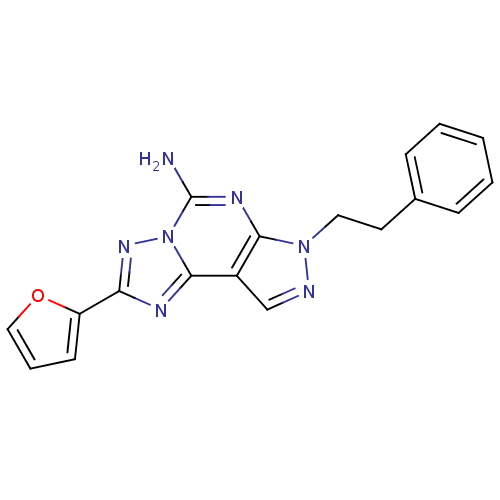
(2-(furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,...)Show InChI InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 7-10 (1999)
Article DOI: 10.1007/pl00005326
BindingDB Entry DOI: 10.7270/Q24F1P91 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202985
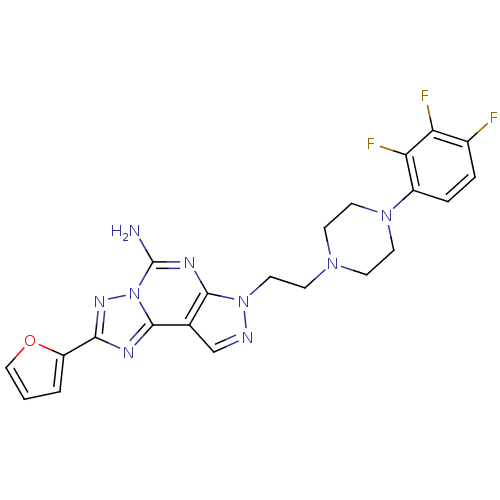
(2-furan-2-yl-7-{2-[4-(2,3,4-trifluoro-phenyl)-pipe...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)c(F)c3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H20F3N9O/c23-14-3-4-15(18(25)17(14)24)32-8-5-31(6-9-32)7-10-33-20-13(12-27-33)21-28-19(16-2-1-11-35-16)30-34(21)22(26)29-20/h1-4,11-12H,5-10H2,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202998

(2-(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(OCCO)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C24H27N9O3/c25-24-28-22-19(23-27-21(29-33(23)24)20-2-1-14-36-20)16-26-32(22)12-9-30-7-10-31(11-8-30)17-3-5-18(6-4-17)35-15-13-34/h1-6,14,16,34H,7-13,15H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202990
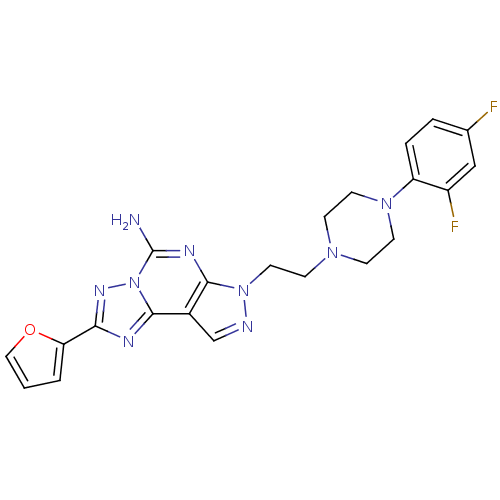
(2-(furan-2-yl)-7-(2-(4-(4-(2-methoxyethoxy)phenyl)...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)cc3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21F2N9O/c23-14-3-4-17(16(24)12-14)31-8-5-30(6-9-31)7-10-32-20-15(13-26-32)21-27-19(18-2-1-11-34-18)29-33(21)22(25)28-20/h1-4,11-13H,5-10H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50013050
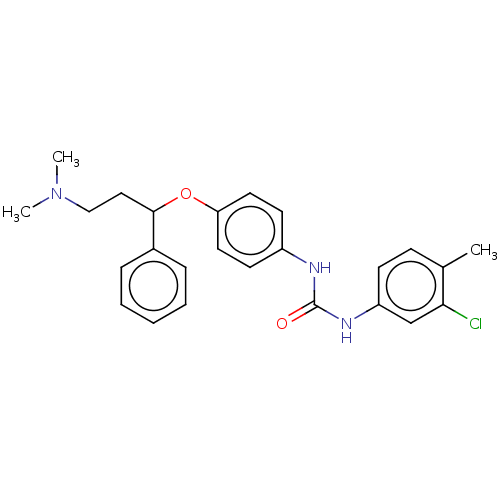
(CHEMBL3261693)Show SMILES CN(C)CCC(Oc1ccc(NC(=O)Nc2ccc(C)c(Cl)c2)cc1)c1ccccc1 Show InChI InChI=1S/C25H28ClN3O2/c1-18-9-10-21(17-23(18)26)28-25(30)27-20-11-13-22(14-12-20)31-24(15-16-29(2)3)19-7-5-4-6-8-19/h4-14,17,24H,15-16H2,1-3H3,(H2,27,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]mesulergine from 5-HT2C receptor in Sprague-Dawley rat choroid plexus membranes after 30 mins by liquid scintillation spectrophot... |
Bioorg Med Chem Lett 24: 2118-22 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.043
BindingDB Entry DOI: 10.7270/Q2G44RV5 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202996
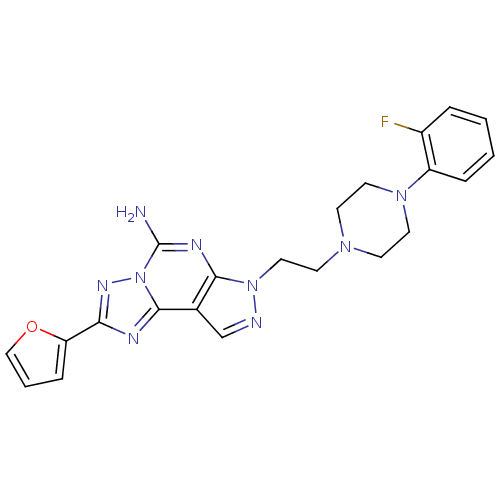
