

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145347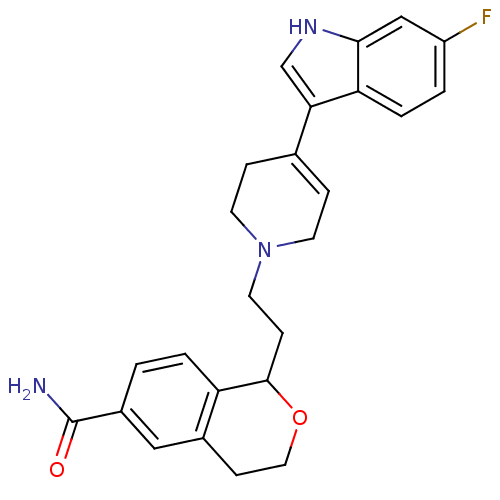 (1-(2-(4-(6-fluoro-1H-indol-3-yl)-5,6-dihydropyridi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM50143037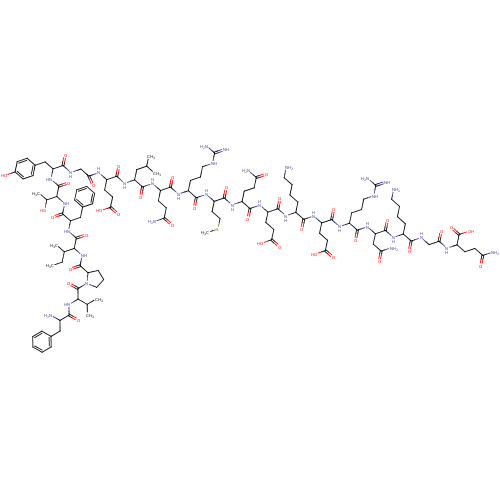 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50451852 (CHEMBL1159649) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145355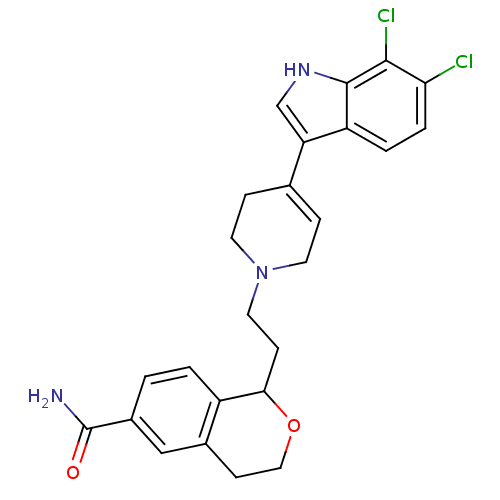 (1-{2-[4-(6,7-Dichloro-1H-indol-3-yl)-3,6-dihydro-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145343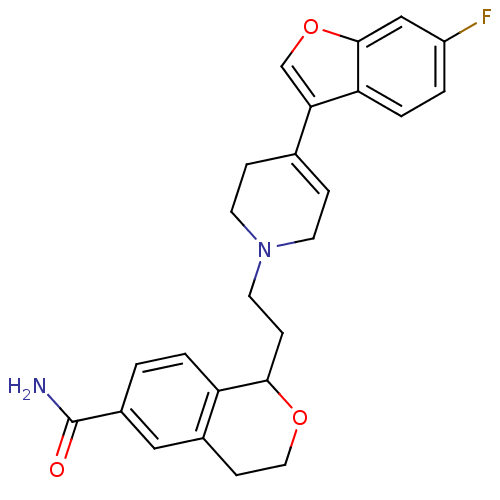 (1-{2-[4-(6-Fluoro-benzofuran-3-yl)-3,6-dihydro-2H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145356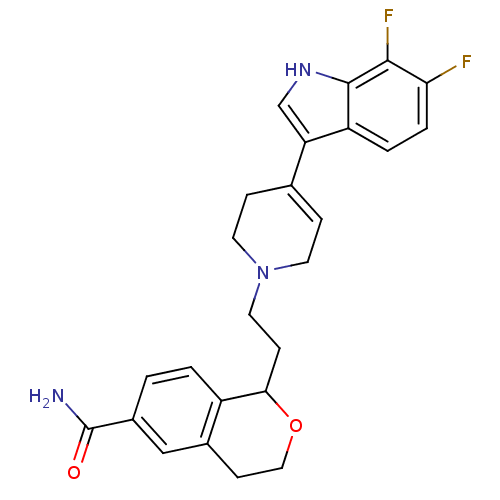 (1-{2-[4-(6,7-Difluoro-1H-indol-3-yl)-3,6-dihydro-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50366907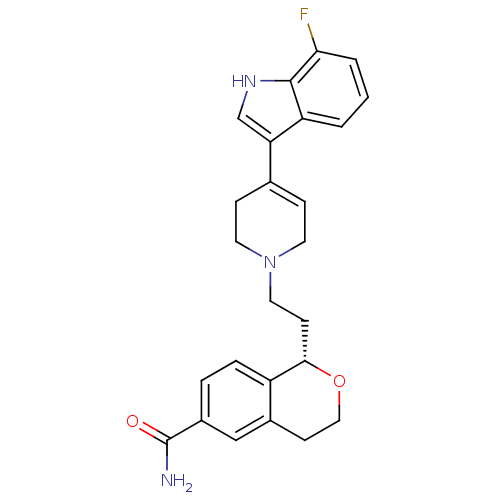 (CHEMBL1169435) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145342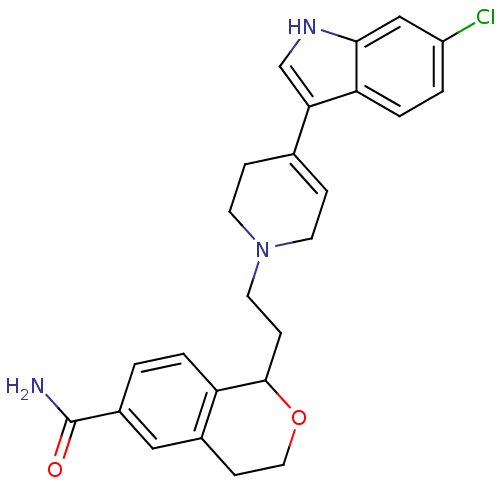 (1-{2-[4-(6-Chloro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM86314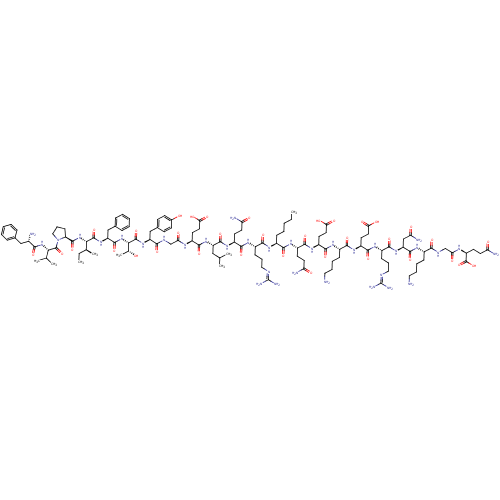 (CAS_0 | NSC_0 | [Nle13]-Motilin) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.447 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145354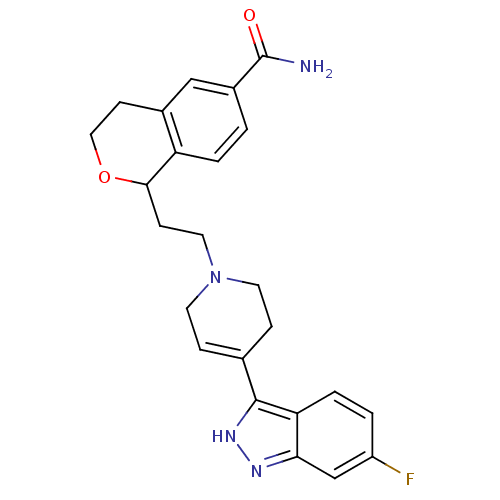 (1-{2-[4-(6-Fluoro-1H-indazol-3-yl)-3,6-dihydro-2H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145350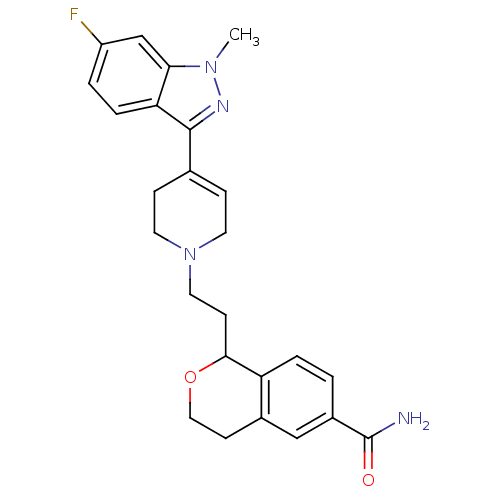 (1-{2-[4-(6-Fluoro-1-methyl-1H-indazol-3-yl)-3,6-di...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000483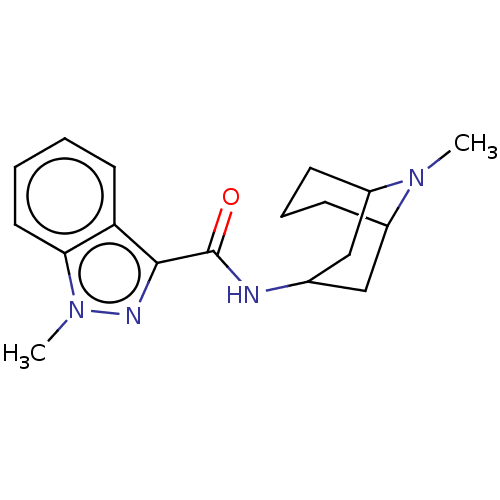 ((BRL 43694)1-Methyl-1H-indazole-3-carboxylic acid ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Beecham Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Binding affinity for central 5-hydroxytryptamine 3 receptor was determined by displacement of [3H]-ketanserin | J Med Chem 33: 1924-9 (1990) BindingDB Entry DOI: 10.7270/Q2QN67CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145349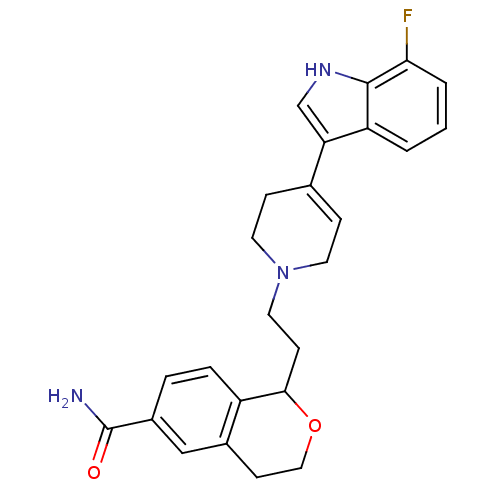 (1-{2-[4-(7-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145338 (1-{2-[(S)-4-(6-Fluoro-1H-indol-3-yl)-2-methyl-pipe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM86314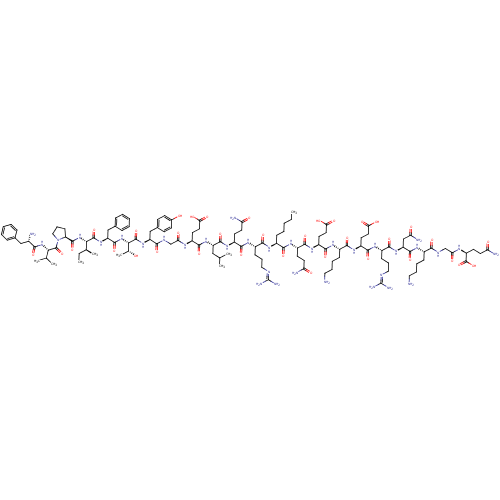 (CAS_0 | NSC_0 | [Nle13]-Motilin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145344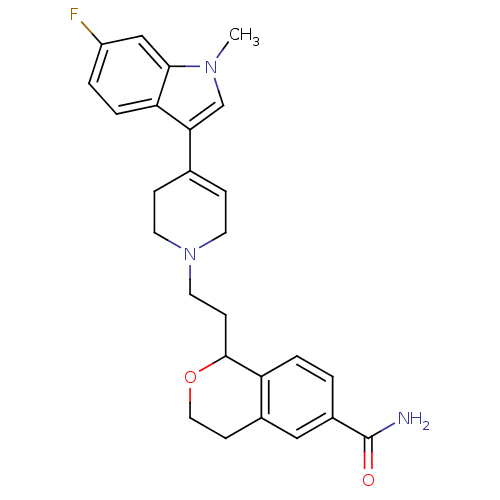 (1-{2-[4-(6-Fluoro-1-methyl-1H-indol-3-yl)-3,6-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM50143037 (CHEMBL411576 | MOTILIN) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145346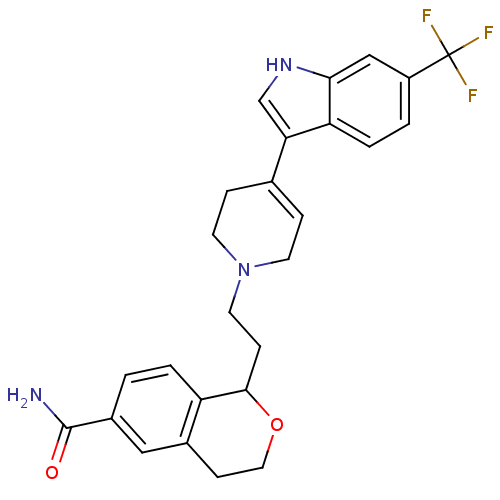 (1-{2-[4-(6-Trifluoromethyl-1H-indol-3-yl)-3,6-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145336 (1-{2-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-3,6-dihyd...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145352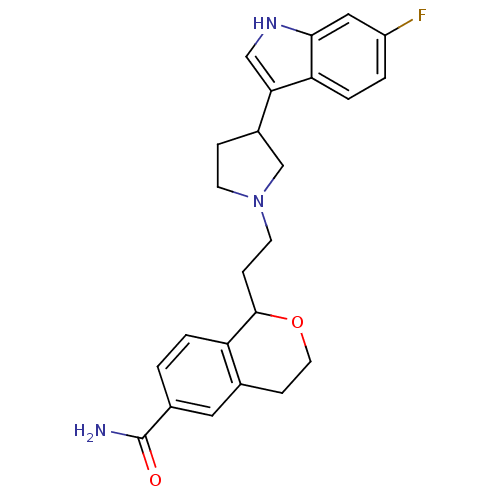 (1-{2-[3-(6-Fluoro-1H-indol-3-yl)-pyrrolidin-1-yl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM50143037 (CHEMBL411576 | MOTILIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM50143037 (CHEMBL411576 | MOTILIN) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145341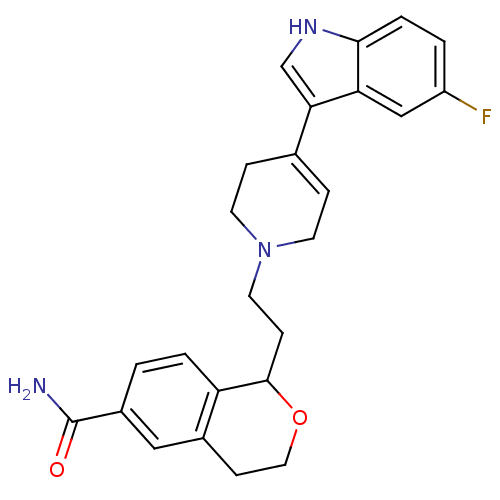 (1-{2-[4-(5-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (RABBIT) | BDBM86314 (CAS_0 | NSC_0 | [Nle13]-Motilin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50451852 (CHEMBL1159649) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50451853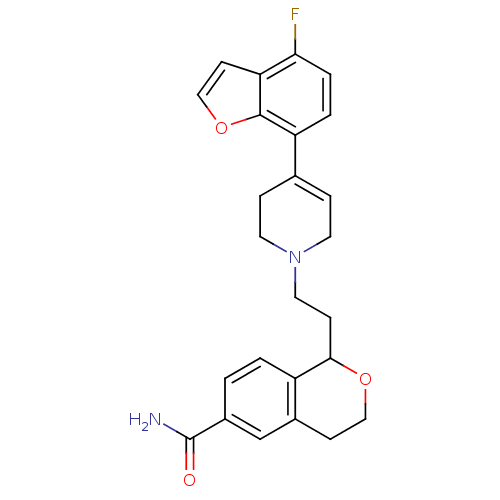 (CHEMBL2112307) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Promotilin (Homo sapiens (Human)) | BDBM86314 (CAS_0 | NSC_0 | [Nle13]-Motilin) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by PDSP Ki Database | Br J Pharmacol 140: 948-54 (2003) Article DOI: 10.1038/sj.bjp.0705505 BindingDB Entry DOI: 10.7270/Q2N58JZF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145337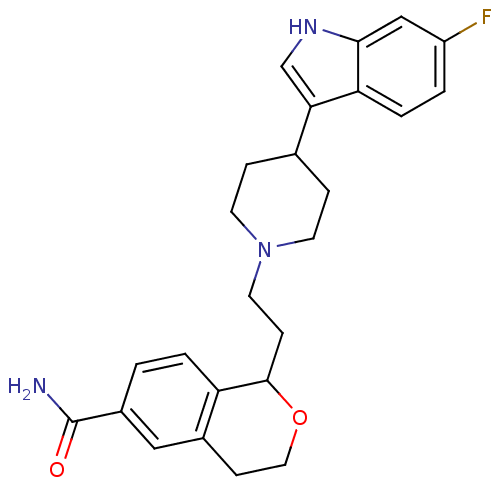 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperidin-1-yl]-e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145352 (1-{2-[3-(6-Fluoro-1H-indol-3-yl)-pyrrolidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145357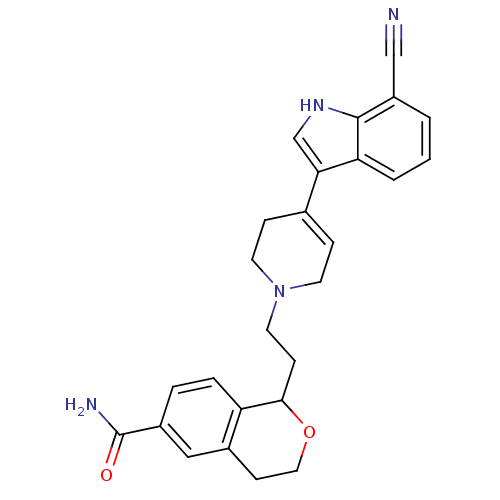 (1-{2-[4-(7-Cyano-1H-indol-3-yl)-3,6-dihydro-2H-pyr...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145354 (1-{2-[4-(6-Fluoro-1H-indazol-3-yl)-3,6-dihydro-2H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145341 (1-{2-[4-(5-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145351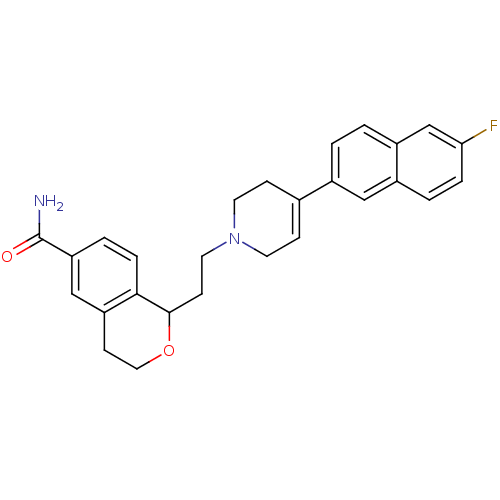 (1-{2-[4-(6-Fluoro-naphthalen-2-yl)-3,6-dihydro-2H-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50366907 (CHEMBL1169435) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145343 (1-{2-[4-(6-Fluoro-benzofuran-3-yl)-3,6-dihydro-2H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145339 (1-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-3,6-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145333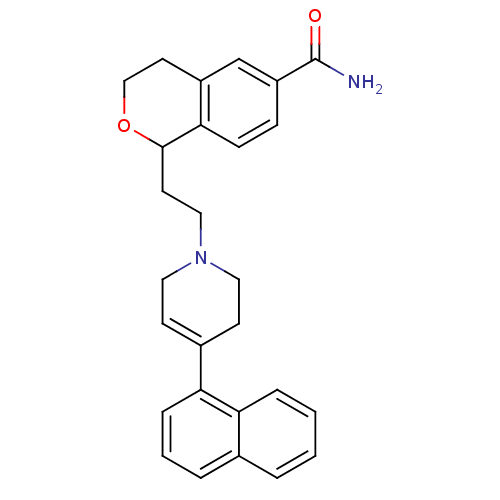 (1-[2-(4-Naphthalen-1-yl-3,6-dihydro-2H-pyridin-1-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145332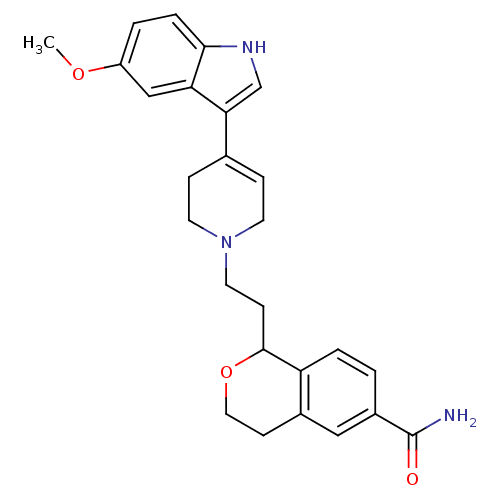 (1-{2-[4-(5-Methoxy-1H-indol-3-yl)-3,6-dihydro-2H-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145336 (1-{2-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-3,6-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145349 (1-{2-[4-(7-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145347 (1-(2-(4-(6-fluoro-1H-indol-3-yl)-5,6-dihydropyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50145333 (1-[2-(4-Naphthalen-1-yl-3,6-dihydro-2H-pyridin-1-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor in L-M(tk-) cells using [3H]GR-125743 as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145334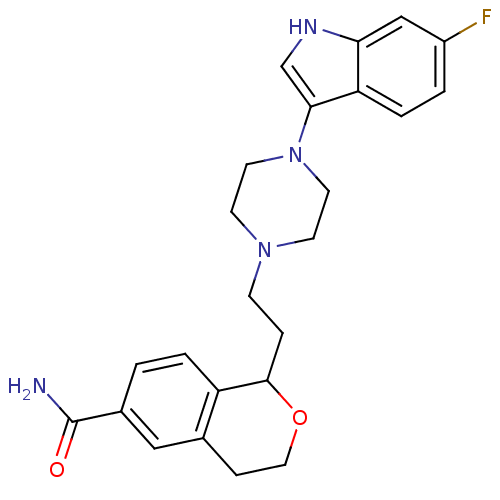 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperazin-1-yl]-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145356 (1-{2-[4-(6,7-Difluoro-1H-indol-3-yl)-3,6-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50366907 (CHEMBL1169435) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145334 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperazin-1-yl]-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50145339 (1-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-3,6-dihyd...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description In vitro binding affinity towards serotonin transporter using [3H]citalopram as radioligand in rat cerebral cortex membranes | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50145337 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperidin-1-yl]-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity towards human 5-hydroxytryptamine 1D receptor in L-M(tk-) cells using [3H]GR-125743 as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50145346 (1-{2-[4-(6-Trifluoromethyl-1H-indol-3-yl)-3,6-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against dopamine receptor D2 in rat caudate membranes using [3H]- raclopride as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50145337 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-piperidin-1-yl]-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co. Ltd Curated by ChEMBL | Assay Description Binding affinity determined against alpha-1 adrenergic receptor in rat cortex membranes using [3H]prazosin as radioligand | Bioorg Med Chem Lett 14: 2469-72 (2004) Article DOI: 10.1016/j.bmcl.2004.03.003 BindingDB Entry DOI: 10.7270/Q2057GG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 602 total ) | Next | Last >> |