 Found 82 hits with Last Name = 'björkling' and Initial = 'f'
Found 82 hits with Last Name = 'björkling' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443803
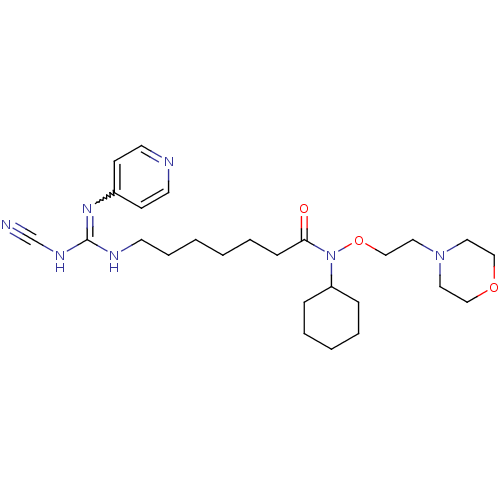
(CHEMBL3094250)Show SMILES O=C(CCCCCCNC(NC#N)=Nc1ccncc1)N(OCCN1CCOCC1)C1CCCCC1 |w:13.13| Show InChI InChI=1S/C26H41N7O3/c27-22-30-26(31-23-11-14-28-15-12-23)29-13-7-2-1-6-10-25(34)33(24-8-4-3-5-9-24)36-21-18-32-16-19-35-20-17-32/h11-12,14-15,24H,1-10,13,16-21H2,(H2,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443800
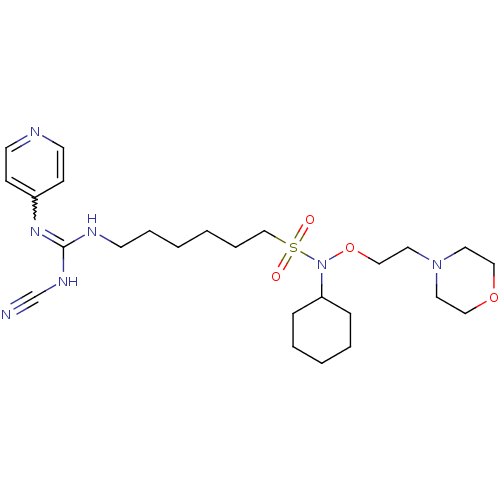
(CHEMBL3094255)Show SMILES O=S(=O)(CCCCCCNC(NC#N)=Nc1ccncc1)N(OCCN1CCOCC1)C1CCCCC1 |w:14.14| Show InChI InChI=1S/C25H41N7O4S/c26-22-29-25(30-23-10-13-27-14-11-23)28-12-6-1-2-7-21-37(33,34)32(24-8-4-3-5-9-24)36-20-17-31-15-18-35-19-16-31/h10-11,13-14,24H,1-9,12,15-21H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268097
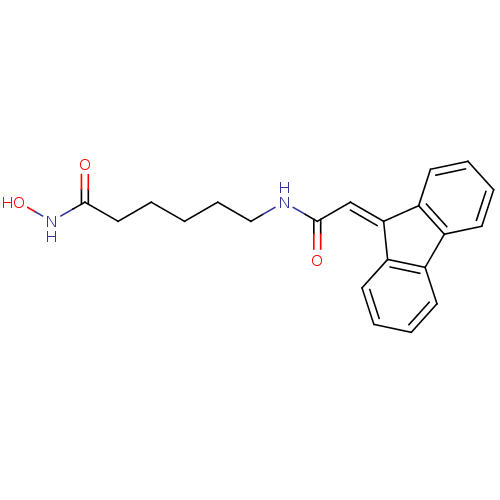
(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443805
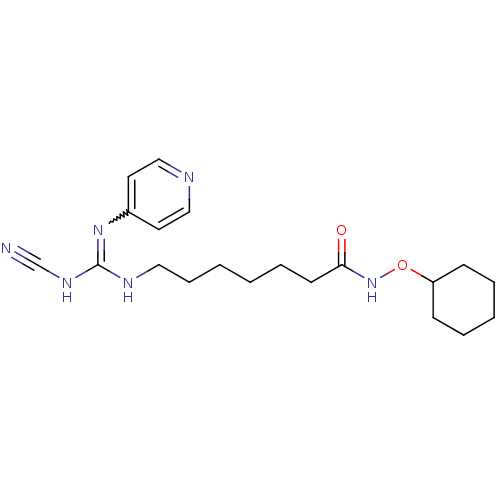
(CHEMBL3094249)Show SMILES O=C(CCCCCCNC(NC#N)=Nc1ccncc1)NOC1CCCCC1 |w:13.13| Show InChI InChI=1S/C20H30N6O2/c21-16-24-20(25-17-11-14-22-15-12-17)23-13-7-2-1-6-10-19(27)26-28-18-8-4-3-5-9-18/h11-12,14-15,18H,1-10,13H2,(H,26,27)(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM81395
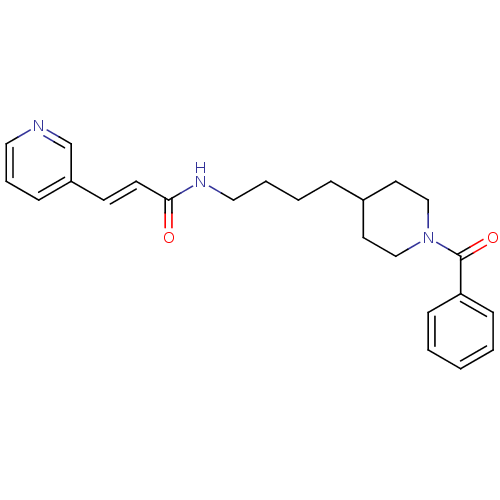
(APO-866)Show SMILES O=C(NCCCCC1CCN(CC1)C(=O)c1ccccc1)\C=C\c1cccnc1 Show InChI InChI=1S/C24H29N3O2/c28-23(12-11-21-8-6-15-25-19-21)26-16-5-4-7-20-13-17-27(18-14-20)24(29)22-9-2-1-3-10-22/h1-3,6,8-12,15,19-20H,4-5,7,13-14,16-18H2,(H,26,28)/b12-11+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443798
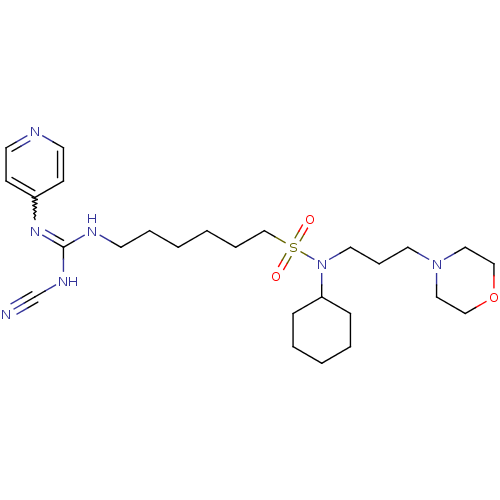
(CHEMBL3094258)Show SMILES O=S(=O)(CCCCCCNC(NC#N)=Nc1ccncc1)N(CCCN1CCOCC1)C1CCCCC1 |w:14.14| Show InChI InChI=1S/C26H43N7O3S/c27-23-30-26(31-24-11-14-28-15-12-24)29-13-6-1-2-7-22-37(34,35)33(25-9-4-3-5-10-25)17-8-16-32-18-20-36-21-19-32/h11-12,14-15,25H,1-10,13,16-22H2,(H2,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443801
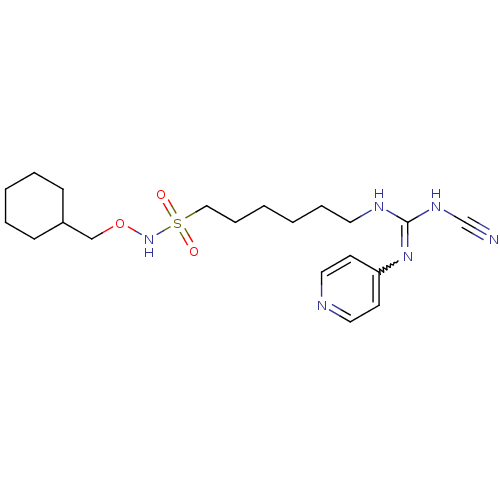
(CHEMBL3094254)Show SMILES O=S(=O)(CCCCCCNC(NC#N)=Nc1ccncc1)NOCC1CCCCC1 |w:14.14| Show InChI InChI=1S/C20H32N6O3S/c21-17-24-20(25-19-10-13-22-14-11-19)23-12-6-1-2-7-15-30(27,28)26-29-16-18-8-4-3-5-9-18/h10-11,13-14,18,26H,1-9,12,15-16H2,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268063
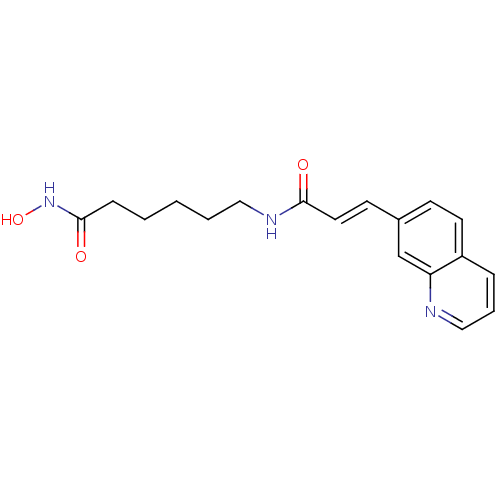
((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268121
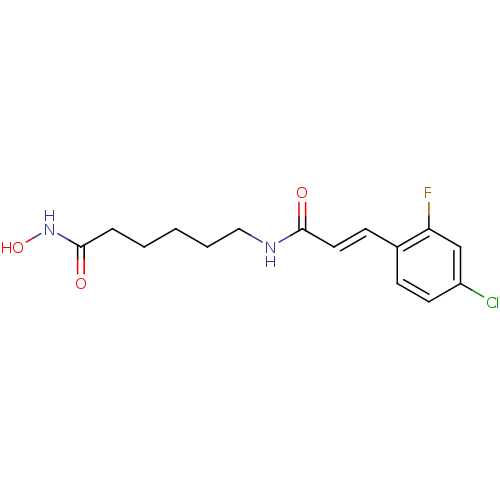
((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268097

(6-{[2-(9H-Fluoren-9-yliden)acetyl]amino}-N-hydroxy...)Show SMILES [#8]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6]-[#6]-[#7]-[#6](=O)\[#6]=[#6]-1\c2ccccc2-c2ccccc-12 Show InChI InChI=1S/C21H22N2O3/c24-20(23-26)12-2-1-7-13-22-21(25)14-19-17-10-5-3-8-15(17)16-9-4-6-11-18(16)19/h3-6,8-11,14,26H,1-2,7,12-13H2,(H,22,25)(H,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50268042
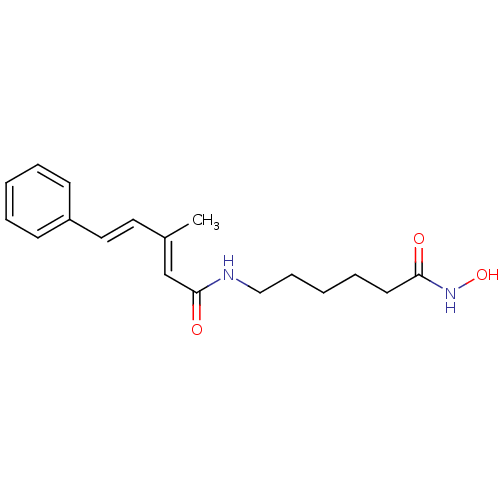
((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443799

(CHEMBL3094257)Show SMILES FCCN(OCC1CCCCC1)S(=O)(=O)CCCCCCNC(NC#N)=Nc1ccncc1 |w:26.27| Show InChI InChI=1S/C22H35FN6O3S/c23-12-16-29(32-18-20-8-4-3-5-9-20)33(30,31)17-7-2-1-6-13-26-22(27-19-24)28-21-10-14-25-15-11-21/h10-11,14-15,20H,1-9,12-13,16-18H2,(H2,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443796
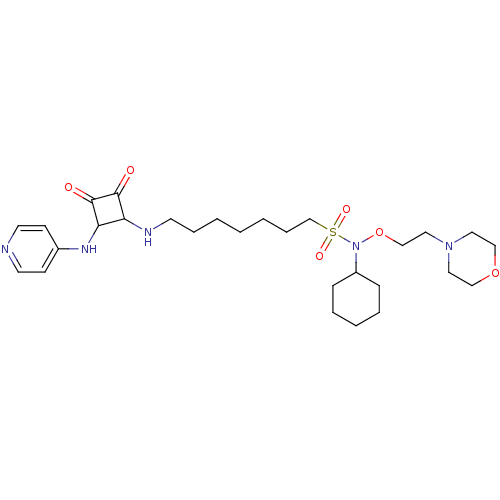
(CHEMBL3094229)Show SMILES O=C1C(NCCCCCCCS(=O)(=O)N(OCCN2CCOCC2)C2CCCCC2)C(Nc2ccncc2)C1=O Show InChI InChI=1S/C28H45N5O6S/c34-27-25(26(28(27)35)31-23-11-14-29-15-12-23)30-13-7-2-1-3-8-22-40(36,37)33(24-9-5-4-6-10-24)39-21-18-32-16-19-38-20-17-32/h11-12,14-15,24-26,30H,1-10,13,16-22H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136890

(CHEMBL152208 | [2-Chloro-4-(4-fluoro-2-methyl-phen...)Show InChI InChI=1S/C21H17ClFNO/c1-13-5-3-4-6-17(13)21(25)18-9-8-16(12-19(18)22)24-20-10-7-15(23)11-14(20)2/h3-12,24H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC9 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443802
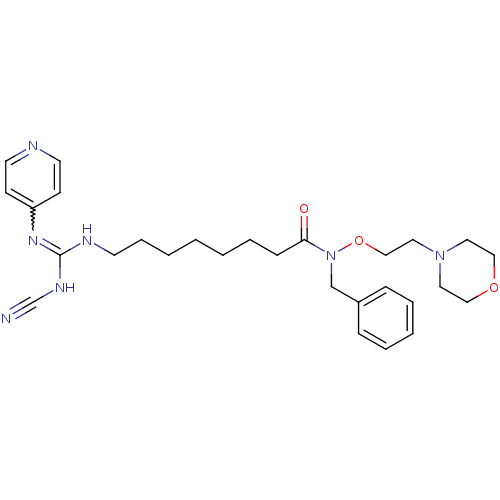