(7-{2-[4-(2-fluoro-phenyl)-piperazin-1-yl]-ethyl}-2...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccccc3F)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H22FN9O/c23-16-4-1-2-5-17(16)30-10-7-29(8-11-30)9-12-31-20-15(14-25-31)21-26-19(18-6-3-13-33-18)28-32(21)22(24)27-20/h1-6,13-14H,7-12H2,(H2,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50203000
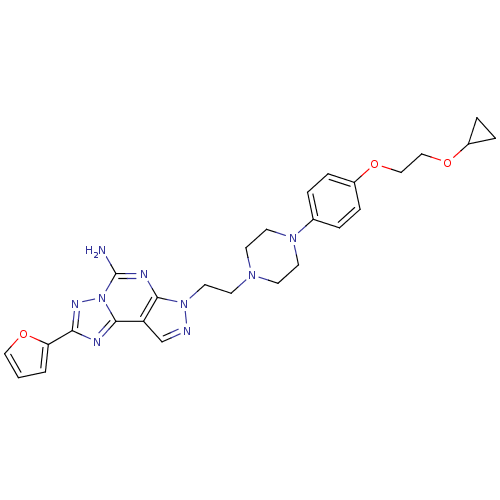
(7-(2-{4-[4-(2-cyclopropoxy-ethoxy)-phenyl]-piperaz...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(OCCOC4CC4)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C27H31N9O3/c28-27-31-25-22(26-30-24(32-36(26)27)23-2-1-15-39-23)18-29-35(25)14-11-33-9-12-34(13-10-33)19-3-5-20(6-4-19)37-16-17-38-21-7-8-21/h1-6,15,18,21H,7-14,16-17H2,(H2,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202989
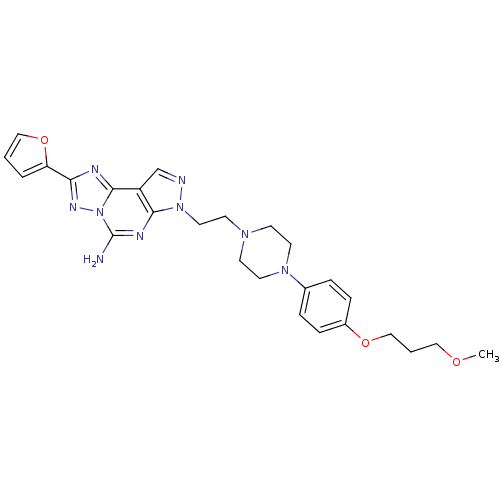
(2-furan-2-yl-7-(2-{4-[4-(3-methoxy-propoxy)-phenyl...)Show SMILES COCCCOc1ccc(cc1)N1CCN(CCn2ncc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C26H31N9O3/c1-36-15-3-17-37-20-7-5-19(6-8-20)33-12-9-32(10-13-33)11-14-34-24-21(18-28-34)25-29-23(22-4-2-16-38-22)31-35(25)26(27)30-24/h2,4-8,16,18H,3,9-15,17H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50026746

(CHEMBL3335471)Show SMILES NCC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(cc(c2)C(F)(F)F)C#N)CC1 |r,wU:5.4,wD:8.8,(44.7,-19.21,;43.93,-20.55,;42.39,-20.55,;41.62,-21.88,;41.62,-19.21,;40.08,-19.21,;39.31,-20.55,;37.77,-20.55,;37,-19.21,;35.46,-19.21,;34.69,-17.88,;33.15,-17.88,;32.38,-19.21,;30.84,-19.21,;30.07,-17.88,;30.84,-16.54,;32.38,-16.54,;28.53,-17.88,;27.76,-19.21,;26.22,-19.21,;25.45,-17.88,;26.22,-16.54,;27.76,-16.54,;25.45,-15.21,;26.22,-13.88,;23.91,-15.21,;24.68,-13.88,;25.45,-20.55,;24.68,-21.88,;37.77,-17.88,;39.31,-17.88,)| Show InChI InChI=1S/C22H30F3N5O/c23-22(24,25)18-11-17(14-26)12-20(13-18)30-9-7-29(8-10-30)6-5-16-1-3-19(4-2-16)28-21(31)15-27/h11-13,16,19H,1-10,15,27H2,(H,28,31)/t16-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D3 receptor expressed in CHO-K1 cells after 120 mins by scintillation counting analysis |
ACS Med Chem Lett 5: 1010-4 (2014)
