

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073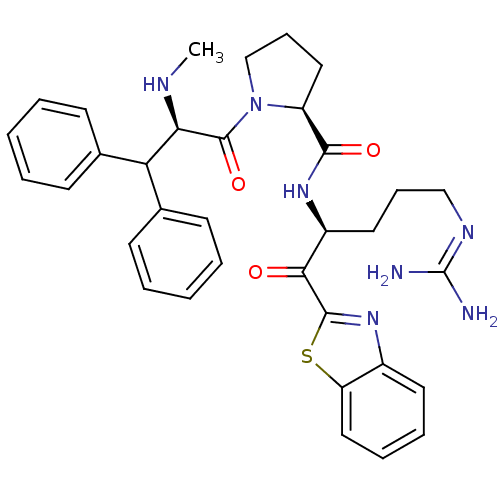 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14073 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | J Med Chem 47: 769-87 (2004) Article DOI: 10.1021/jm030493t BindingDB Entry DOI: 10.7270/Q2251HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065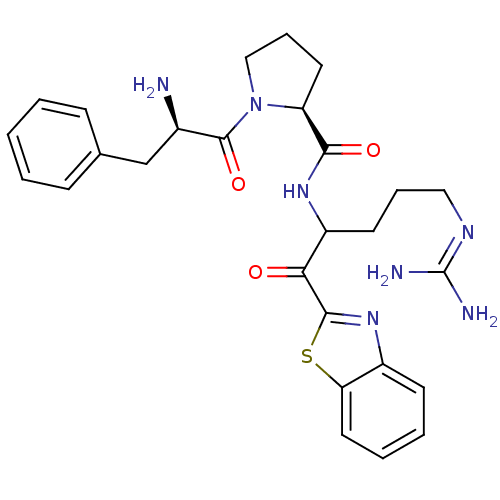 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14127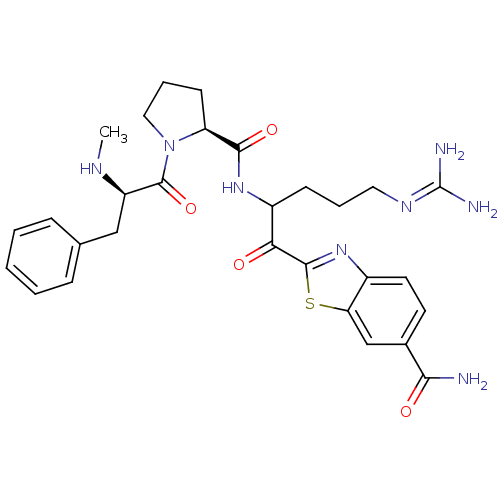 (2-(5-carbamimidamido-2-{[(2S)-1-[(2R)-2-(methylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00700 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328697 ((R)-5-guanidino-N-(2-((S)-5-guanidino-1-oxo-1-(thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14076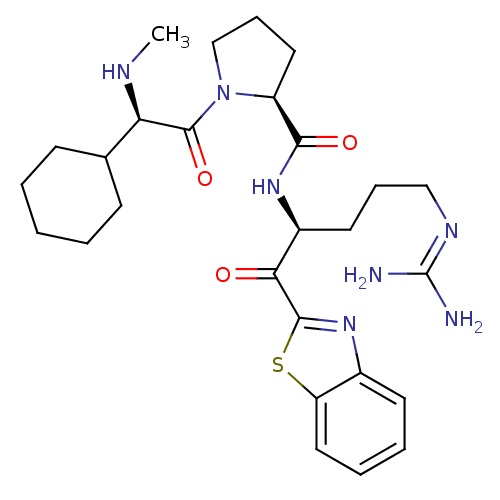 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | 5.30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50139746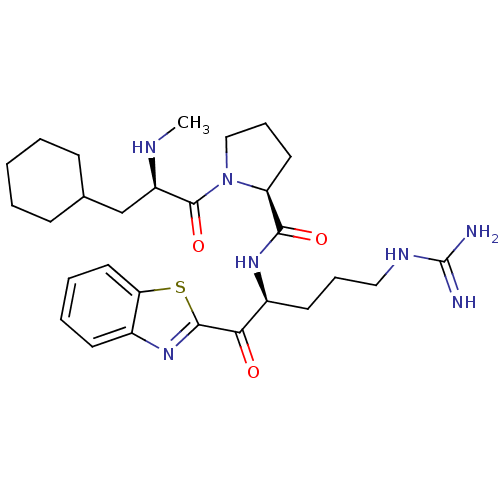 ((S)-1-((R)-3-Cyclohexyl-2-methylamino-propionyl)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | J Med Chem 47: 769-87 (2004) Article DOI: 10.1021/jm030493t BindingDB Entry DOI: 