(CHEMBL3094251)Show SMILES O=C(CCCCCCCNC(NC#N)=Nc1ccncc1)N(Cc1ccccc1)OCCN1CCOCC1 |w:14.14| Show InChI InChI=1S/C28H39N7O3/c29-24-32-28(33-26-12-15-30-16-13-26)31-14-8-3-1-2-7-11-27(36)35(23-25-9-5-4-6-10-25)38-22-19-34-17-20-37-21-18-34/h4-6,9-10,12-13,15-16H,1-3,7-8,11,14,17-23H2,(H2,30,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136887

(CHEMBL356455 | [4-(2-Amino-phenylamino)-2-chloro-p...)Show InChI InChI=1S/C20H17ClN2O2/c1-25-19-9-5-2-6-15(19)20(24)14-11-10-13(12-16(14)21)23-18-8-4-3-7-17(18)22/h2-12,23H,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268042

((2E,4E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-methyl-5...)Show InChI InChI=1S/C18H24N2O3/c1-15(11-12-16-8-4-2-5-9-16)14-18(22)19-13-7-3-6-10-17(21)20-23/h2,4-5,8-9,11-12,14,23H,3,6-7,10,13H2,1H3,(H,19,22)(H,20,21)/b12-11+,15-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50268121

((E)-3-(4-Chloro-2-fluorophenyl)-N-[6-(hydroxyamino...)Show InChI InChI=1S/C15H18ClFN2O3/c16-12-7-5-11(13(17)10-12)6-8-14(20)18-9-3-1-2-4-15(21)19-22/h5-8,10,22H,1-4,9H2,(H,18,20)(H,19,21)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC7 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136897
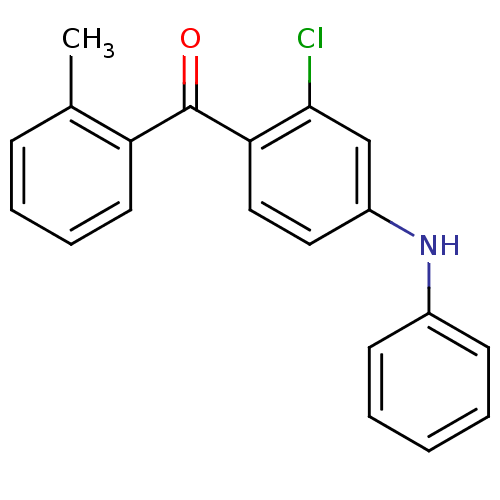
((2-Chloro-4-phenylamino-phenyl)-o-tolyl-methanone ...)Show InChI InChI=1S/C20H16ClNO/c1-14-7-5-6-10-17(14)20(23)18-12-11-16(13-19(18)21)22-15-8-3-2-4-9-15/h2-13,22H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50268063