Article DOI: 10.1021/ml500201u
BindingDB Entry DOI: 10.7270/Q2CC128M |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50054820
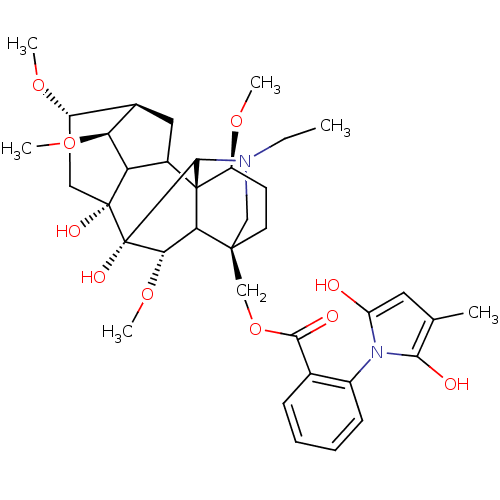
(Methyllycaconitine | [(1S,4S,5R,6S,8R,9R,13S,16S,1...)Show SMILES CCN1C[C@]2(COC(=O)c3ccccc3-n3c(O)cc(C)c3O)CC[C@H](OC)[C@@]34C5C[C@H]6[C@H](OC)C5[C@](O)(C[C@@H]6OC)[C@](O)([C@@H](OC)C23)C14 |wU:4.4,42.46,36.39,44.48,39.43,wD:28.55,32.34,25.27,31.32,TLB:28:29:32:36.38.39,1:2:47:25.23.24,25:28:42.44:4.2.3,THB:42:48:47:25.23.24,29:28:42.44:4.2.3,2:48:36.35.29:47.44,(3.83,-6.7,;5.61,-4.92,;8.1,-4.92,;7.12,-7.5,;8.22,-6.42,;8.22,-7.94,;7.82,-9.45,;8.61,-10.78,;9.94,-10.01,;8.62,-12.33,;7.29,-13.1,;7.29,-14.64,;8.62,-15.41,;9.95,-14.64,;9.95,-13.1,;11.49,-13.09,;12.9,-13.7,;13.3,-15.19,;13.93,-12.55,;13.14,-11.22,;13.91,-9.88,;11.64,-11.55,;10.86,-10.2,;6.88,-5.65,;6.87,-4.11,;8.21,-3.34,;8.2,-1.8,;6.87,-1.03,;9.54,-4.11,;10.5,-3.18,;10.3,-1.71,;11.64,-1.06,;12.67,-2.15,;14.21,-2.15,;14.96,-3.46,;11.97,-3.45,;12.65,-4.9,;13.98,-5.67,;16.6,-3.1,;16.6,-1.56,;17.69,-.47,;19.02,-1.24,;11.93,-6.28,;12.7,-7.59,;10.46,-6.53,;10.06,-8.01,;11.39,-8.78,;9.55,-5.63,;9.53,-2.57,)| Show InChI InChI=1S/C37H50N2O10/c1-7-38-17-34(18-49-32(42)20-10-8-9-11-23(20)39-26(40)14-19(2)31(39)41)13-12-25(46-4)36-22-15-21-24(45-3)16-35(43,27(22)28(21)47-5)37(44,33(36)38)30(48-6)29(34)36/h8-11,14,21-22,24-25,27-30,33,40-41,43-44H,7,12-13,15-18H2,1-6H3/t21-,22?,24+,25+,27?,28+,29?,30+,33?,34+,35-,36+,37+/m1/s1 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
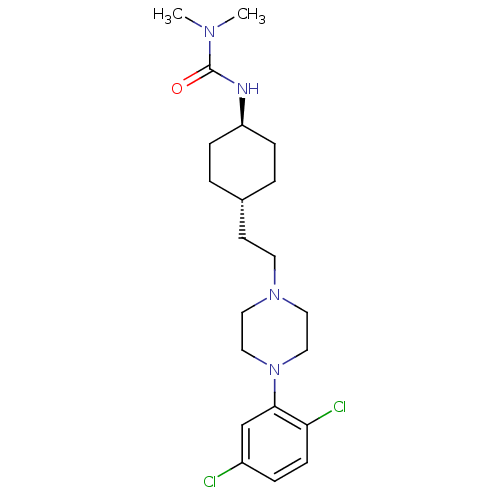
(Rattus norvegicus (Rat)) | BDBM50382310
(CHEMBL2024675)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(Cl)ccc2Cl)CC1 |r,wU:6.5,wD:9.9,(23.12,-11.63,;24.45,-12.4,;24.45,-13.94,;25.79,-11.64,;25.79,-10.1,;27.12,-12.41,;28.45,-11.64,;28.45,-10.1,;29.78,-9.32,;31.11,-10.1,;32.45,-9.33,;33.78,-10.1,;35.11,-9.34,;36.44,-10.12,;37.77,-9.36,;37.78,-7.82,;36.45,-7.04,;35.11,-7.8,;39.12,-7.06,;39.13,-5.52,;40.46,-4.76,;40.46,-3.22,;41.8,-5.54,;41.79,-7.08,;40.45,-7.84,;40.44,-9.38,;31.11,-11.64,;29.78,-12.4,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-18-6-3-16(4-7-18)9-10-26-11-13-27(14-12-26)20-15-17(22)5-8-19(20)23/h5,8,15-16,18H,3-4,6-7,9-14H2,1-2H3,(H,24,28)/t16-,18- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]spiperone from rat recombinant dopamine D3 receptor expressed in Sf9 cells |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50026747

(CHEMBL3335472)Show SMILES NS(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(cc(c2)C(F)(F)F)C#N)CC1 |r,wU:5.4,wD:8.8,(31.51,-21.3,;30.02,-21.7,;30.02,-23.24,;28.69,-22.47,;29.25,-20.36,;27.71,-20.36,;26.94,-21.7,;25.4,-21.7,;24.63,-20.36,;23.09,-20.36,;22.32,-19.03,;20.78,-19.03,;20.01,-20.36,;18.47,-20.36,;17.7,-19.03,;18.47,-17.7,;20.01,-17.7,;16.16,-19.03,;15.39,-20.36,;13.85,-20.36,;13.08,-19.03,;13.85,-17.7,;15.39,-17.7,;13.08,-16.36,;12.31,-15.02,;13.85,-15.02,;11.54,-16.36,;13.08,-21.7,;12.31,-23.03,;25.4,-19.03,;26.94,-19.03,)| Show InChI InChI=1S/C20H28F3N5O2S/c21-20(22,23)17-11-16(14-24)12-19(13-17)28-9-7-27(8-10-28)6-5-15-1-3-18(4-2-15)26-31(25,29)30/h11-13,15,18,26H,1-10H2,(H2,25,29,30)/t15-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D3 receptor expressed in CHO-K1 cells after 120 mins by scintillation counting analysis |
ACS Med Chem Lett 5: 1010-4 (2014)
Article DOI: 10.1021/ml500201u
BindingDB Entry DOI: 10.7270/Q2CC128M |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202779
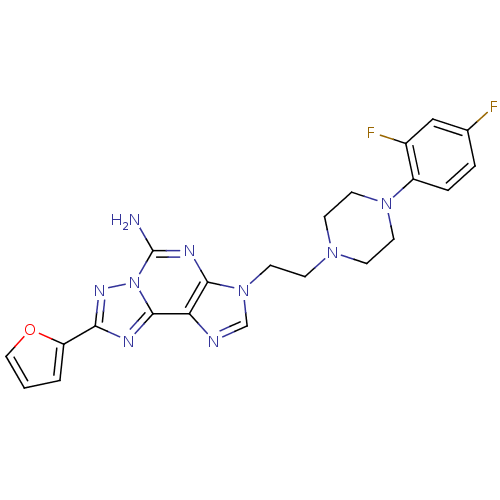
(3-(2-(4-(2,4-difluorophenyl)piperazin-1-yl)ethyl)-...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(F)cc3F)cnc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H21F2N9O/c23-14-3-4-16(15(24)12-14)31-8-5-30(6-9-31)7-10-32-13-26-18-20(32)28-22(25)33-21(18)27-19(29-33)17-2-1-11-34-17/h1-4,11-13H,5-10H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
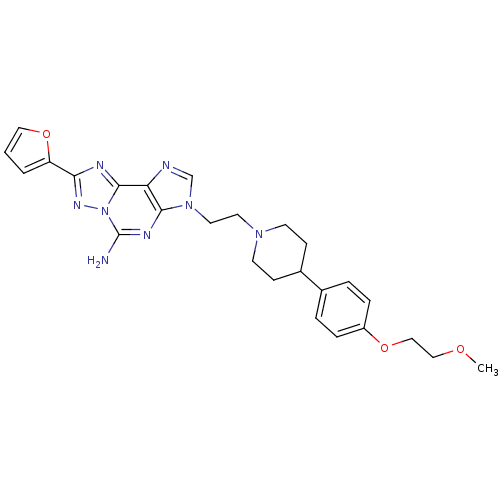
(Homo sapiens (Human)) | BDBM50202790
(8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...)Show SMILES COCCOc1ccc(cc1)C1CCN(CCn2cnc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C26H30N8O3/c1-35-15-16-36-20-6-4-18(5-7-20)19-8-10-32(11-9-19)12-13-33-17-28-22-24(33)30-26(27)34-25(22)29-23(31-34)21-3-2-14-37-21/h2-7,14,17,19H,8-13,15-16H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50007872
