

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160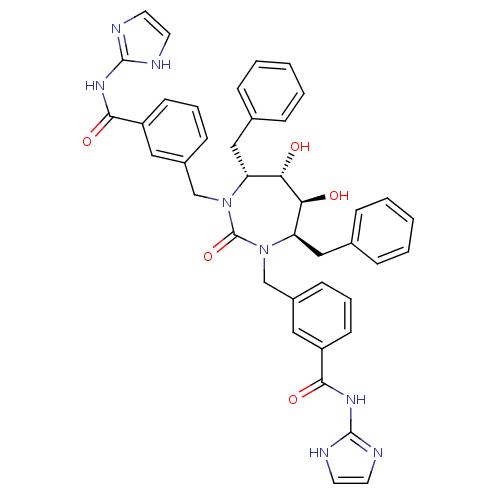 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055590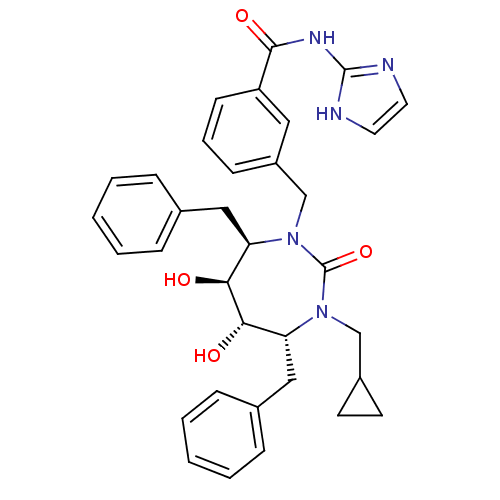 (3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-cyclopropylmethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155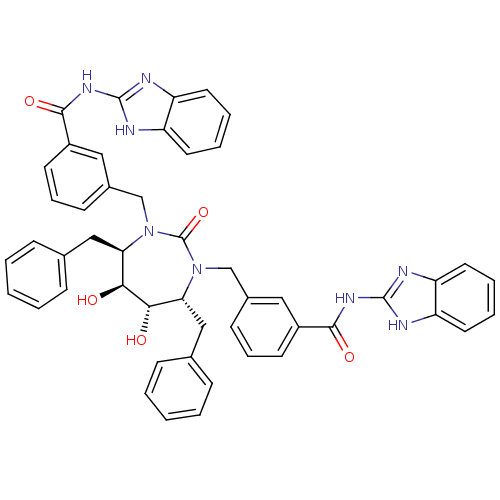 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055587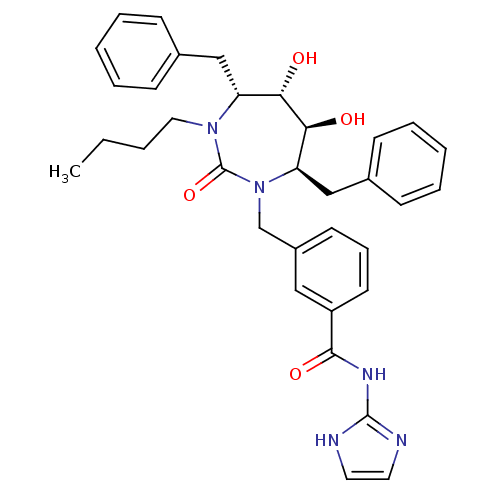 (3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-butyl-5,6-dihydrox...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM154 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055593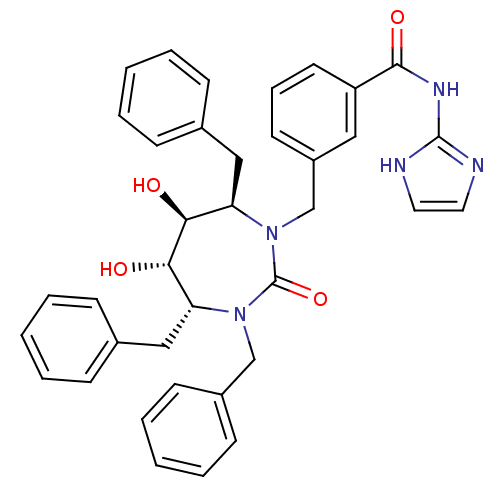 (CHEMBL292905 | N-(1H-Imidazol-2-yl)-3-((4R,5S,6S,7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055588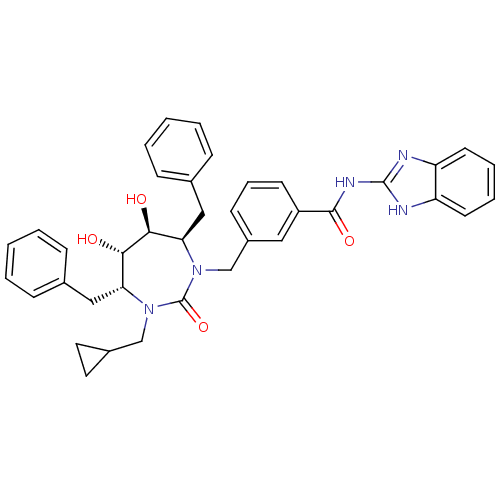 (CHEMBL301219 | N-(1H-Benzoimidazol-2-yl)-3-((4R,5S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50097626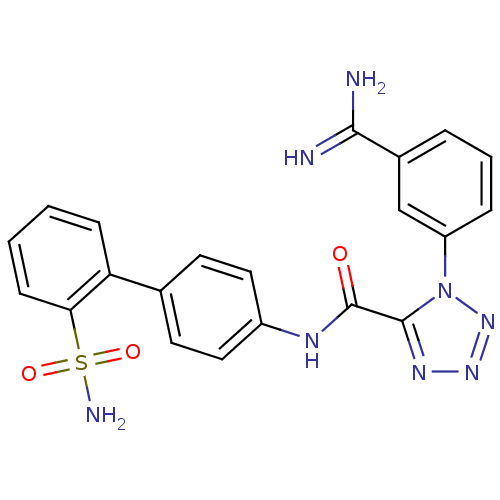 (1-(3-Carbamimidoyl-phenyl)-1H-tetrazole-5-carboxyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055589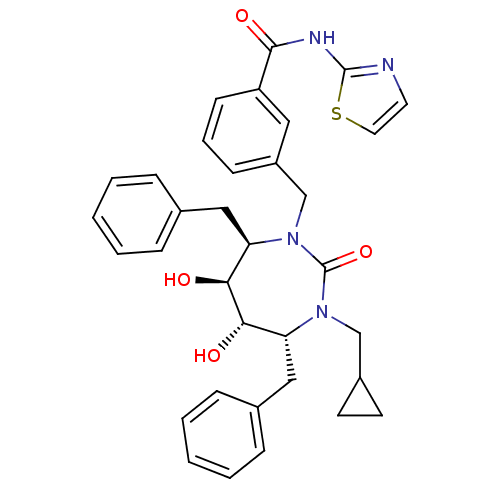 (3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-cyclopropylmethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055592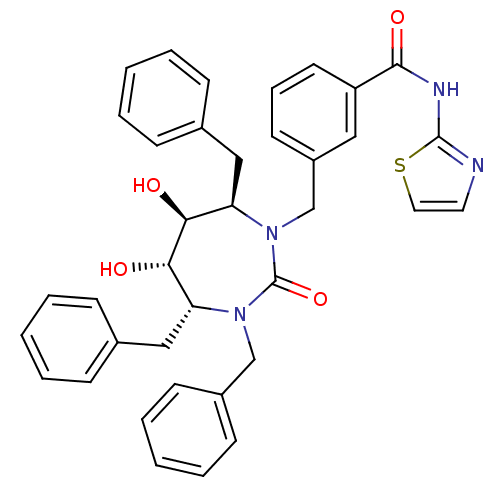 (CHEMBL56117 | N-Thiazol-2-yl-3-((4R,5S,6S,7R)-3,4,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM19023 (1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1727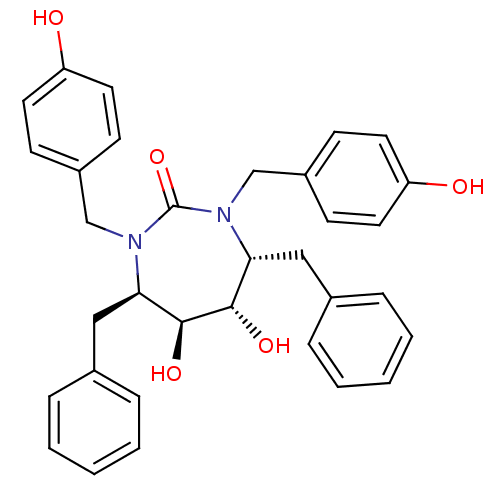 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1726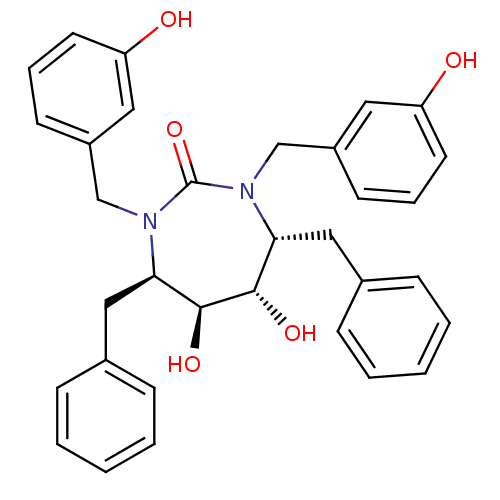 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1730 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12657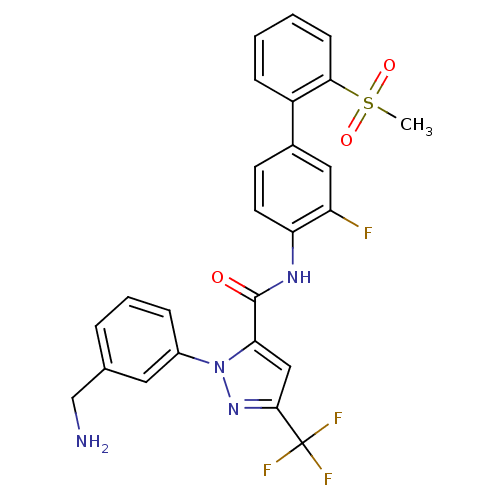 (1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM577 ((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12676 (1-(3-Aminobenzisoxazol-5-yl)-3-trifluoromethyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50055591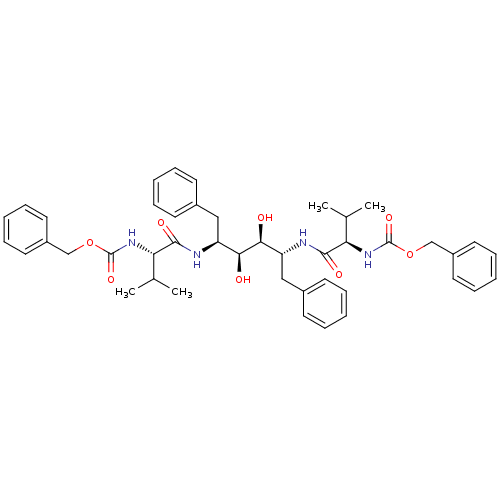 (CHEMBL56387 | {(R)-1-[(1R,2S,3S,4S)-1-Benzyl-4-((S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307102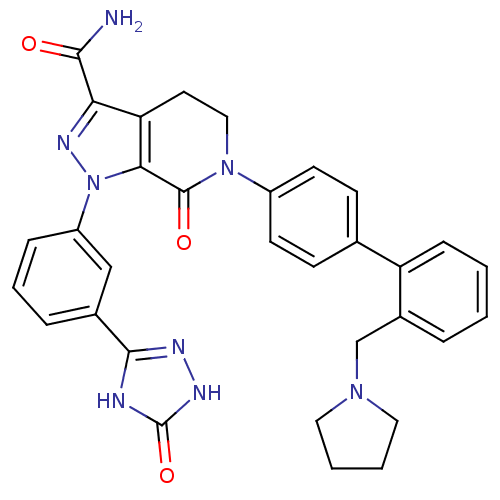 (7-oxo-1-(3-(5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM150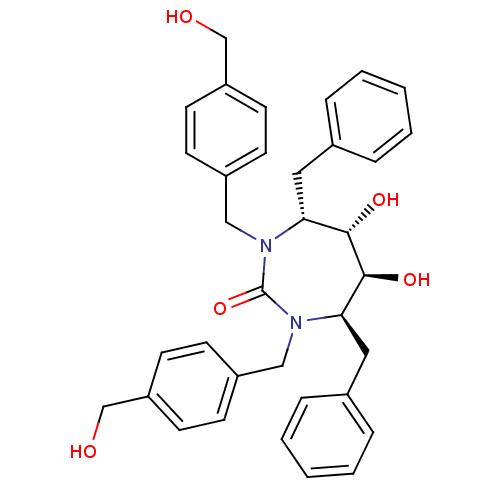 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM151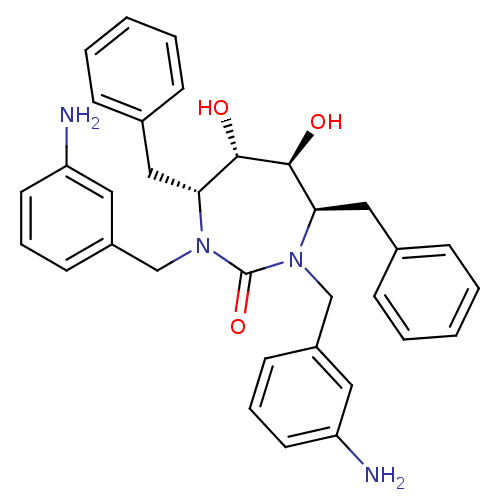 ((4R,5S,6S,7R)-1,3-bis[(3-aminophenyl)methyl]-4,7-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123033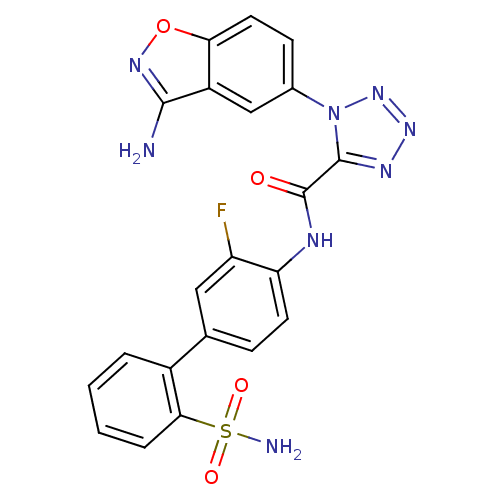 (1-(3-Amino-benzo[d]isoxazol-5-yl)-1H-tetrazole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123037 (1-(3-Amino-benzo[d]isoxazol-5-yl)-1H-tetrazole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307100 (1-(3-(5-OXO-4,5-DIHYDRO-1H-1,2,4-TRIAZOL-3-YL)PHEN...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307093 (CHEMBL591955 | N-(3-fluoro-2'-(methylsulfonyl)biph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1702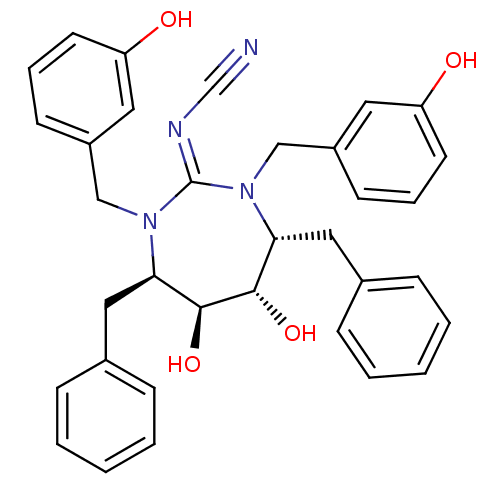 (Cyclic Cyanoguanidine deriv. 8r | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.720 | -54.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307099 ((R)-6-(2'-((3-hydroxypyrrolidin-1-yl)methyl)biphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123038 (1-(3-Amino-benzo[d]isoxazol-5-yl)-1H-tetrazole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1723 ((4R,5S,6S,7R)-1,3-bis[(4-aminophenyl)methyl]-4,7-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307098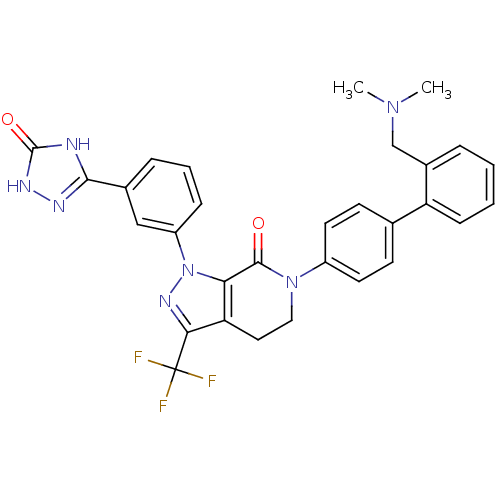 (6-(2'-((dimethylamino)methyl)biphenyl-4-yl)-1-(3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123035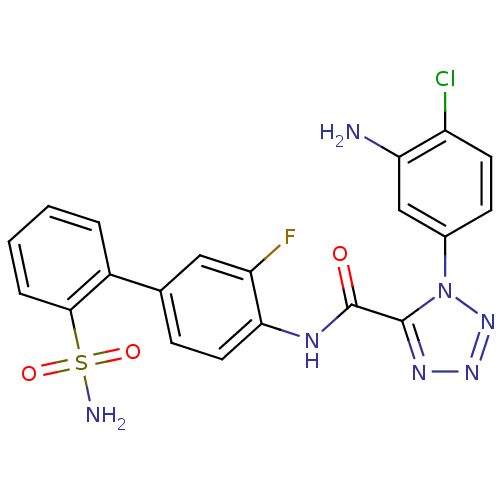 (1-(3-Amino-4-chloro-phenyl)-1H-tetrazole-5-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1693 (Cyclic Cyanoguanidine deriv. 8i | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.5 | -52.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1706 (Cyclic Cyanoguanidine deriv. 8v | [4R-(4a,5a,6b,7b...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -52.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM32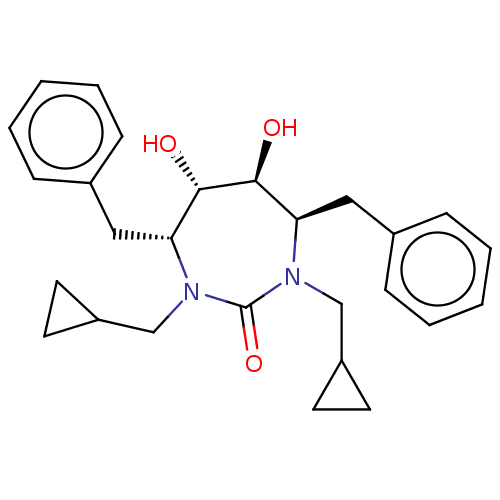 ((4R,5S,6S,7R)-4,7-dibenzyl-1,3-bis(cyclopropylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to inhibit the purified wild-type HIV-1 Protease | J Med Chem 40: 181-91 (1997) Article DOI: 10.1021/jm960586t BindingDB Entry DOI: 