((E)-N-[6-(Hydroxyamino)-6-oxohexyl]-3-(7-quinoliny...)Show InChI InChI=1S/C18H21N3O3/c22-17(20-11-3-1-2-6-18(23)21-24)10-8-14-7-9-15-5-4-12-19-16(15)13-14/h4-5,7-10,12-13,24H,1-3,6,11H2,(H,20,22)(H,21,23)/b10-8+ | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Organic Synthesis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 |
Eur J Med Chem 44: 1067-85 (2009)
Article DOI: 10.1016/j.ejmech.2008.06.020
BindingDB Entry DOI: 10.7270/Q2BG2NTX |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443793

(CHEMBL3094236)Show SMILES O=C(CCCCCCNC(=S)NCc1cccnc1)N(OCCN1CCOCC1)C1CCCCC1 Show InChI InChI=1S/C26H43N5O3S/c32-25(12-6-1-2-7-14-28-26(35)29-22-23-9-8-13-27-21-23)31(24-10-4-3-5-11-24)34-20-17-30-15-18-33-19-16-30/h8-9,13,21,24H,1-7,10-12,14-20,22H2,(H2,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136886
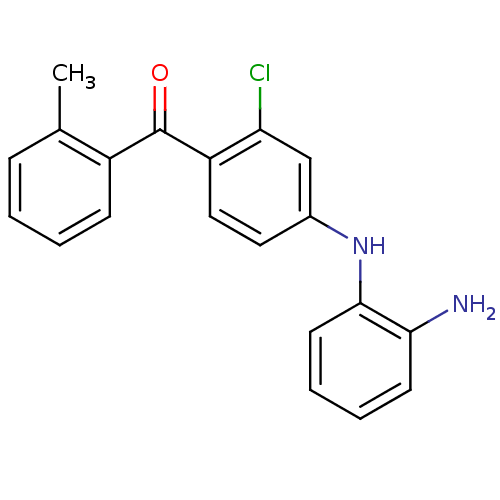
(CHEMBL152767 | [4-(2-Amino-phenylamino)-2-chloro-p...)Show InChI InChI=1S/C20H17ClN2O/c1-13-6-2-3-7-15(13)20(24)16-11-10-14(12-17(16)21)23-19-9-5-4-8-18(19)22/h2-12,23H,22H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136884
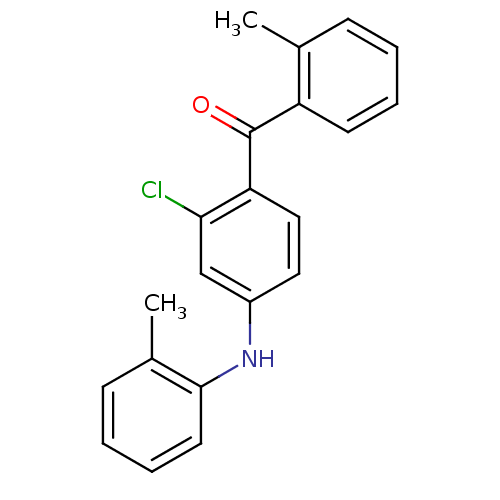
((2-Chloro-4-o-tolylamino-phenyl)-o-tolyl-methanone...)Show InChI InChI=1S/C21H18ClNO/c1-14-7-3-5-9-17(14)21(24)18-12-11-16(13-19(18)22)23-20-10-6-4-8-15(20)2/h3-13,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443791