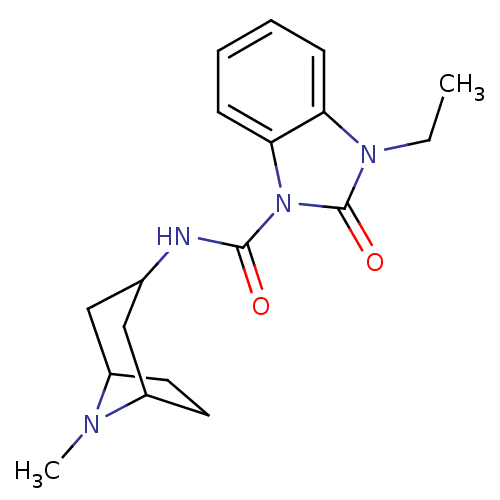
(3-Ethyl-2-oxo-2,3-dihydro-benzoimidazole-1-carboxy...)Show SMILES CCn1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:12:13:20:16.17| Show InChI InChI=1S/C18H24N4O2/c1-3-21-15-6-4-5-7-16(15)22(18(21)24)17(23)19-12-10-13-8-9-14(11-12)20(13)2/h4-7,12-14H,3,8-11H2,1-2H3,(H,19,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50026745

(CHEMBL3335470)Show SMILES NC(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cc(cc(c2)C(F)(F)F)C#N)CC1 |r,wU:4.3,wD:7.7,(20.37,-9.78,;21.14,-8.45,;22.68,-8.45,;20.37,-7.12,;18.83,-7.12,;18.06,-8.45,;16.52,-8.45,;15.75,-7.12,;14.21,-7.12,;13.44,-5.78,;11.9,-5.78,;11.13,-7.12,;9.59,-7.12,;8.82,-5.78,;9.59,-4.45,;11.13,-4.45,;7.28,-5.78,;6.51,-7.12,;4.97,-7.12,;4.2,-5.78,;4.97,-4.45,;6.51,-4.45,;4.2,-3.12,;4.97,-1.78,;2.66,-3.12,;3.43,-1.78,;4.2,-8.45,;3.43,-9.78,;16.52,-5.78,;18.06,-5.78,)| Show InChI InChI=1S/C21H28F3N5O/c22-21(23,24)17-11-16(14-25)12-19(13-17)29-9-7-28(8-10-29)6-5-15-1-3-18(4-2-15)27-20(26)30/h11-13,15,18H,1-10H2,(H3,26,27,30)/t15-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Displacement of [3H]-raclopride from human D3 receptor expressed in CHO-K1 cells after 120 mins by scintillation counting analysis |
ACS Med Chem Lett 5: 1010-4 (2014)
Article DOI: 10.1021/ml500201u
BindingDB Entry DOI: 10.7270/Q2CC128M |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50056404
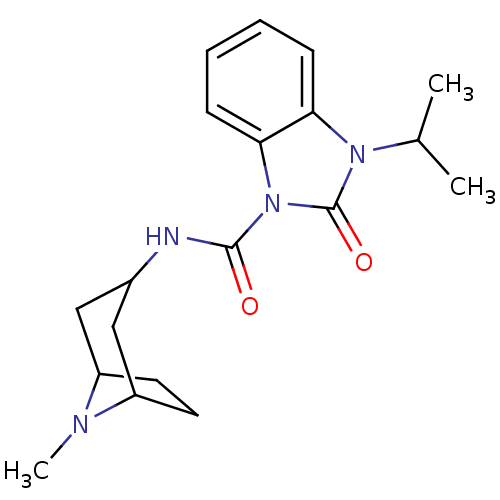
(3-Isopropyl-2-oxo-2,3-dihydro-benzoimidazole-1-car...)Show SMILES CC(C)n1c2ccccc2n(C(=O)NC2CC3CCC(C2)N3C)c1=O |TLB:13:14:21:17.18| Show InChI InChI=1S/C19H26N4O2/c1-12(2)22-16-6-4-5-7-17(16)23(19(22)25)18(24)20-13-10-14-8-9-15(11-13)21(14)3/h4-7,12-15H,8-11H2,1-3H3,(H,20,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50203001
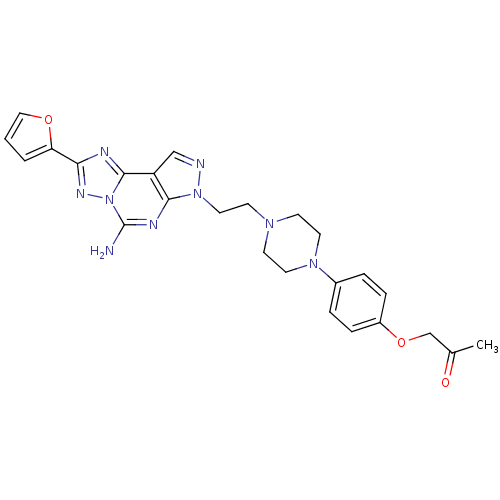
(1-(4-{4-[2-(5-amino-2-furan-2-yl-pyrazolo[4,3-e][1...)Show SMILES CC(=O)COc1ccc(cc1)N1CCN(CCn2ncc3c4nc(nn4c(N)nc23)-c2ccco2)CC1 Show InChI InChI=1S/C25H27N9O3/c1-17(35)16-37-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-23-20(15-27-33)24-28-22(21-3-2-14-36-21)30-34(24)25(26)29-23/h2-7,14-15H,8-13,16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1376-80 (2007)
