

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245983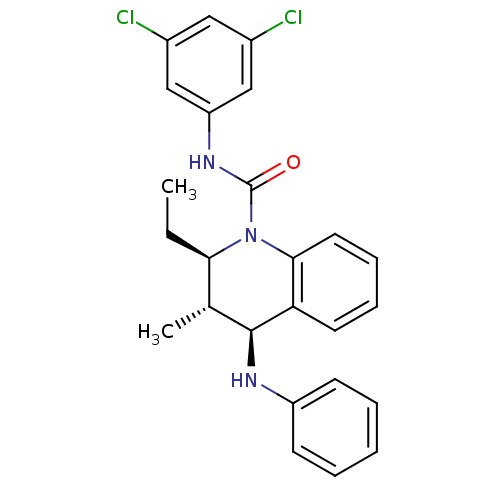 ((2R,3S,4S)-2-Ethyl-3-methyl-N-[3,5-(chloro)phenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245937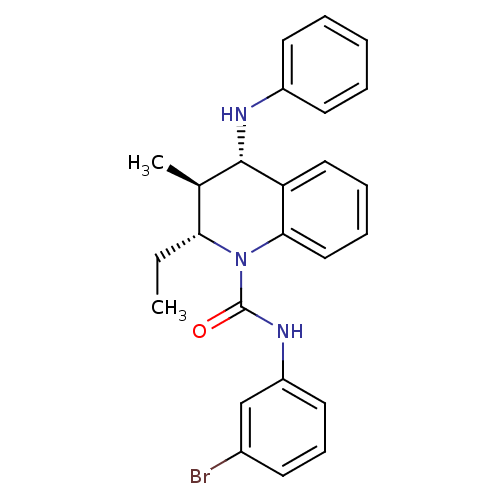 ((2R,3S,4S)-N-(3-bromophenyl)-2-ethyl-3-methyl-4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245772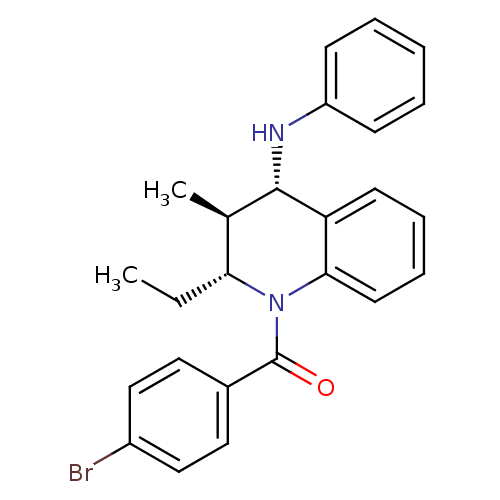 ((4-bromophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246518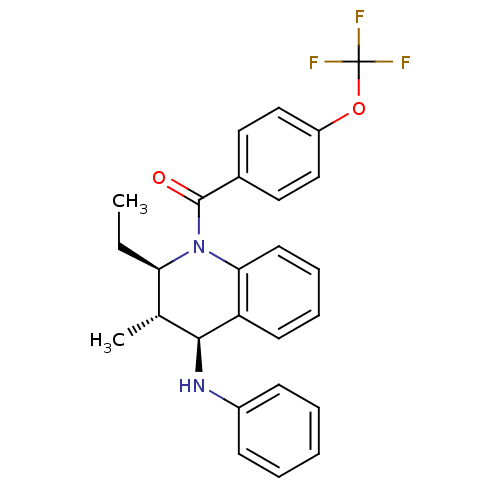 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245982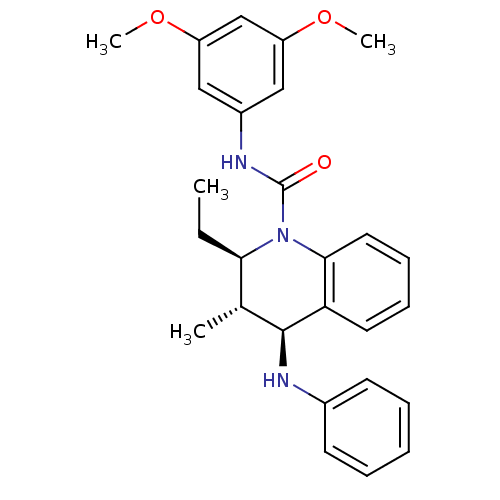 ((2R,3S,4S)-N-(3,5-dimethoxyphenyl)-2-ethyl-3-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245934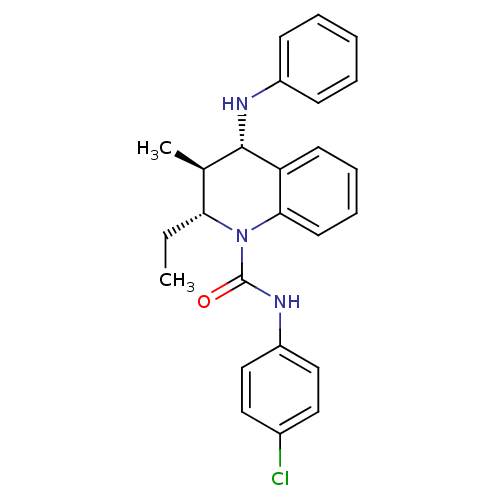 ((2R,3S,4S)-N-(4-chlorophenyl)-2-ethyl-3-methyl-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245835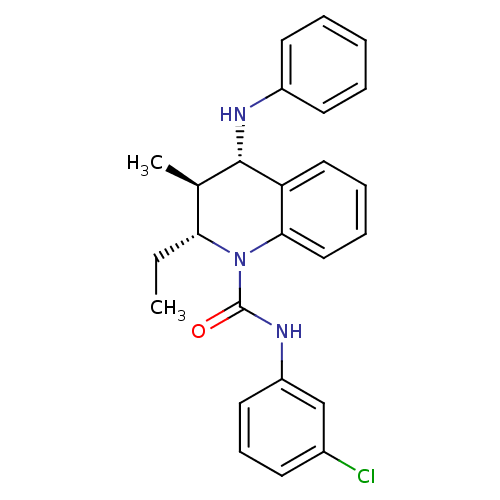 ((2R,3S,4S)-N-(3-chlorophenyl)-2-ethyl-3-methyl-4-(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50089354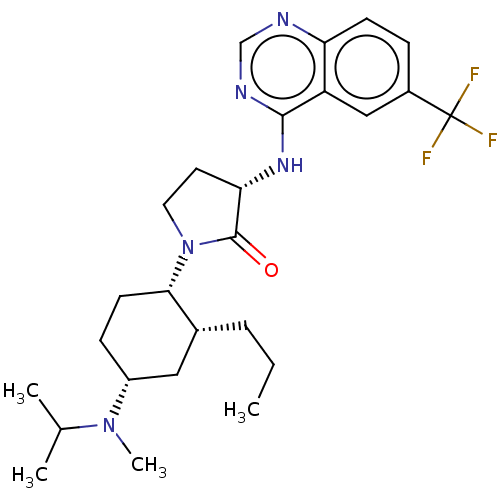 (CHEMBL3577945) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M1 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246517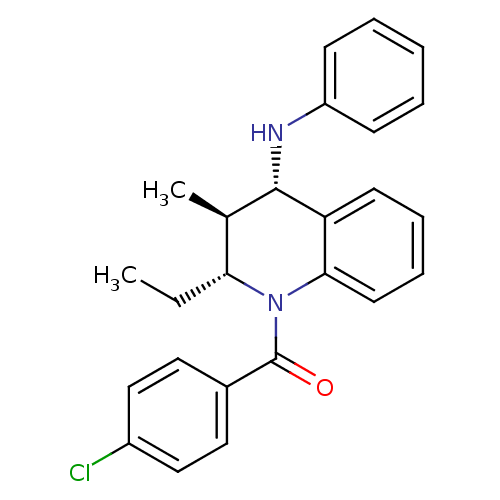 ((4-chlorophenyl)((2R,3S,4S)-2-ethyl-3-methyl-4-(ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246516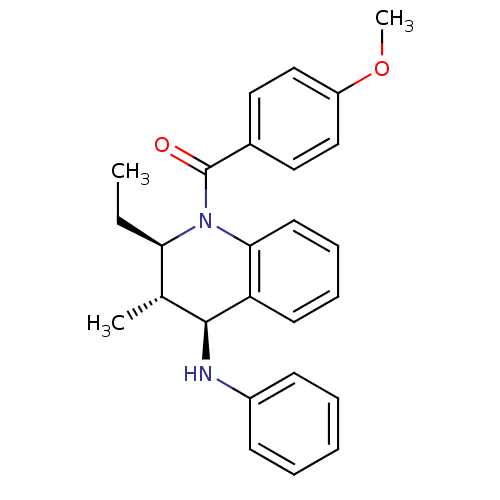 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245981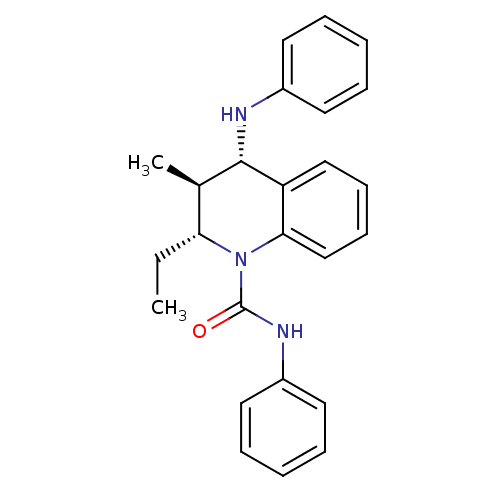 ((2R,3S,4S)-2-ethyl-3-methyl-N-phenyl-4-(phenylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245935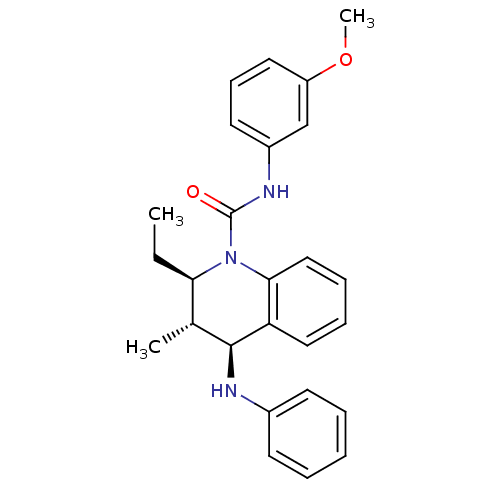 ((2R,3S,4S)-2-ethyl-N-(3-methoxyphenyl)-3-methyl-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50246468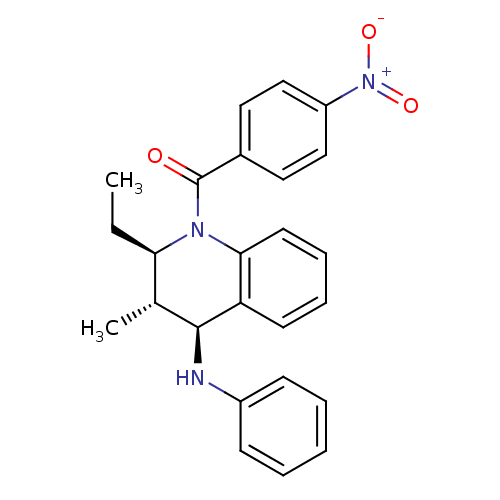 (((2R,3S,4S)-2-ethyl-3-methyl-4-(phenylamino)-3,4-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50245984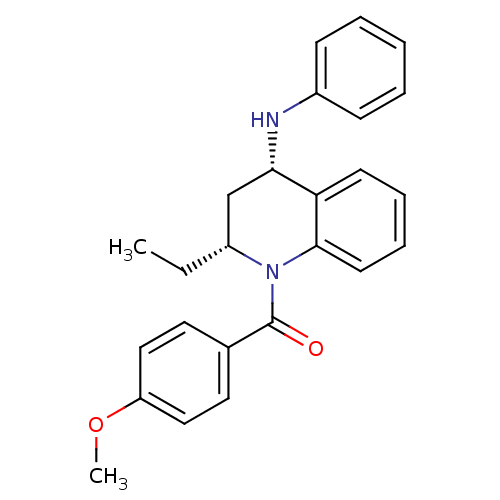 (CHEMBL516508 | cis-(2(R)-ethyl-4-(phenylamino)-3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [33P]2-Mes-ADP from human recombinant P2Y1 receptor expressed in human U20S cells | Bioorg Med Chem Lett 18: 6222-6 (2008) Article DOI: 10.1016/j.bmcl.2008.09.102 BindingDB Entry DOI: 10.7270/Q26Q1X42 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M4 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M2 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM141485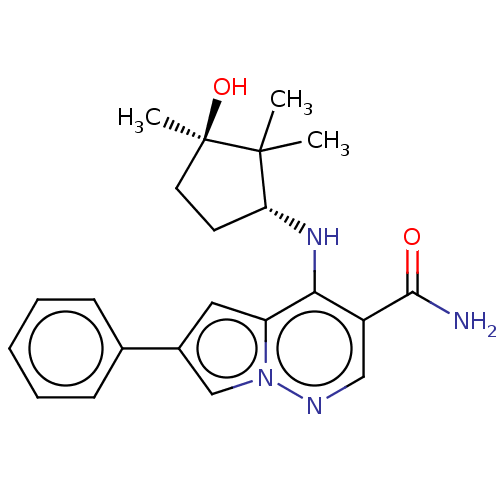 (US8921368, 293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 ul prepared from 15 ul additions of enzyme and substr... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245763 (US9428511, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141459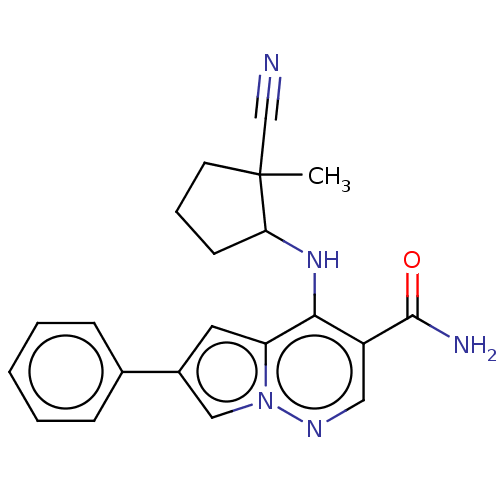 (US8921368, 123) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141485 (US8921368, 293) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245770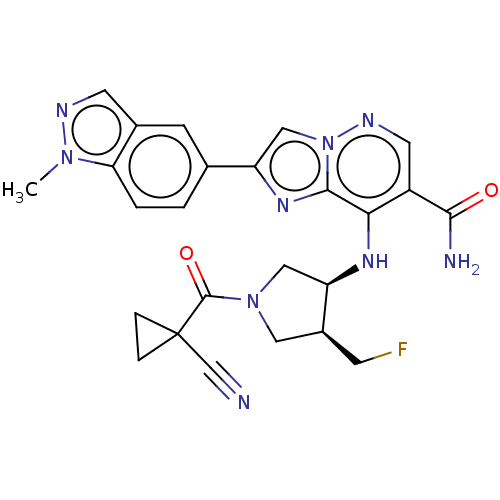 (US9428511, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141480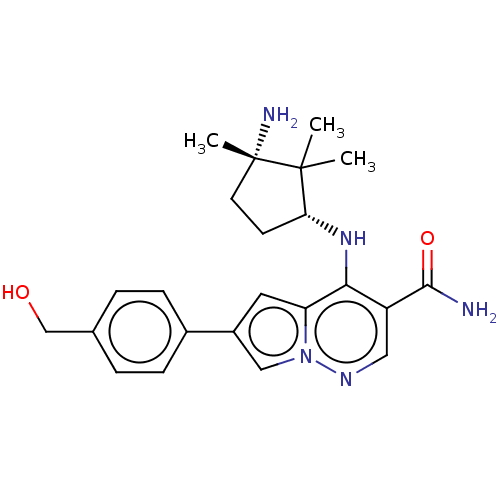 (US8921368, 271) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141498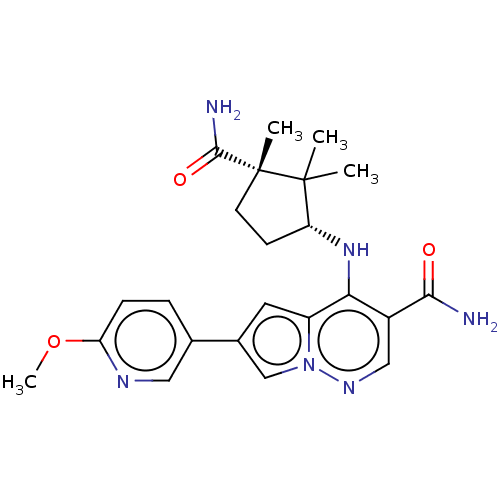 (US8921368, 338) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557876 (CHEMBL4764460) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 30 mins by calcein-AM dye based fluor... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00082 BindingDB Entry DOI: 10.7270/Q24J0JS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245761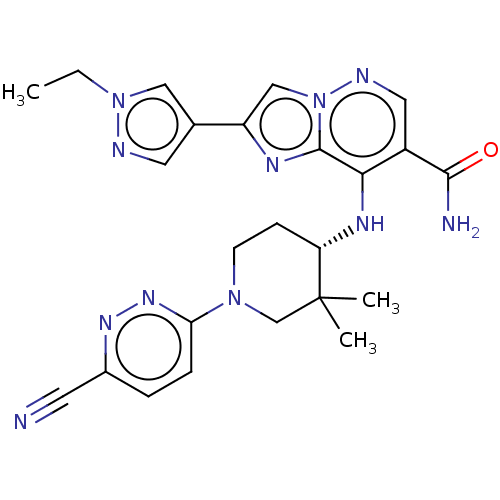 (US9428511, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141456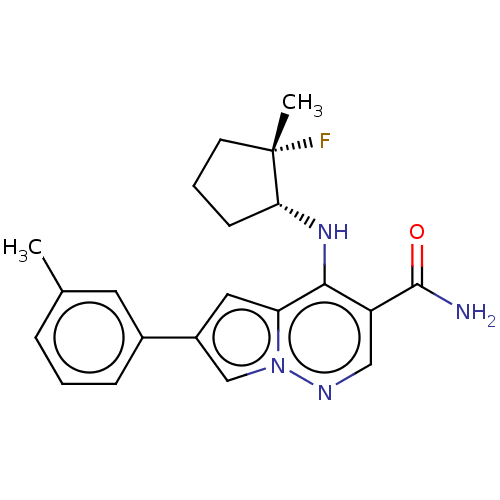 (US8921368, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141497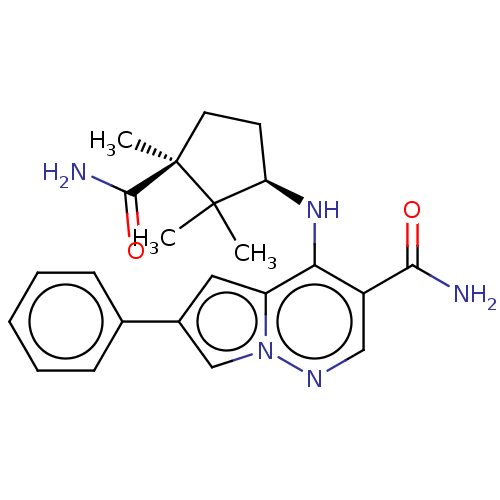 (US8921368, 337) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50509862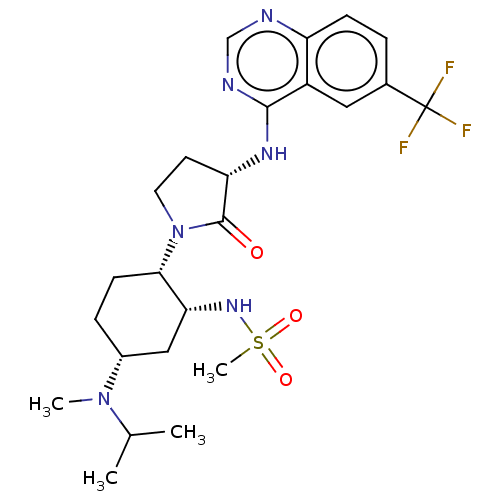 (CHEMBL4457723) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of [125I]MCP1 from CCR2 in human PBMC measured after 30 mins | ACS Med Chem Lett 10: 300-305 (2019) Article DOI: 10.1021/acsmedchemlett.8b00439 BindingDB Entry DOI: 10.7270/Q22R3W0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089366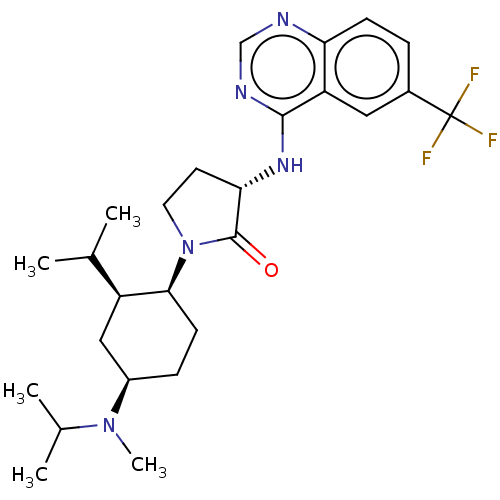 (CHEMBL3577948) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245762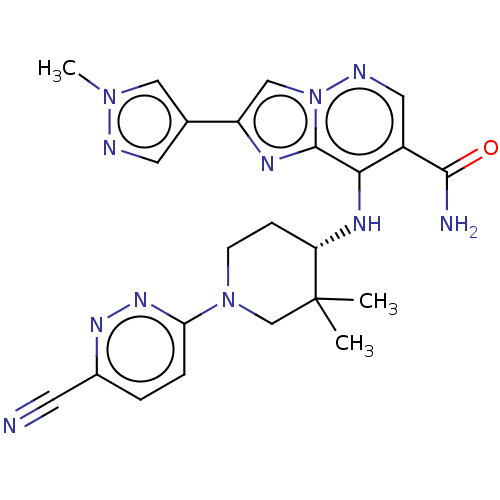 (US9428511, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245772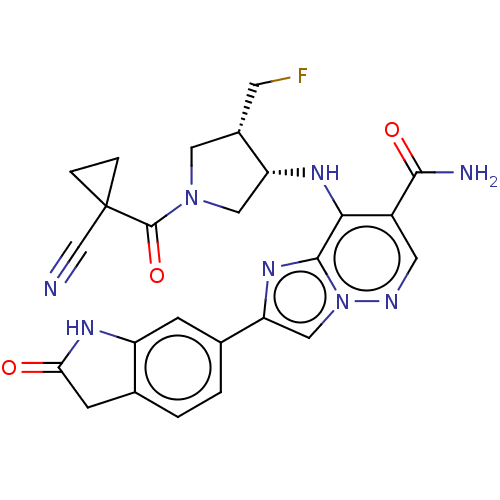 (US9428511, 64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089356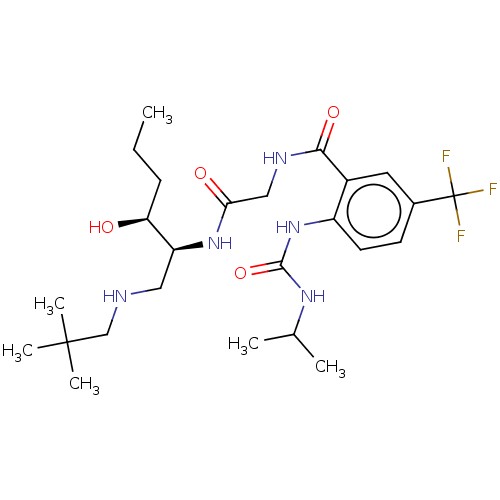 (CHEMBL3577933) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089355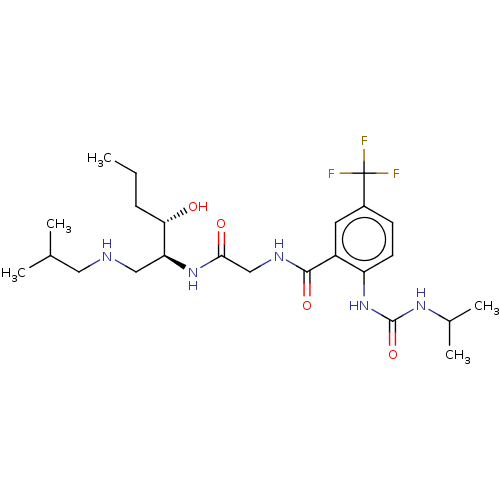 (CHEMBL3577932) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50435764 (CHEMBL2392692) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human soluble epoxide hydrolase using PHOME as substrate assessed as formation of 6-methoxy-2-naphthaldehyde incubated for 10 mins prio... | Bioorg Med Chem Lett 23: 3584-8 (2013) Article DOI: 10.1016/j.bmcl.2013.04.019 BindingDB Entry DOI: 10.7270/Q2J967S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245779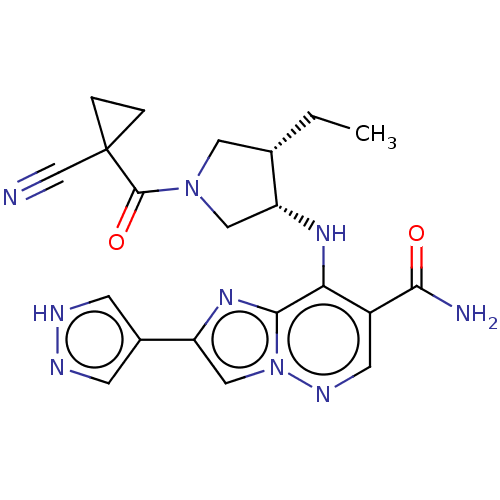 (US9428511, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141488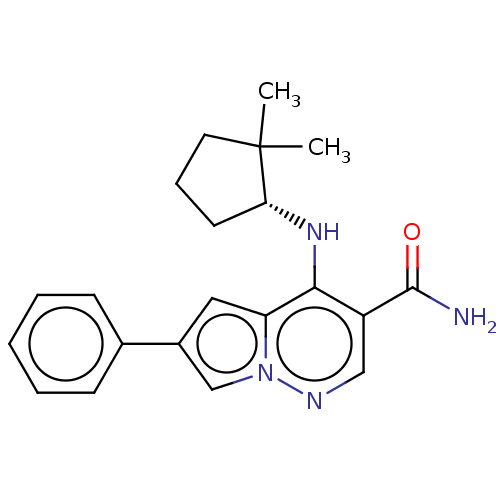 (US8921368, 304) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141473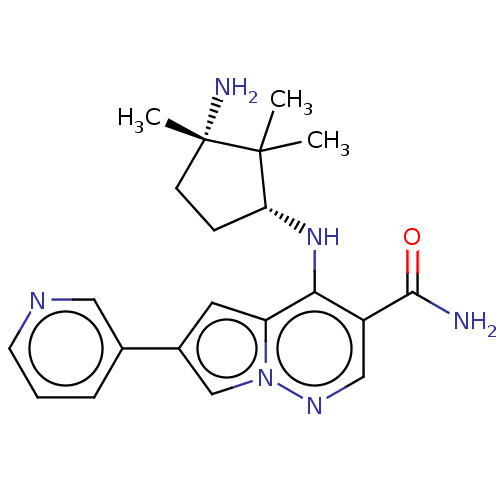 (US8921368, 192) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245762 (US9428511, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245760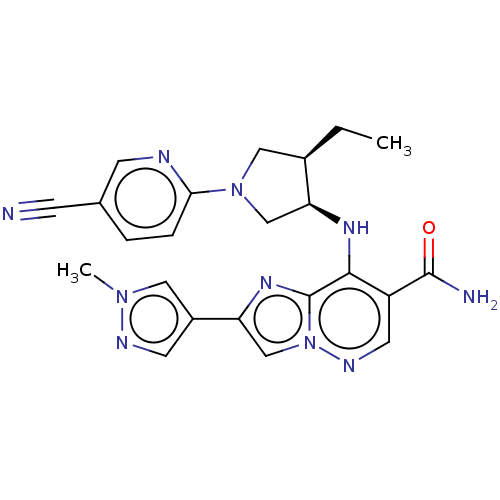 (US9428511, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141457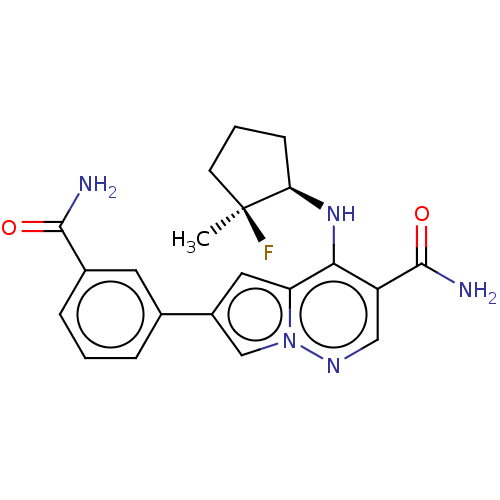 (US8921368, 94) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM141463 (US8921368, 159) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description