10.7270/Q2251HN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50320472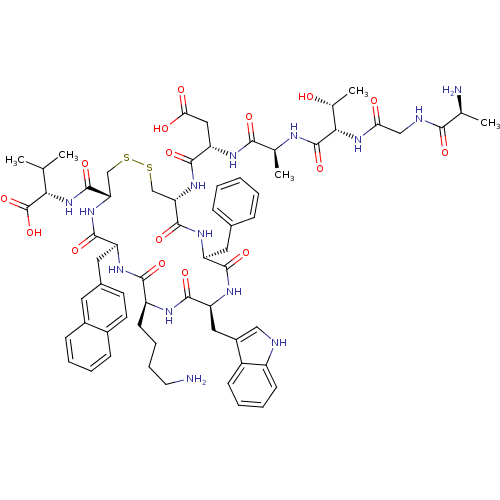 ((3S,6S,9S,15S)-3-((4R,7S,10S,13S,16S,19R)-13-((1H-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cells | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50095523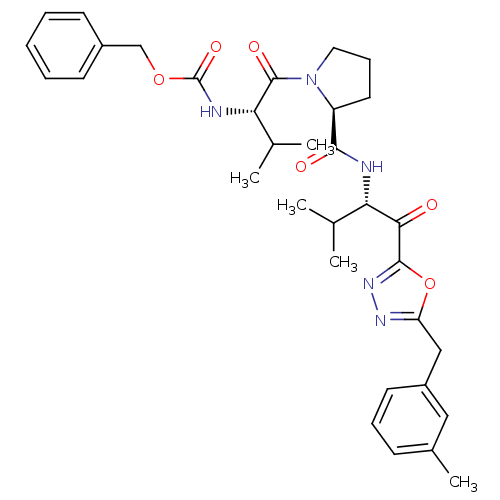 (CHEMBL285231 | [(S)-2-methyl-1-((S)-2-{(S)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50140304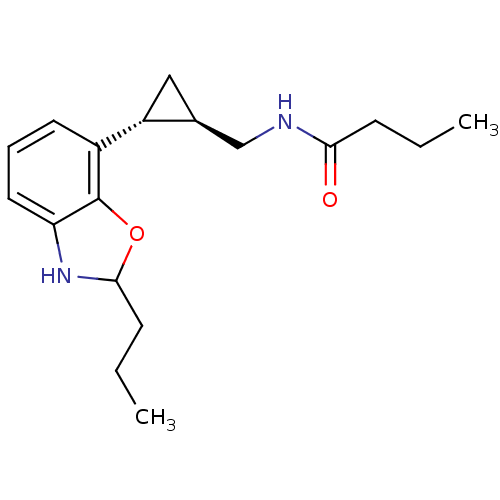 (CHEMBL19211 | N-[(1R,2R)-2-(2-Propyl-2,3-dihydro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50140301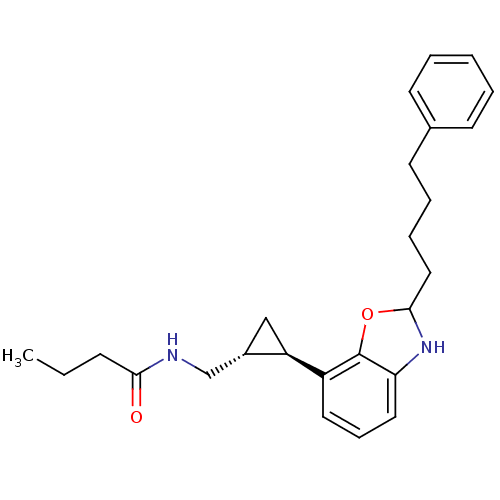 (CHEMBL19026 | N-{(1R,2R)-2-[2-(4-Phenyl-butyl)-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452037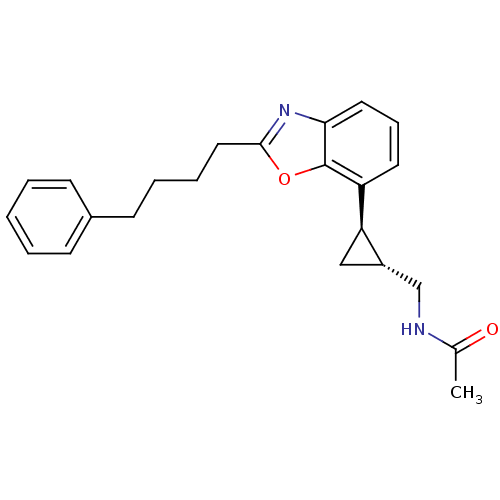 (CHEMBL2112700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452032 (CHEMBL2112727) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50142220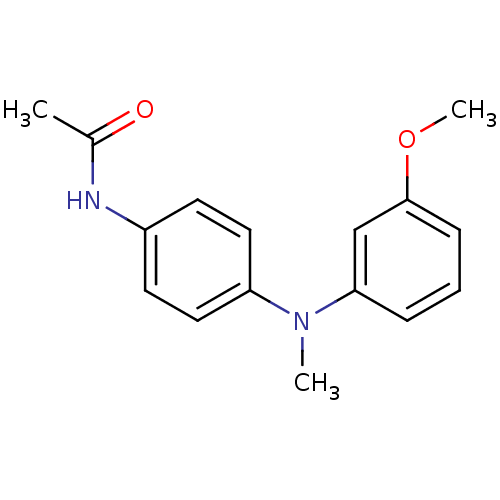 (CHEMBL8168 | N-{4-[(3-Methoxy-phenyl)-methyl-amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1023-6 (2004) Article DOI: 10.1016/j.bmcl.2003.11.030 BindingDB Entry DOI: 10.7270/Q2HT2NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452034 (CHEMBL2112724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452036 (CHEMBL2112734) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452035 (CHEMBL2112722) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50452033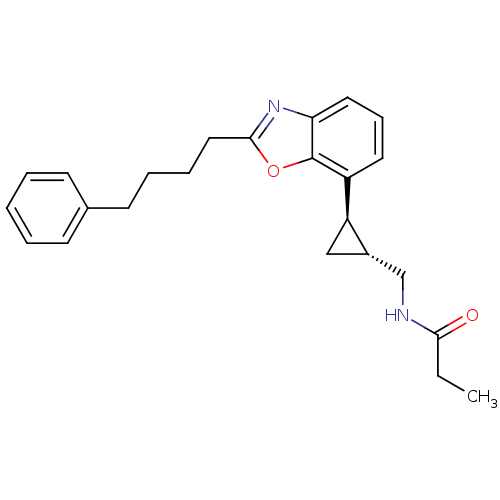 (CHEMBL2112690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50374350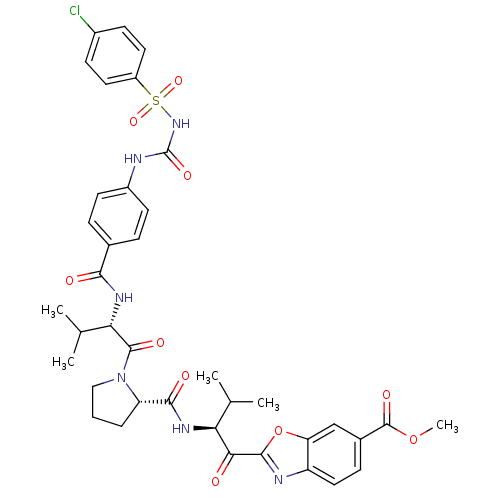 (CHEMBL403098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50452033 (CHEMBL2112690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029246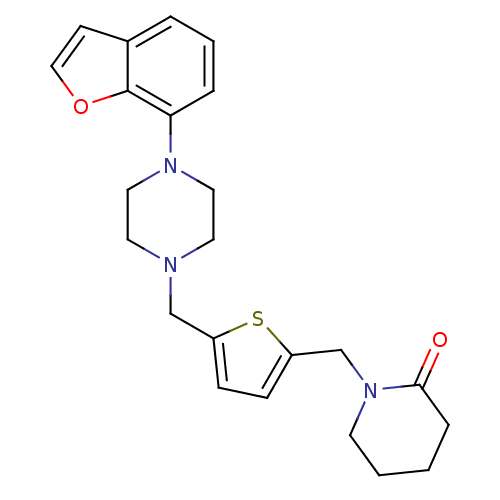 (1-[5-(4-Benzofuran-7-yl-piperazin-1-ylmethyl)-thio...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity was determined against 5-hydroxytryptamine 1A receptor using [3H]WB-4101 | J Med Chem 38: 4198-210 (1995) BindingDB Entry DOI: 10.7270/Q2GX4C61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50076232 ((1S,7S)-7-Amino-7-benzyl-8-oxo-hexahydro-pyrazolo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50140299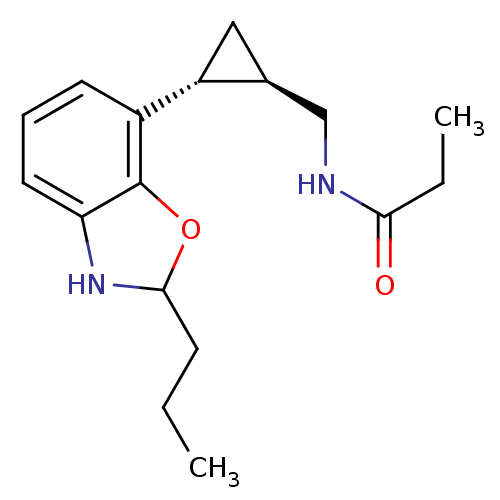 (CHEMBL276921 | N-[(1R,2R)-2-(2-Propyl-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serotonin 2 (5-HT2) receptor (RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50069019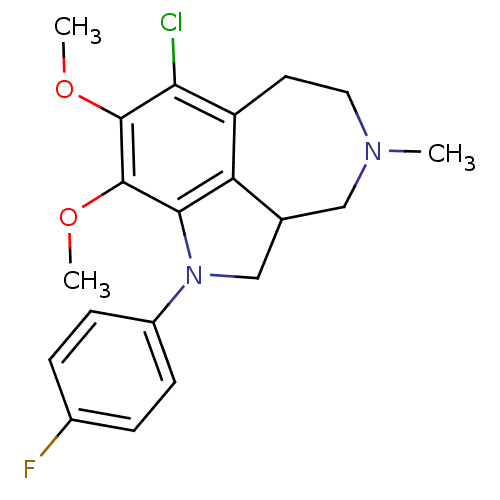 (7-Chloro-1-(4-fluoro-phenyl)-8,9-dimethoxy-4-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity for 5-hydroxytryptamine 2 receptor | Bioorg Med Chem Lett 8: 983-8 (1999) BindingDB Entry DOI: 10.7270/Q2Q81C7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50161518 (1-(oxazolo[4,5-b]pyridin-2-yl)-6-phenylhexan-1-one...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50142200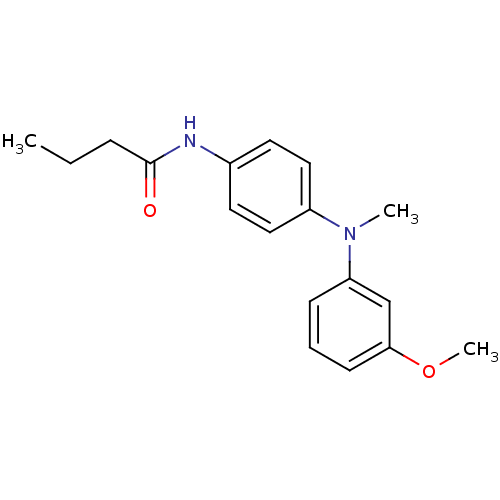 (CHEMBL266549 | N-{4-[(3-Methoxy-phenyl)-methyl-ami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1023-6 (2004) Article DOI: 10.1016/j.bmcl.2003.11.030 BindingDB Entry DOI: 10.7270/Q2HT2NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50123504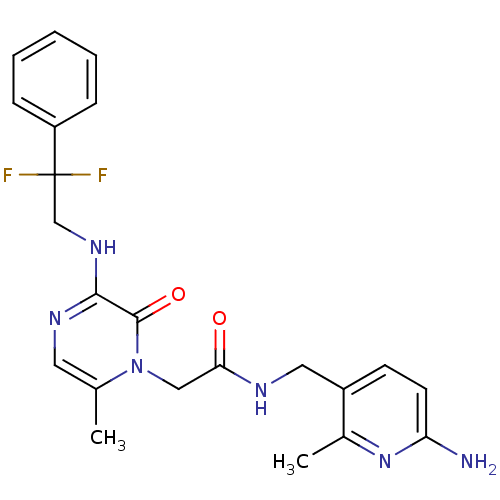 (CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Binding affinity to human thrombin | Bioorg Med Chem Lett 18: 2865-70 (2008) Article DOI: 10.1016/j.bmcl.2008.03.087 BindingDB Entry DOI: 10.7270/Q2FJ2HP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50031203 (2-{(S)-2-[((S)-1-{(S)-2-[4-(4-Chloro-benzenesulfon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14066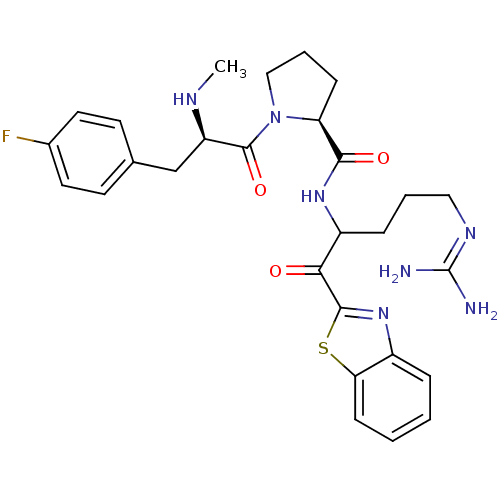 