10.7270/Q2ST7NZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307097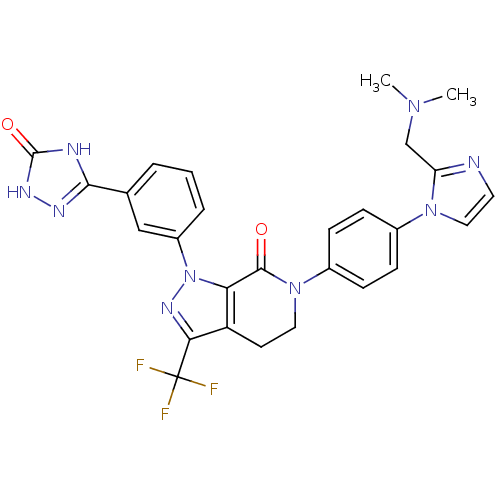 (6-(4-(2-((dimethylamino)methyl)-1H-imidazol-1-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1692 (Cyclic Cyanoguanidine deriv. 8h | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -51.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50307096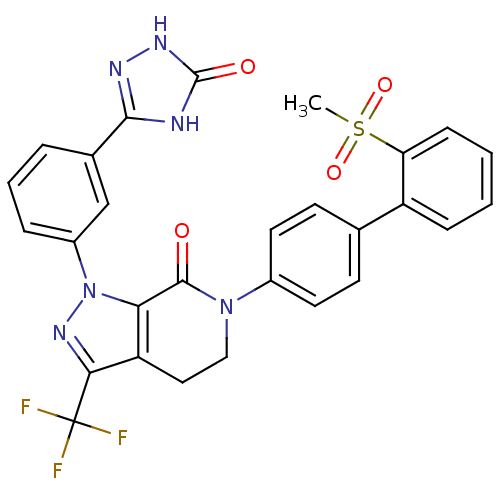 (6-(2'-(METHYLSULFONYL)BIPHENYL-4-YL)-1-(3-(5-OXO-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 20: 1373-7 (2010) Article DOI: 10.1016/j.bmcl.2010.01.011 BindingDB Entry DOI: 10.7270/Q2FQ9WQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1703 (Cyclic Cyanoguanidine deriv. 8s | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.60 | -51.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1688 (Cyclic Cyanoguanidine deriv. 8d | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1720 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123039 (1-(3-Amino-benzo[d]isoxazol-5-yl)-1H-tetrazole-5-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123043 (1-(3-Amino-4-chloro-phenyl)-1H-tetrazole-5-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1724 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-3-[(3-cyanophenyl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123041 (1-(3-Amino-4-chloro-phenyl)-1H-tetrazole-5-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1690 (Cyclic Cyanoguanidine deriv. 8f | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.80 | -50.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50123036 (1-(3-Amino-4-chloro-phenyl)-1H-tetrazole-5-carboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Co. Curated by ChEMBL | Assay Description Inhibition of human purified factor Xa | Bioorg Med Chem Lett 13: 369-73 (2003) BindingDB Entry DOI: 10.7270/Q2BG2NC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1694 (Cyclic Cyanoguanidine deriv. 8j | [4R-(4a,5a,6B,7B...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
The DuPont Merck Pharmaceutical Company | Assay Description Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... | J Med Chem 41: 1446-55 (1998) Article DOI: 10.1021/jm970524i BindingDB Entry DOI: 10.7270/Q2K935QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |