

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118030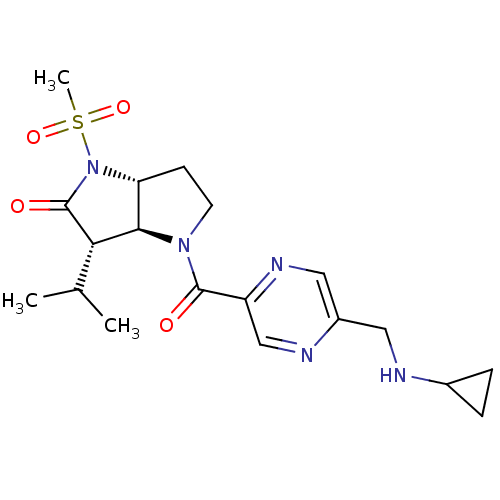 (4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288943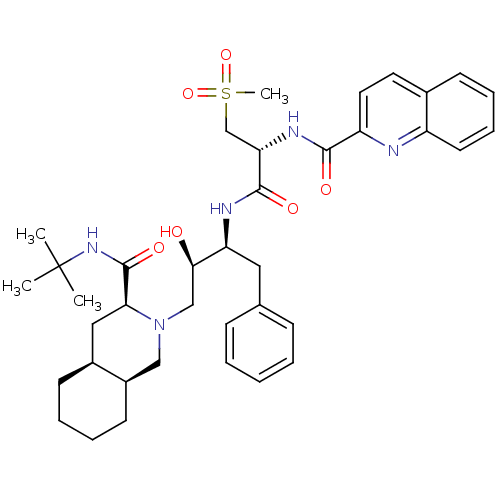 (CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288941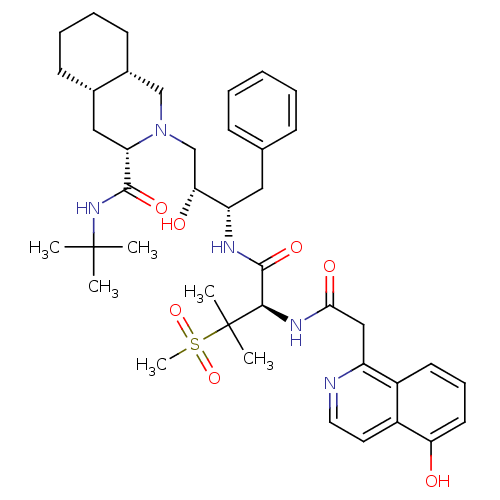 ((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028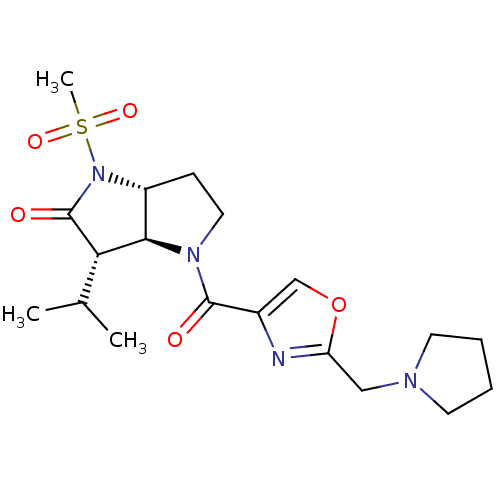 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | 4.90 | 3.05E+4 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118028 (3-Isopropyl-1-methanesulfonyl-4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288942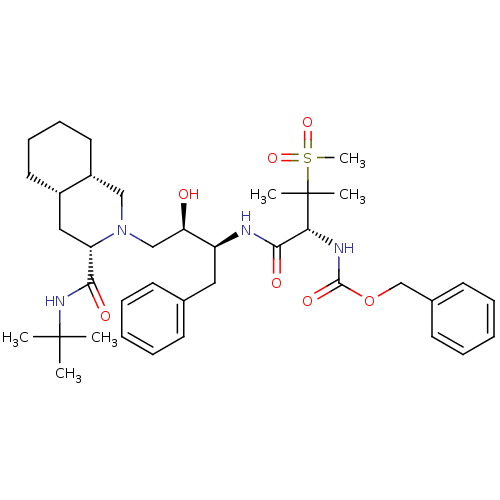 (CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288939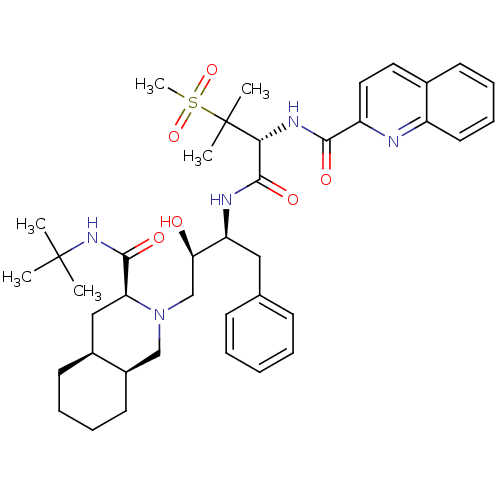 (CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | 0.00000207 | 6.63E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118029 (3-Isopropyl-1-methanesulfonyl-4-(4-piperidin-1-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50058235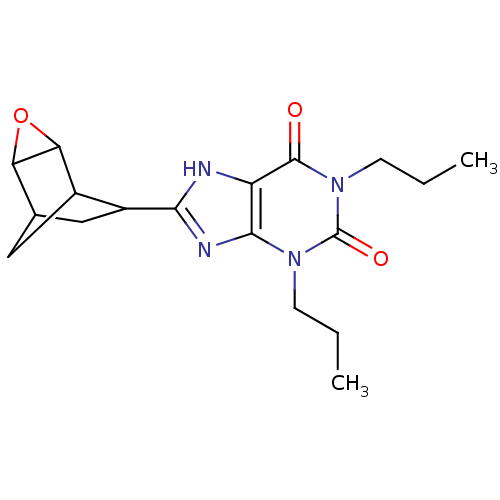 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of binding to membranes from HEK293 cells expressing human Adenosine A1 receptor | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9294 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50058235 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor of rat forebrain | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290520 (((R)-1-{(S)-1-[(2R,3S)-3-(2-tert-Butylsulfamoyl-et...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003019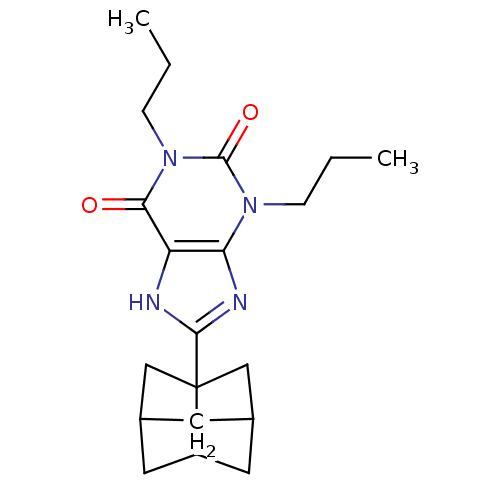 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Inhibition of binding to membranes from HEK293 cells expressing human Adenosine A1 receptor | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290527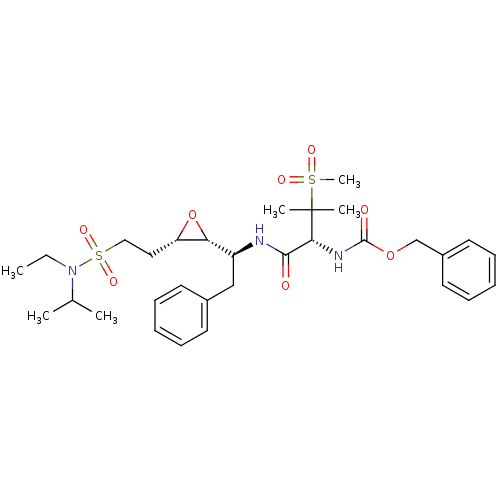 (CHEMBL86971 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108418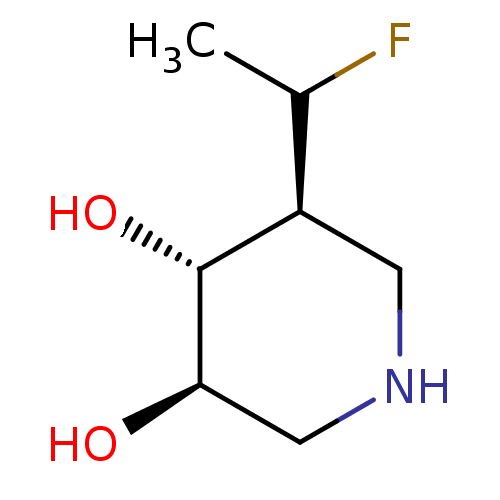 (US8604206, 13 | US8604206, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | 17.1 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50003019 (8-(Hexahydro-2,5-methano-pentalen-3a-yl)-1,3-dipro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor from Guinea pig membranes | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027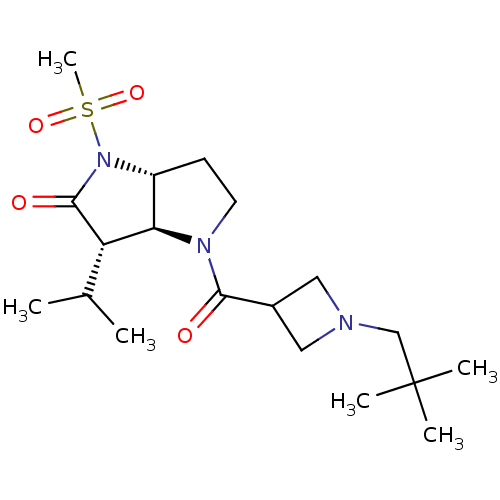 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50118027 (4-[1-(2,2-Dimethyl-propyl)-azetidine-3-carbonyl]-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.73 | n/a | n/a | n/a | n/a | 0.0000109 | 6.31E+3 | n/a | n/a |