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | -58.9 | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273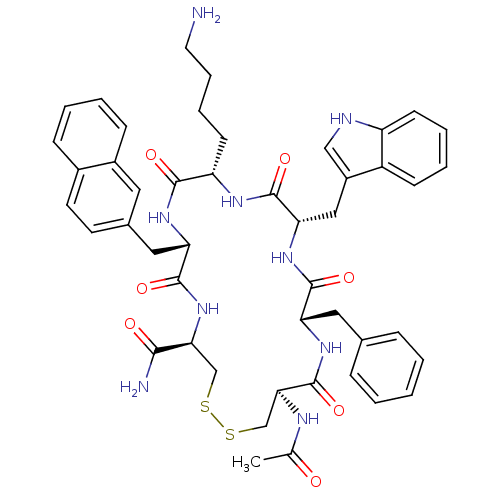 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assay | J Med Chem 52: 7432-45 (2009) Article DOI: 10.1021/jm900683d BindingDB Entry DOI: 10.7270/Q2S75GD7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50142231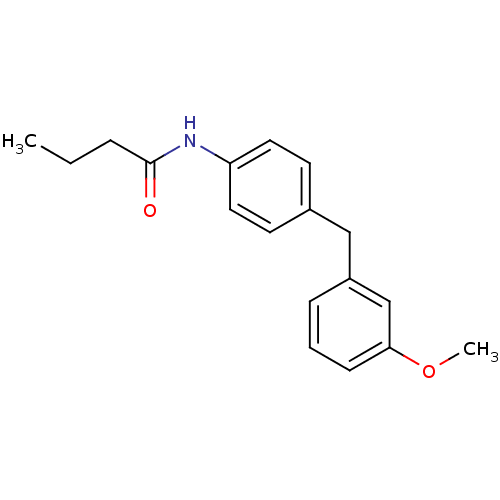 (CHEMBL7867 | N-[4-(3-Methoxy-benzyl)-phenyl]-butyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1023-6 (2004) Article DOI: 10.1016/j.bmcl.2003.11.030 BindingDB Entry DOI: 10.7270/Q2HT2NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302273 ((4R,7S,10S,13S,16S,19R)-13-((1H-indol-3-yl)methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]U2 from rat urotensin 2 receptor expressed in CHO cells | J Med Chem 53: 2695-708 (2010) Article DOI: 10.1021/jm901294u BindingDB Entry DOI: 10.7270/Q20G3K9N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50140293 (CHEMBL19128 | N-[(1R,2R)-2-(2-Propyl-2,3-dihydro-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50029326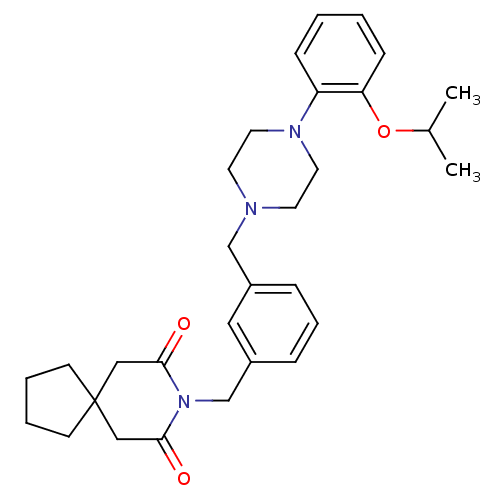 (8-{3-[4-(2-Isopropoxy-phenyl)-piperazin-1-ylmethyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Affinity