Kinase reactions consisted of 5 ng of JAK3 enzyme, 30 uM CSKtide substrate, 0.2 uCi gamma-33P ATP, 8 uM ATP in 30 ul kinase buffer (50 mM Hepes, pH ... | US Patent US8921368 (2014) BindingDB Entry DOI: 10.7270/Q2HH6HS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364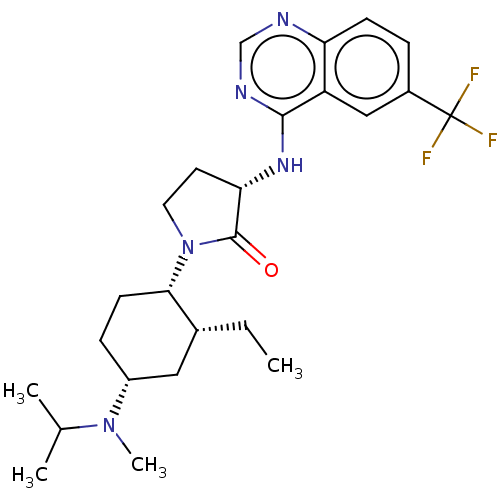 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antagonist activity against CCR2 in human PBMC assessed as inhibition of MCP1-induced chemotaxis at 37 degC | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50089364 (CHEMBL3577946) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of [125I]hMCP1 binding to CCR2 in human PBMC incubated for 30 mins at room temperature | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245763 (US9428511, 55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245761 (US9428511, 53) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM245734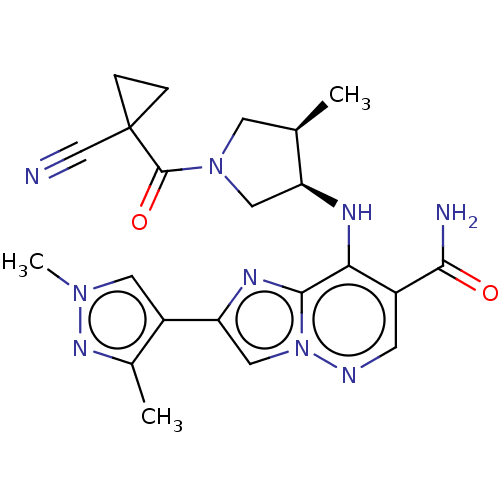 (US9428511, 26) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50557877 (CHEMBL4790208) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at CCR2 in human THP1 cells assessed as reduction in MCP1-induced chemotaxis measured after 30 mins by calcein-AM dye based fluor... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00082 BindingDB Entry DOI: 10.7270/Q24J0JS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245722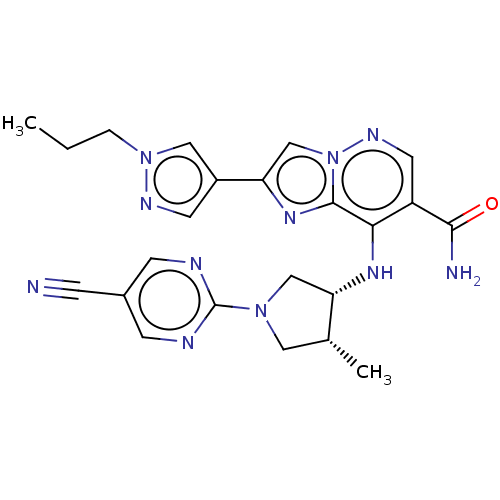 (US9428511, 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM245751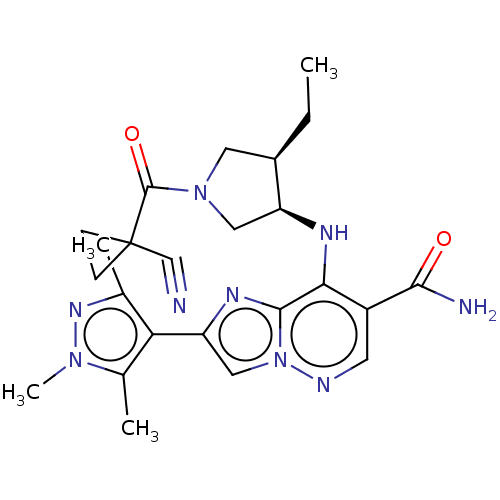 (US9428511, 43) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Bristol-Myers Squibb Company US Patent | Assay Description The assay reactions were performed in U-bottom 384-well plates. The final assay volume was 30 μl prepared from 15 μl additions of enzyme and su... | US Patent US9428511 (2016) BindingDB Entry DOI: 10.7270/Q27080B9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1022 total ) | Next | Last >> |