GSK Curated by ChEMBL | Assay Description The compound was evaluated for its inhibitory activity against human neutrophil elastase (HNE) using whole blood assay | J Med Chem 45: 3878-90 (2002) BindingDB Entry DOI: 10.7270/Q2HM596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068673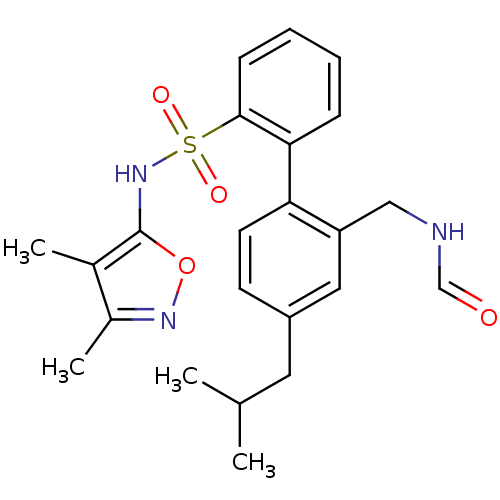 (2'-Formylaminomethyl-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50058235 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor from Guinea pig membranes | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50058235 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor from Guinea pig membranes | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288940 (CHEMBL154416 | Quinoline-2-carboxylic acid {(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against P2 site in HIV protease. | Bioorg Med Chem Lett 6: 585-588 (1996) Article DOI: 10.1016/0960-894X(96)00086-8 BindingDB Entry DOI: 10.7270/Q2KH0N9Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068706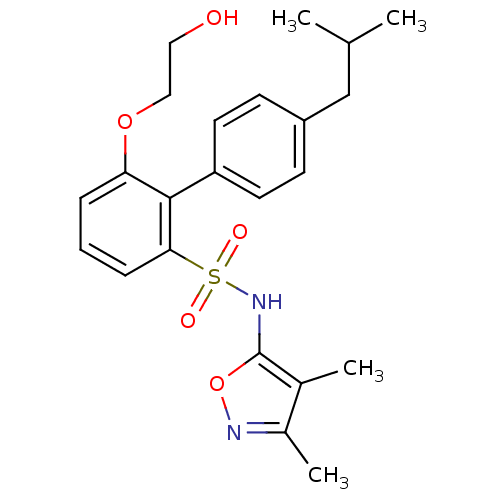 (6-(2-Hydroxy-ethoxy)-4'-isobutyl-biphenyl-2-sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor from Guinea pig membranes | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290525 (CHEMBL92097 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor from Guinea pig membranes | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14381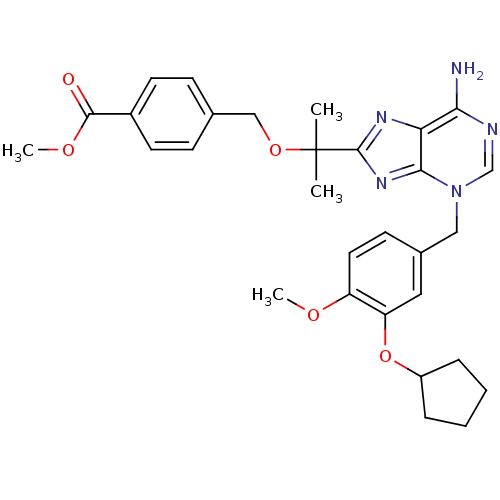 (4-{2-[6-Amino-3-(3-cyclopentyloxy-4-methoxy-benzyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108417 (US8604206, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.06 | n/a | 5.80 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290523 (CHEMBL327222 | Thiophene-2-carboxylic acid [(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290524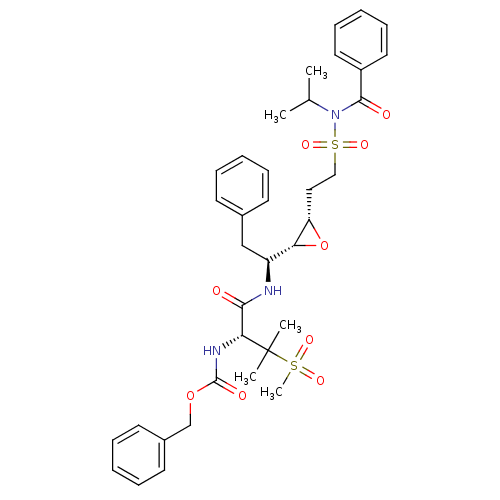 (CHEMBL404933 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Benzoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14376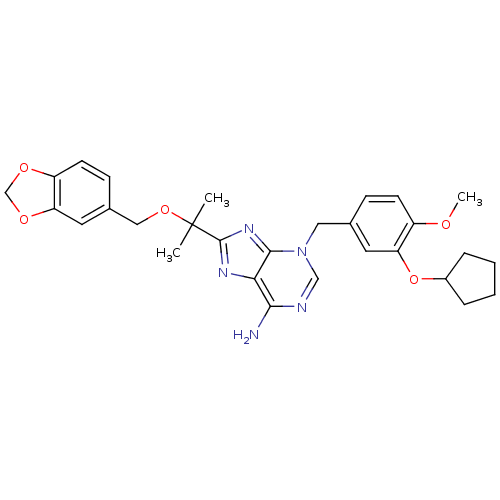 (3-(3-Cyclopentyloxy-4-methoxy-benzyl)-8-[1-(3,4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor of rat forebrain | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM108418 (US8604206, 13 | US8604206, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4 | n/a | 7 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amicus Therapeutics, Inc. US Patent | Assay Description The enzyme inhibition assays used monitored the ability of a test compound to bind and prevent the hydrolysis of a fluorogenic substrate in a concent... | US Patent US8604206 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14365 (8-(1-Benzyloxy-1-methyl-ethyl)-3-(3-cyclopentyloxy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14379 (3-(3-Cyclopentyloxy-4-methoxy-benzyl)-8-[1-(4-tert...