Article DOI: 10.1016/j.bmcl.2006.11.083
BindingDB Entry DOI: 10.7270/Q27H1J8J |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202769
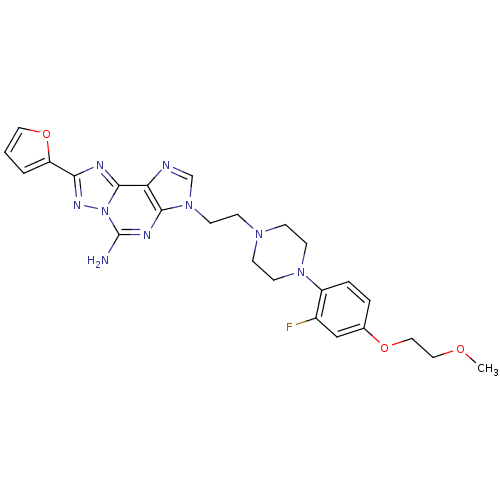
(3-(2-(4-(2-fluoro-4-(2-methoxyethoxy)phenyl)pipera...)Show SMILES COCCOc1ccc(N2CCN(CCn3cnc4c3nc(N)n3nc(nc43)-c3ccco3)CC2)c(F)c1 Show InChI InChI=1S/C25H28FN9O3/c1-36-13-14-37-17-4-5-19(18(26)15-17)33-9-6-32(7-10-33)8-11-34-16-28-21-23(34)30-25(27)35-24(21)29-22(31-35)20-3-2-12-38-20/h2-5,12,15-16H,6-11,13-14H2,1H3,(H2,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21190

(4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...)Show InChI InChI=1S/C16H15N7O2/c17-14-20-15(18-8-7-10-3-5-11(24)6-4-10)21-16-19-13(22-23(14)16)12-2-1-9-25-12/h1-6,9,24H,7-8H2,(H3,17,18,19,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 359: 7-10 (1999)
Article DOI: 10.1007/pl00005326
BindingDB Entry DOI: 10.7270/Q24F1P91 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50108022
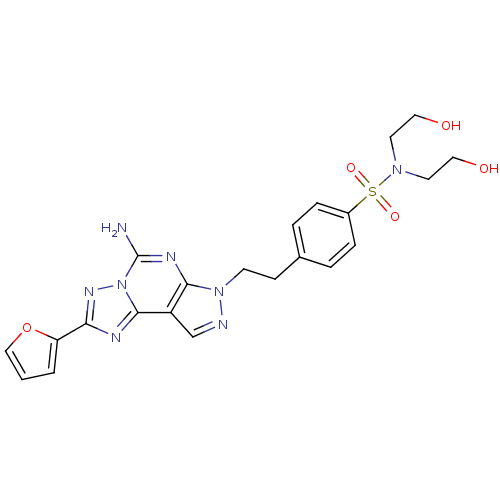
(4-(2-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,...)Show SMILES Nc1nc2n(CCc3ccc(cc3)S(=O)(=O)N(CCO)CCO)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C22H24N8O5S/c23-22-26-20-17(21-25-19(27-30(21)22)18-2-1-13-35-18)14-24-29(20)8-7-15-3-5-16(6-4-15)36(33,34)28(9-11-31)10-12-32/h1-6,13-14,31-32H,7-12H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università degli Studi di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]-SCH- 58261 binding at human Adenosine A2A receptor expressed in HEK-293 cells; ranges from 0.69-0.92 |
J Med Chem 45: 115-26 (2001)
BindingDB Entry DOI: 10.7270/Q29Z946T |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50382290

(CARIPRAZINE HYDROCHLORIDE | RGH-188 HCL)Show SMILES CN(C)C(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc(Cl)c2Cl)CC1 |r,wU:6.5,wD:9.9,(.89,-12.18,;2.22,-12.95,;2.22,-14.49,;3.56,-12.18,;3.56,-10.64,;4.89,-12.95,;6.22,-12.18,;6.22,-10.64,;7.55,-9.86,;8.88,-10.64,;10.22,-9.87,;11.55,-10.65,;12.88,-9.88,;14.21,-10.66,;15.54,-9.9,;15.55,-8.36,;14.22,-7.58,;12.88,-8.35,;16.89,-7.6,;18.25,-8.33,;19.56,-7.51,;19.51,-5.97,;18.16,-5.25,;18.11,-3.71,;16.84,-6.06,;15.49,-5.33,;8.88,-12.18,;7.55,-12.94,)| Show