(CHEMBL3094238)Show SMILES O=C(NCCCCCCS(=O)(=O)N(CCN1CCOCC1)CC1CCCCC1)NCc1cccnc1 Show InChI InChI=1S/C26H45N5O4S/c32-26(29-22-25-11-8-12-27-21-25)28-13-6-1-2-7-20-36(33,34)31(23-24-9-4-3-5-10-24)15-14-30-16-18-35-19-17-30/h8,11-12,21,24H,1-7,9-10,13-20,22-23H2,(H2,28,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136898

(CHEMBL152820 | [4-(2-Amino-phenylamino)-2-methyl-p...)Show InChI InChI=1S/C21H20N2O/c1-14-7-3-4-8-17(14)21(24)18-12-11-16(13-15(18)2)23-20-10-6-5-9-19(20)22/h3-13,23H,22H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50435350
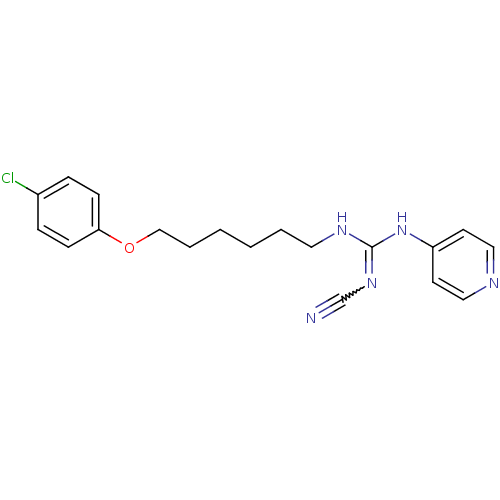
(CHEMBL17289)Show SMILES Clc1ccc(OCCCCCCNC(Nc2ccncc2)=NC#N)cc1 |w:21.22| Show InChI InChI=1S/C19H22ClN5O/c20-16-5-7-18(8-6-16)26-14-4-2-1-3-11-23-19(24-15-21)25-17-9-12-22-13-10-17/h5-10,12-13H,1-4,11,14H2,(H2,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136900
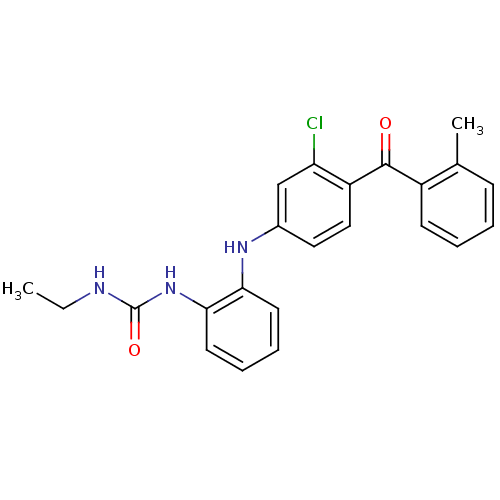
(1-{2-[3-Chloro-4-(2-methyl-benzoyl)-phenylamino]-p...)Show SMILES CCNC(=O)Nc1ccccc1Nc1ccc(C(=O)c2ccccc2C)c(Cl)c1 Show InChI InChI=1S/C23H22ClN3O2/c1-3-25-23(29)27-21-11-7-6-10-20(21)26-16-12-13-18(19(24)14-16)22(28)17-9-5-4-8-15(17)2/h4-14,26H,3H2,1-2H3,(H2,25,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |
Nicotinamide phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50443797
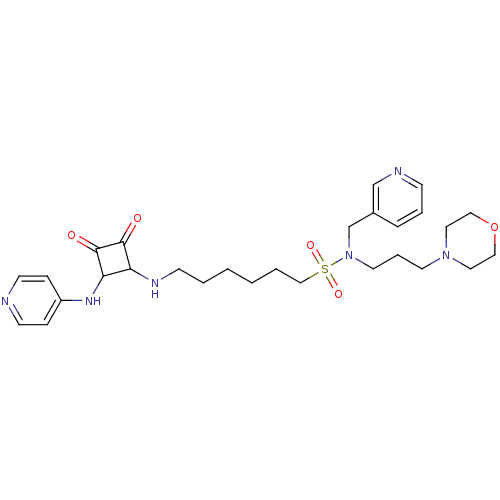