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290526 (CHEMBL328036 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068674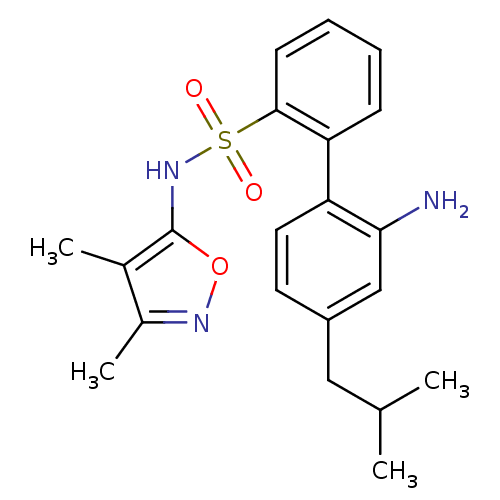 (2'-Amino-4'-isobutyl-biphenyl-2-sulfonic acid (3,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal alpha-glucosidase (Homo sapiens (Human)) | BDBM50583383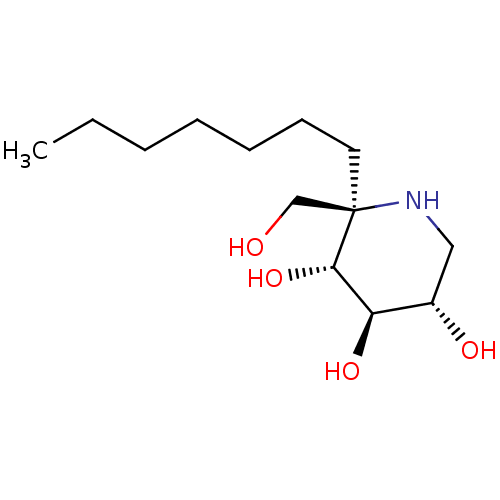 (CHEMBL5028005 | US20230339856, Compound (IIb3)) | PDB NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01673 BindingDB Entry DOI: 10.7270/Q23J3HVM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14370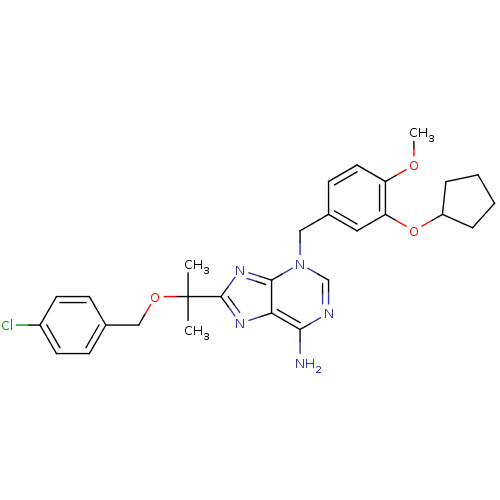 (8-{1-[(4-Chlorobenzyl)oxy]-1-methyl-ethyl}-3-[3-cy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14382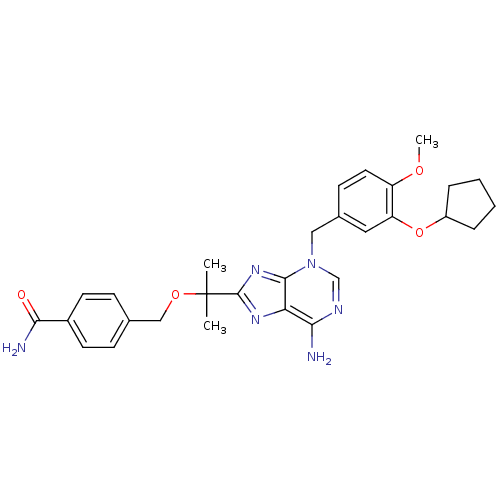 (4-{2-[6-Amino-3-(3-cyclopentyloxy-4-methoxy-benzyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14379 (3-(3-Cyclopentyloxy-4-methoxy-benzyl)-8-[1-(4-tert...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068720 (6-Hydroxy-4'-isobutyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50068713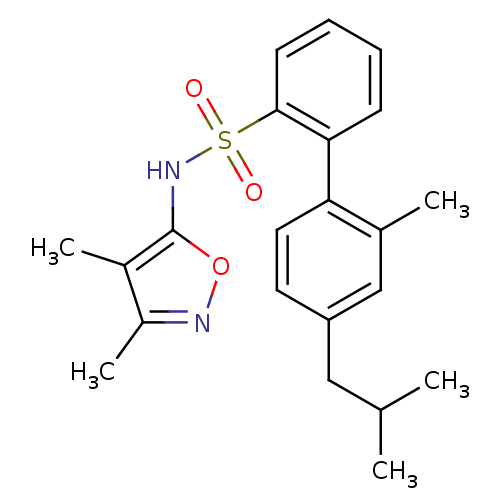 (4'-Isobutyl-2'-methyl-biphenyl-2-sulfonic acid (3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Ability to inhibit binding of [125I]ET1 to membranes prepared from A10 rat thoracic aorta smooth muscle Endothelin A receptor | J Med Chem 41: 5198-218 (1999) Article DOI: 10.1021/jm970872k BindingDB Entry DOI: 10.7270/Q2J9672R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50290522 (CHEMBL313237 | Quinoline-2-carboxylic acid [(R)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 7: 2635-2638 (1997) Article DOI: 10.1016/S0960-894X(97)10054-3 BindingDB Entry DOI: 10.7270/Q2G44Q84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14367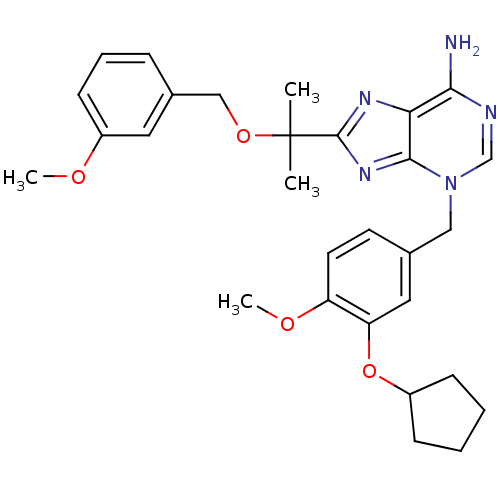 (3-(3-Cyclopentyloxy-4-methoxy-benzyl)-8-[1-(3-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM14366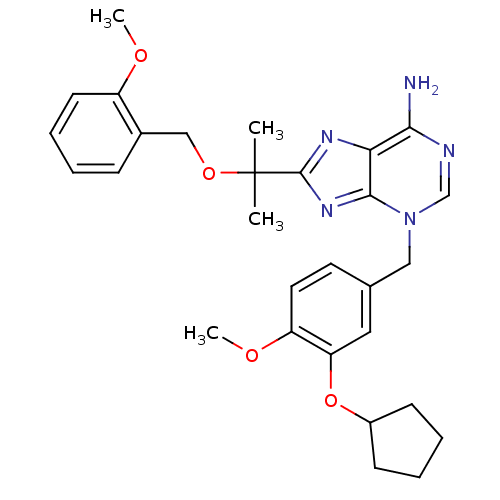 (3-(3-Cyclopentyloxy-4-methoxy-benzyl)-8-[1-(2-meth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50058235 (8-(3-Oxa-tricyclo[3.2.1.0*2,4*]oct-6-yl)-1,3-dipro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CV Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Adenosine A1 receptor of rat forebrain | J Med Chem 40: 1773-8 (1997) Article DOI: 10.1021/jm970013w BindingDB Entry DOI: 10.7270/Q28K786M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM14381 (4-{2-[6-Amino-3-(3-cyclopentyloxy-4-methoxy-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue Pharma LP | Assay Description PDE4 enzyme activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using SPA kit. [3H]-AMP was captured by the SPA beads, and qu... | J Med Chem 48: 1237-43 (2005) Article DOI: 10.1021/jm030603w BindingDB Entry DOI: 10.7270/Q2GT5KF0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4190 total ) | Next | Last >> |