InChI InChI=1S/C21H32Cl2N4O/c1-25(2)21(28)24-17-8-6-16(7-9-17)10-11-26-12-14-27(15-13-26)19-5-3-4-18(22)20(19)23/h3-5,16-17H,6-15H2,1-2H3,(H,24,28)/t16-,17- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc
Curated by ChEMBL
| Assay Description
Binding affinity to human dopamine D2S receptor |
Bioorg Med Chem Lett 22: 3437-40 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.104
BindingDB Entry DOI: 10.7270/Q2ZW1MZN |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Richter Gedeon Nyrt.
US Patent
| Assay Description
Alpha-1 receptor binding studies were performed according to the methods described by Greengrass and Bremner (Eur. J. Pharmacol., 55:323-326, 1979) o... |
US Patent US8802672 (2014)
BindingDB Entry DOI: 10.7270/Q2FF3R1T |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202766
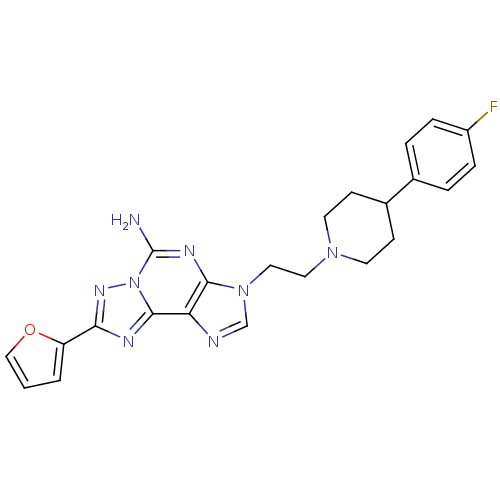
(3-(2-(4-(4-fluorophenyl)piperidin-1-yl)ethyl)-8-(f...)Show SMILES Nc1nc2n(CCN3CCC(CC3)c3ccc(F)cc3)cnc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C23H23FN8O/c24-17-5-3-15(4-6-17)16-7-9-30(10-8-16)11-12-31-14-26-19-21(31)28-23(25)32-22(19)27-20(29-32)18-2-1-13-33-18/h1-6,13-14,16H,7-12H2,(H2,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202783
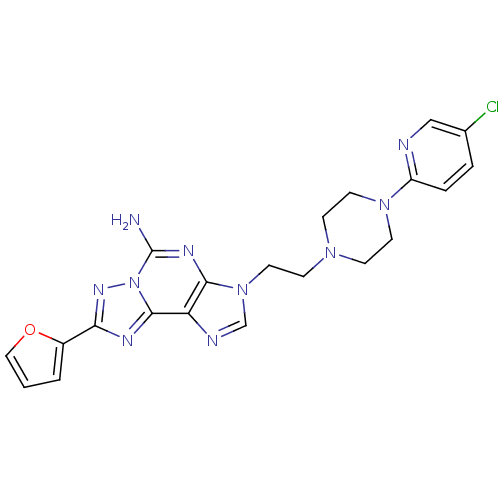
(3-(2-(4-(5-chloropyridin-2-yl)piperazin-1-yl)ethyl...)Show SMILES Nc1nc2n(CCN3CCN(CC3)c3ccc(Cl)cn3)cnc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C21H21ClN10O/c22-14-3-4-16(24-12-14)30-8-5-29(6-9-30)7-10-31-13-25-17-19(31)27-21(23)32-20(17)26-18(28-32)15-2-1-11-33-15/h1-4,11-13H,5-10H2,(H2,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50202773
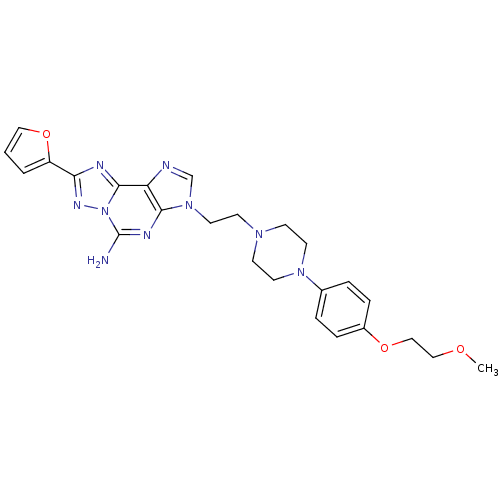
(8-(furan-2-yl)-3-(2-(4-(4-(2-methoxyethoxy)phenyl)...)Show SMILES COCCOc1ccc(cc1)N1CCN(CCn2cnc3c2nc(N)n2nc(nc32)-c2ccco2)CC1 Show InChI InChI=1S/C25H29N9O3/c1-35-15-16-36-19-6-4-18(5-7-19)32-11-8-31(9-12-32)10-13-33-17-27-21-23(33)29-25(26)34-24(21)28-22(30-34)20-3-2-14-37-20/h2-7,14,17H,8-13,15-16H2,1H3,(H2,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonist activity at human adenosine A2A receptor |