(CHEMBL3094224)Show SMILES O=C1C(NCCCCCCS(=O)(=O)N(CCCN2CCOCC2)Cc2cccnc2)C(Nc2ccncc2)C1=O Show InChI InChI=1S/C28H40N6O5S/c35-27-25(26(28(27)36)32-24-8-12-29-13-9-24)31-11-3-1-2-4-20-40(37,38)34(22-23-7-5-10-30-21-23)15-6-14-33-16-18-39-19-17-33/h5,7-10,12-13,21,25-26,31H,1-4,6,11,14-20,22H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Topotarget A/S
Curated by ChEMBL
| Assay Description
Inhibition of NAMPT in human HepG2 cells using [14C]-nicotinamide/PRPP as substrate assessed as formation of [14C]-nicotinamide mononucleotide after ... |
J Med Chem 56: 9071-88 (2013)
Article DOI: 10.1021/jm4009949
BindingDB Entry DOI: 10.7270/Q2CV4K6W |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM15244
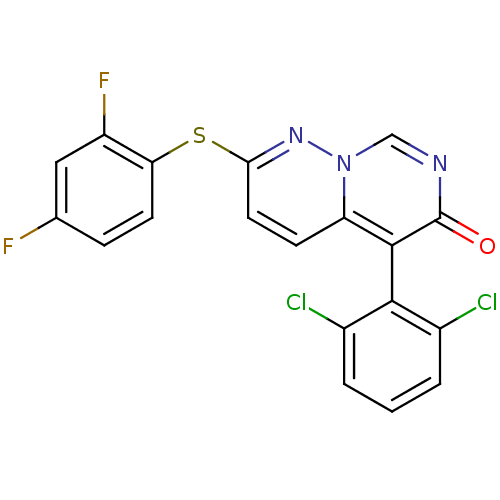
(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50136903
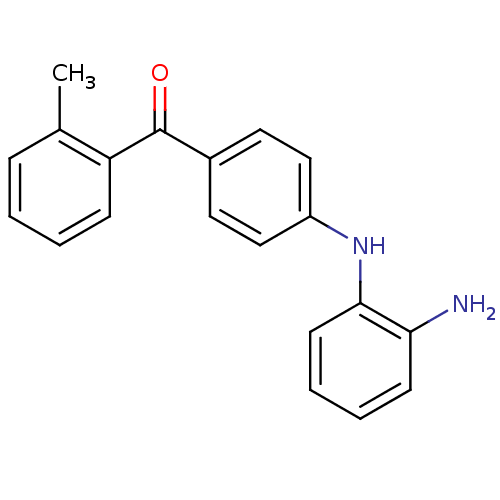
(CHEMBL357732 | [4-(2-Amino-phenylamino)-phenyl]-o-...)Show InChI InChI=1S/C20H18N2O/c1-14-6-2-3-7-17(14)20(23)15-10-12-16(13-11-15)22-19-9-5-4-8-18(19)21/h2-13,22H,21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
LEO Pharma
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity determined against human mitogen-activated protein kinase p38 alpha |
J Med Chem 46: 5651-62 (2003)
Article DOI: 10.1021/jm030851s
BindingDB Entry DOI: 10.7270/Q2B56KFK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data