against the 5-hydroxytryptamine receptor 1A using [3H]WB-4101. | J Med Chem 38: 4211-22 (1995) BindingDB Entry DOI: 10.7270/Q2C53MH2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50140308 (CHEMBL18845 | N-{(1R,2R)-2-[2-(4-Phenyl-butyl)-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14123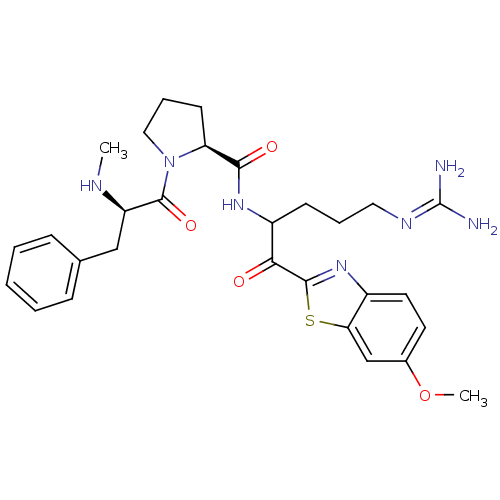 ((2S)-N-[5-carbamimidamido-1-(6-methoxy-1,3-benzoth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50374357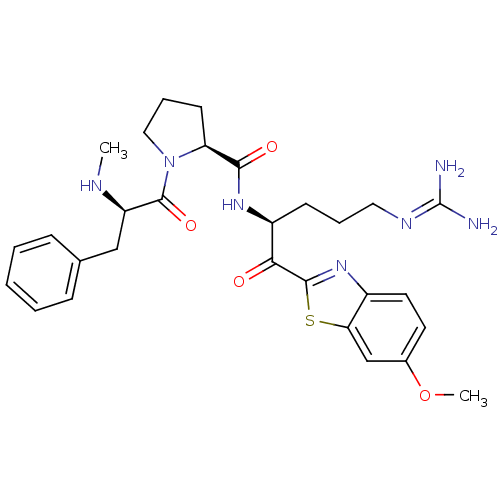 (CHEMBL404043) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50136402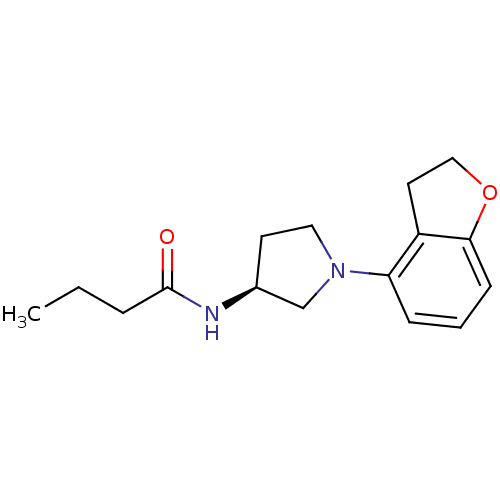 (CHEMBL136531 | N-[(S)-1-(2,3-Dihydro-benzofuran-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells. | Bioorg Med Chem Lett 13: 4381-4 (2003) BindingDB Entry DOI: 10.7270/Q2X066FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50140302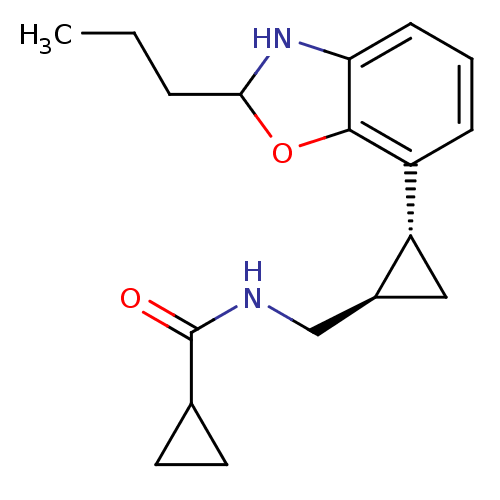 (CHEMBL279343 | Cyclopropanecarboxylic acid [(1R,2R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity towards Dopamine receptor D1 | J Med Chem 30: 1433-54 (1987) BindingDB Entry DOI: 10.7270/Q2D50NJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14125 ((2S)-N-[5-carbamimidamido-1-(6-fluoro-1,3-benzothi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50374356 (CHEMBL404042) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human alpha-thrombin | Bioorg Med Chem 16: 1562-95 (2008) Article DOI: 10.1016/j.bmc.2007.11.015 BindingDB Entry DOI: 10.7270/Q21J9BNH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50367554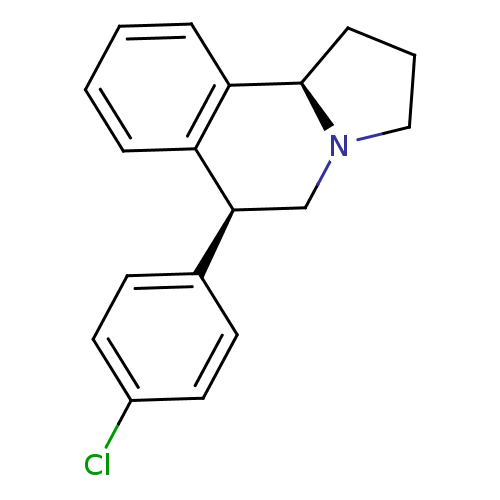 (CHEMBL1743782) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of uptake of tritiated norepinephrine (NE) in rat synaptosomes | J Med Chem 30: 1433-54 (1987) BindingDB Entry DOI: 10.7270/Q2D50NJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transporter (Rattus norvegicus) | BDBM50028288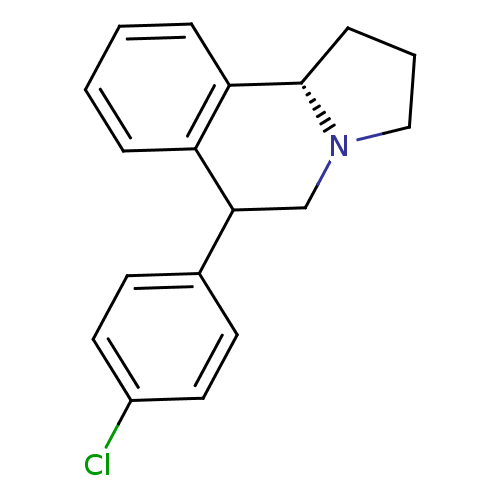 (6-(4-Chloro-phenyl)-1,2,3,5,6,10b-hexahydro-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of norepinephrine (NE) into rat brain synaptosomes | J Med Chem 27: 943-6 (1984) BindingDB Entry DOI: 10.7270/Q2VT1SPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50142214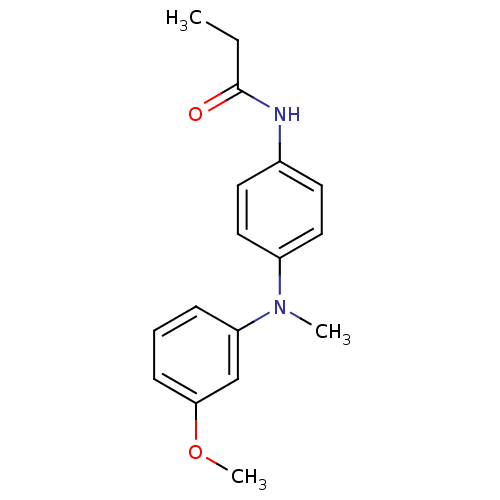 (CHEMBL7832 | N-{4-[(3-Methoxy-phenyl)-methyl-amino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1023-6 (2004) Article DOI: 10.1016/j.bmcl.2003.11.030 BindingDB Entry DOI: 10.7270/Q2HT2NSG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14068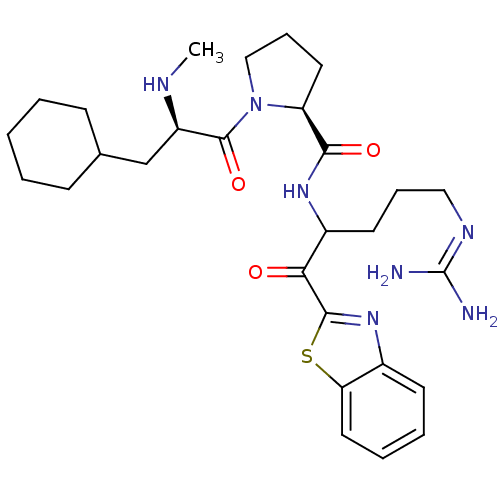 ((2S)-N-[1-(1,3-benzothiazol-2-yl)-5-carbamimidamid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.180 | -57.9 | 48 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50140302 (CHEMBL279343 | Cyclopropanecarboxylic acid [(1R,2R...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1A (Homo sapiens (Human)) | BDBM50136400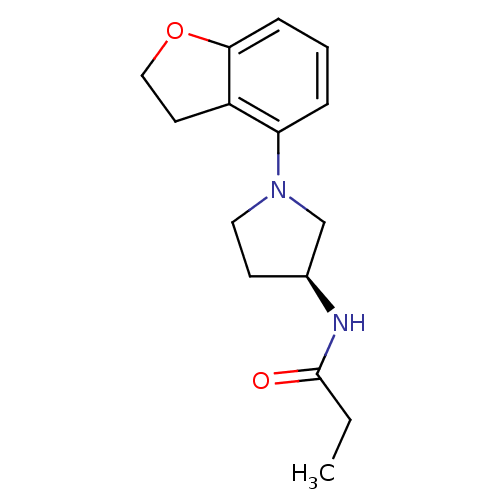 (CHEMBL343825 | N-[(S)-1-(2,3-Dihydro-benzofuran-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells. | Bioorg Med Chem Lett 13: 4381-4 (2003) BindingDB Entry DOI: 10.7270/Q2X066FK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melatonin receptor type 1B (Homo sapiens (Human)) | BDBM50452034 (CHEMBL2112724) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding of 2-[125I]-iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B | Bioorg Med Chem Lett 14: 1197-200 (2004) Article DOI: 10.1016/j.bmcl.2003.12.052 BindingDB Entry DOI: 10.7270/Q2CF9PJC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4098 total ) | Next | Last >> |