Bioorg Med Chem Lett 17: 1659-62 (2007)
Article DOI: 10.1016/j.bmcl.2006.12.104
BindingDB Entry DOI: 10.7270/Q2RF5TPQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50064700
(4-(3-(5-amino-2-(furan-2-yl)-7H-pyrazolo[4,3-e][1,...)Show SMILES Nc1nc2n(CCCc3ccc(O)cc3)ncc2c2nc(nn12)-c1ccco1 Show InChI InChI=1S/C19H17N7O2/c20-19-23-17-14(18-22-16(24-26(18)19)15-4-2-10-28-15)11-21-25(17)9-1-3-12-5-7-13(27)8-6-12/h2,4-8,10-11,27H,1,3,9H2,(H2,20,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGS- 21680 from rat striatal membranes Adenosine A2A receptor. Parenthesis indicate 95% confidence limits. |
J Med Chem 41: 2126-33 (1998)
Article DOI: 10.1021/jm9708689
BindingDB Entry DOI: 10.7270/Q2251H9K |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM50004566

(9-Chloro-2-furan-2-yl-[1,2,4]triazolo[1,5-c]quinaz...)Show InChI InChI=1S/C13H8ClN5O/c14-7-3-4-9-8(6-7)12-17-11(10-2-1-5-20-10)18-19(12)13(15)16-9/h1-6H,(H2,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Ferrara
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CGS- 21680 from rat striatal membranes Adenosine A2A receptor. Parenthesis indicate 95% confidence limits. |
J Med Chem 41: 2126-33 (1998)
Article DOI: 10.1021/jm9708689
BindingDB Entry DOI: 10.7270/Q2251H9K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(GUINEA PIG) | BDBM50000492

((zacopride)4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl...)Show SMILES COc1cc(N)c(Cl)cc1C(=O)NC1CN2CCC1CC2 |(27.19,-33.96,;28.52,-34.73,;28.53,-36.27,;27.2,-37.04,;27.2,-38.58,;25.86,-39.35,;28.53,-39.36,;28.53,-40.89,;29.87,-38.58,;29.86,-37.03,;31.19,-36.26,;31.19,-34.72,;32.53,-37.02,;33.86,-36.25,;35.2,-37.02,;36.52,-36.25,;36.52,-34.71,;35.19,-33.94,;33.85,-34.71,;34.61,-36.04,;35.74,-34.91,)| Show InChI InChI=1S/C15H20ClN3O2/c1-21-14-7-12(17)11(16)6-10(14)15(20)18-13-8-19-4-2-9(13)3-5-19/h6-7,9,13H,2-5,8,17H2,1H3,(H,18,20) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Discovery Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 267: 961-70 (1993)
BindingDB Entry DOI: 10.7270/Q2125R5V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data